Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.2 Pretoria Fev. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i2.16681
RESEARCH
Risk stratification of hospital admissions for COVID-19 pneumonia by chest radiographic scoring in a Johannesburg tertiary hospital
H C LabuschagneI; J VenturasII, III; H MoodleyIV
IMB ChB, Dip HIV Man (SA); Department of Radiology, Charlotte Maxeke Johannesburg Academic Hospital, and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB, FCP (SA); Department of Internal Medicine, Charlotte Maxeke Johannesburg Academic Hospital, and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMB ChB, FCP (SA); Department of Respiratory Medicine, Waikato District Health Board, Hamilton, New Zealand
IVMB ChB, MMed Diag Rad; Department of Radiology, Charlotte Maxeke Johannesburg Academic Hospital, and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Chest radiographic scoring systems for COVID-19 pneumonia have been developed. However, little is published on the utility of these scoring systems in low- and middle-income countries.
OBJECTIVES. To perform risk stratification of COVID-19 pneumonia in Johannesburg, South Africa (SA), by comparing the Brixia score with clinical parameters, disease course and clinical outcomes. To assess inter-rater reliability and developing predictive models of the clinical outcome using the Brixia score and clinical parameters.
METHODS. Retrospective investigation was conducted of adult participants with established COVID-19 pneumonia admitted at a tertiary institution from 1 May to 30 June 2020. Two radiologists, blinded to clinical data, assigned Brixia scores. Brixia scores were compared with clinical parameters, length of stay and clinical outcomes (discharge/death). Inter-rater agreement was determined. Multivariable logistic regression extracted variables predictive of in-hospital demise.
RESULTS. The cohort consisted of 263 patients, 51% male, with a median age of 47 years (interquartile range (IQR) = 20; 95% confidence interval (CI) 46.5 - 49.9). Hypertension (38.4%), diabetes (25.1%), obesity (19.4%) and HIV (15.6%) were the most common comorbidities. The median length of stay for 258 patients was 7.5 days (IQR = 7; 95% CI 8.2 - 9.7) and 6.5 days (IQR = 8; 95% CI 6.5 - 12.5) for intensive care unit stay. Fifty (19%) patients died, with a median age of 55 years (IQR = 23; 95% CI 50.5 - 58.7) compared with survivors, of median age 46 years (IQR = 20; 95% CI 45 - 48.6) (p=0.01). The presence of one or more comorbidities resulted in a higher death rate (23% v. 9.2%; p=0.01) than without comorbidities. The median Brixia score for the deceased was higher (14.5) than for the discharged patients (9.0) (p<0.001). Inter-rater agreement for Brixia scores was good (intraclass correlation coefficient 0.77; 95% CI 0.6 - 0.85; p<0.001). A model combining Brixia score, age, male gender and obesity (sensitivity 84%; specificity 63%) as well as a model with Brixia score and C-reactive protein (CRP) count (sensitivity 81%; specificity 63%) conferred the highest risk for in-hospital mortality.
CONCLUSION. We have demonstrated the utility of the Brixia scoring system in a middle-income country setting and developed the first SA risk stratification models incorporating comorbidities and a serological marker. When used in conjunction with age, male gender, obesity and CRP, the Brixia scoring system is a promising and reliable risk stratification tool. This may help inform the clinical decision pathway in resource-limited settings like ours during future waves of COVID-19.
SARS-CoV-2 is the novel viral pathogen responsible for the potentially serious pulmonary infection known as COVID-19 pneumonia, now a global pandemic.[1,2] The clinical disease progression varies from asymptomatic to severe pneumonia with multiorgan dysfunction that may result in death.[2,3] The benchmark test for COVID-19 is a nucleic acid amplification test (NAAT) using reverse transcriptase polymerase chain reaction (RT-PCR) to detect SARS-CoV-2 in respiratory samples.[4,5] However, it has limitations, including false negative rates of up to 58%,[6] difficulties associated with large-scale testing in low- and middle-income countries and lengthy turnaround times during the height of the pandemic.[7,8] Thus various radiological imaging modalities have proven to be integral ancillary tools in the triage and management of COVID-19.[9,10]
The most sensitive imaging tool to detect COVID-19-related lung changes is computed tomography (CT), and quantification methods to help assess disease severity have been developed.[11,12] The sensitivity for typical COVID-19 features using CT is as high as 98%,[13] but CT is not universally used as first-line imaging, particularly in resource-constrained environments, owing to various factors that include the unavailability of CT, logistics required to transport patients while limiting cross-infection and disinfection of the CT scanner in between patients. The sensitivity for detecting COVID-19-related lung changes using chest radiographs (CXR) is low compared with CT, with reported sensitivities ranging from 38% to 89%.[12,14,15] Despite CXR sensitivity being inferior to CT, it has been widely used as first-line imaging both internationally and locally owing to its availability, ability to be performed at the bedside, cost-effectiveness, lower radiation dose than CT, fast patient throughput and rapid disinfection.[16,17] Locally, CXR has been employed as the first-line imaging tool, with CT reserved for specific scenarios and problem-solving situations.
The CXR abnormalities during COVID-19 chest infection span a range of findings. The most commonly described abnormalities are ground-glass opacification and areas of lung consolidation[17,18] usually with bilateral involvement, lower zone and peripheral predilection.[12,17] Pleural effusion, pneumothorax and cavitation have also been reported. However, these are not common.[17,18] A recent South African (SA) study corroborated ground-glass opacification and consolidation as the most frequent radiographic findings, the majority of which were bilateral.[19] This study reported the presence of pleural effusion in 29.1% of cases,[19] which is high compared with international publications, and was considered unusual.[12,18,19]
CXR scoring systems have been developed and implemented as triaging and risk stratification tools in multiple settings with promising results, helping to quantify disease severity, prognosticating disease course and outcome and ultimately aiding clinical decision-making.[16,20-26] These scoring systems are based on airspace opacities and interstitial findings.'[6,20,22]
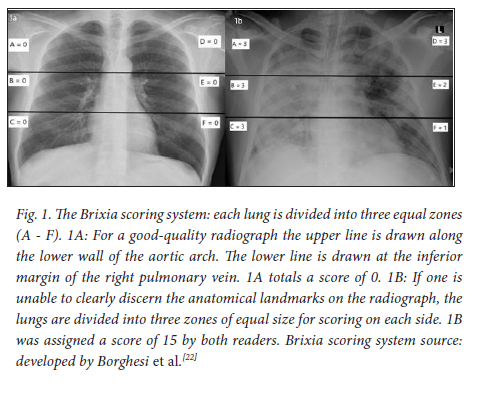
The Brixia scoring tool, an 18-point scoring system that divides the lungs into six equal zones, incorporates the character of lung abnormalities and has defined a clear threshold score (>8 points) for severe disease.[22,23] Borghesi et al.[22]pioneered the Brixia scoring system and conducted a study that determined that the Brixia score, age and immunosuppression were factors that conferred the highest risk of death.[20] Their study did not include obesity or laboratory markers.[20] In addition, the Brixia scoring system consistently conferred good, and in certain instances, near-perfect inter-rater reliability in local and international studies.[16,19,27] To the authors' best knowledge, Brixia is the most widely investigated CXR scoring tool for COVID-19 pneumonia. When compared with various other scoring systems, Brixia demonstrated superior inter-rater reliability,[22,24] was quicker to use[27] and is the only chest radiographic scoring system designed specifically with COVID-19 in mind, with a clear threshold score for severe disease.[23] Table 1 compares these scoring systems.
The utility of the Brixia scoring system in a SA setting has only been evaluated once, and while that study found that higher Brixia scores were related to increased risk of demise, the study did not compare the scores with comorbid conditions or laboratory markers.[19] In addition to the authors' best knowledge, other international studies utilising the Brixia scoring tool have not found obesity, specific anthropometric parameters, diabetes mellitus and poor glycaemic control to have a powerful predictive power of final patient outcome.[16,20]
Locally and internationally, ethnicity and comorbid disease, such as obesity, diabetes mellitus and HIV, have been associated with increased COVID-19 mortality.'32-341 It has been shown that HIV-positive participants not receiving antiretroviral therapy are specifically at increased risk of death from COVID-19.'341 Obesity's role in the disease course and prognostication of hospitalised individuals with COVID-19 has been assessed, with multiple studies implicating it as a potential risk factor for progression to severe disease'35,361 and possibly higher risk of death.'371 A meta-analysis by Booth et al.'381 evaluating data from 76 studies found that severe obesity was a commonly reported comorbidity for adverse outcome. Early local epidemiological data indicated that around 5.5% of SA patients who died from COVID-19 suffered from obesity.[39] It has been demonstrated that diabetes mellitus and substandard glycaemic control may confer a worse prognosis in those hospitalised with COVID-19.[40,41]
The study focus was to evaluate the utility of the Brixia scoring system combined with participant demographics, comorbidities and laboratory parameters for predicting disease course and patient outcome (in-hospital demise v. discharge) in the SA context, where we have a high prevalence of HIV co-infection,[42] among other comorbidities.
Methods
Ethical considerations
Ethical clearance was secured from the Human Research Ethics committee of the University of the Witwatersrand (ref. no. M2011113).
Population and sampling strategy
We identified hospitalised participants with RT-PCR confirmed COVID-19 pneumonia from a period spanning 1 May 2020 to 30 June 2020. Inclusion criteria comprised the following: (i) patient >18 years; (if) a CXR, either mobile anteroposterior or erect posteroanterior, must have been performed while in the hospital receiving treatment for RT-PCR-confirmed COVID-19 pneumonia; and (iii) the clinical outcome, defined as either death or discharge from the hospital for each participant. A total of 263 participants were retrospectively enrolled in the study.
Data collection
Participant demographic information, clinical parameters and laboratory data were collected. These data consisted of the participant sex, age, comorbidities (to meet the case definition of obesity a documented body mass index of >30 kg/m2 must have been recorded), laboratory data, need for ventilatory support (both non-invasive and invasive techniques), length of stay, days spent in intensive care unit (ICU) and recovery or demise. The corresponding CXR for each participant was retrieved from the hospital picture archiving and communication system (PACS) (Philips Intellispace PACS Radiology version 4.4.532.1, SA).
Image acquisition and scoring
All images were obtained using mobile computed radiographic and digital radiographic systems with tube voltages ranging from 60 to 70 kVp and exposure times of 3.2 mAs - 6.4 mAs. No grids were employed. All CXRs were obtained at the bedside as anterior-posterior projections to limit the risk of departmental contamination and cross-infection.
All participants had CXRs performed either on admission or based on clinical need as determined by the attending physician's local expertise - for example, if clinical deterioration was apparent. Where a participant had more than one radiograph, the radiograph with the most severe findings was chosen for the Brixia score calculation.
Each radiograph was independently scored by two general radiologists (2 and 15 years' experience) who were blinded to all clinical data, except for RT-PCR-confirmed COVID-19 pneumonia. Readers were instructed to score radiographs utilising the Brixia scoring system.'16,20,221 Readers were also required to comment on the technical quality of each CXR image as either good or poor, and needed to note if a pleural effusion was present.
Statistical analysis
IBM SPSS version 28 (IBM Corp., USA) was used for the statistical analysis. The Shapiro-Wilk test showed that the data deviated from a normal distribution, and continuous variables were therefore expressed as median with interquartile range (IQR), while categorical data variables were expressed in frequencies and percentages. Interrater agreement between the radiologists for the Brixia scores was determined through the intraclass correlation coefficient (ICC). To investigate the association between demographic and clinical factors and patient outcomes, the Pearson χ2 and Mann-Whitney U tests were used for categorical and continuous variables, respectively. Factors found to be significantly associated with patient outcomes were then included in binary logistic regression modelling to determine whether they were significant predictors of patient death. The logistic regression modelling consisted of initial univariate analyses to select the patient and clinical factors to include as covariates in the subsequent multivariate modelling. This selection was based on a less strict cut-off of p<0.20 to avoid excluding variables that could be associated with patient death. Confounding was assessed by backward elimination of non-significant predictors, and the Hosmer and Lemeshow test ascertained if the final model was a good fit.
Results
Within the study period, 303 patients tested RT-PCR positive, and 265 had CXRs. Those without CXRs were excluded. Two further participants were excluded, the first owing to age (<18 years), while the second participant's final clinical outcome (in-hospital demise v. discharge) could not be traced. Ultimately 263 participants were enrolled for the study. The demographic, clinical and laboratory details are summarised in Tables 2 and 3. The study population was almost evenly split by sex, with 134 males (51%) and 129 females (49%). The median age was 47 years (IQR = 20; 95% CI 46.5 - 59.9). Most participants were black (221; 84%). There were 7 (5.4%) pregnant participants. The most common comorbid condition was hypertension (101; 38.4%), followed by diabetes (66; 25.1%), obesity (51; 19.4%) and HIV (41; 15.6%). Only 6 (2.3%) participants were noted to have active pulmonary tuberculosis.
The clinical course is summarised in Table 4, while the final clinical outcome (in-hospital demise v. discharge) is summarised in Tables 5A and 5B. The length of stay in hospital was recorded for 258 of the individuals, with the median length of stay found to be 7.5 days (IQR = 7; 95% CI 8.2 - 9.7). A total of 54.1% of patients required some level of respiratory support, most frequently via a rebreather mask (95; 36.7%).
Some 15.9% of participants were in the ICU, and the median length of stay in ICU was 6.5 days (IQR = 8; 95% CI 6.5 -12.5). Twenty-two of the 30 intubated participants died (73.3%; p<0.001), v. 28 participants from the 233 non-intubated group (12%; p<0.001).
In total, 50 (19%) patients died in hospital, and these patients were older than those who survived, with a median age of 55 years (IQR = 23; 95% CI 50.5 - 58.7) for those who died v. 46 years (IQR = 20; 95% CI 45 - 48.6) for those who lived (p=0.01). There was a higher mortality among males (24%) than females (14%) (p=0.04), and among patients with one or more comorbid conditions compared with those without (23% v. 9.2%; p=0.01). In addition, a higher proportion of patients with obesity, chronic kidney disease and underlying chronic cardiovascular disease died compared with those without these conditions (p<0.05). However, neither HIV co-infection nor diabetes mellitus were linked to increased mortality. Deceased patients had significantly higher C-reactive protein (CRP) counts, lactate dehydrogenase (LDH) levels, and neutrophil counts (p<0.001). Lymphopenia was a common finding, and the lymphocyte count was lower for the participants who died (p=0.05). HBa1c value for diabetic participants who died was higher (11.3% v. 9.5%, p=0.07) but this difference was not statistically significant.
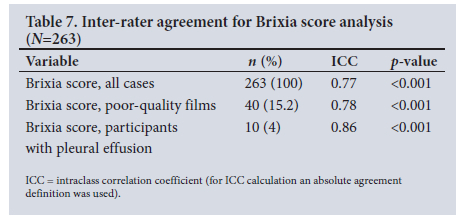
Brixia scores assigned by the readers ranged from 0 to 18 (Fig. 1A-B). The averaged median Brixia scores for patients who died were significantly higher than for those who survived: median score 14.5 (IQR =7.5; 95% CI 11.6 - 14.4) v. 9.0 (IQR = 8.8; 95% CI 8.4 - 9.8), respectively (p=0.001). The inter-rater agreement for Brixia score calculation between readers was good (ICC 0.77; 95% CI 0.6 -0.85; p<0.001) (Tables 6 and 7). Notably, even when readers indicated that the radiograph was of a technically poor quality, this did not significantly alter agreement (ICC 0.78; 95% CI 0.62 - 0.88; p<0.001) (Table 7). Only 10 patients were assessed to have a pleural effusion. The ICC for this small cohort was 0.86 (Table 7).
The multivariable logistic regression analysis is summarised in Table 8. Brixia score, age, male gender and obesity were found to be significant independent predictors of patient death. CRP was the only laboratory marker found to have significant predictive power for in-hospital death. Receiver operating curve (ROC) analysis was conducted for the predictive variables (Figs 2A-C). The models with the best predictive power for in-hospital demise incorporated Brixia score, age, male gender and obesity (area under curve (AUC) = 0.78; 95% CI 0.70 - 0.85; p<0.001; sensitivity 84%; specificity 63%) as well as a curve combining Brixia score and CRP (AUC = 0.78; 95% CI 0.71 - 0.85; p<0.001; sensitivity 81%; specificity 63%). The optimum cut-off values for Brixia score and age along the curve were 8 points and 43 years, respectively.
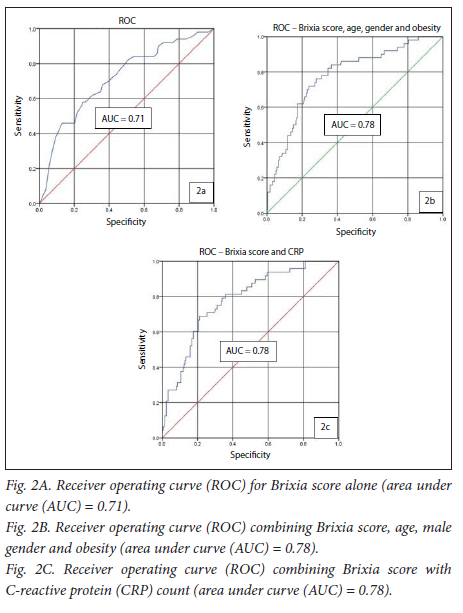

The same analysis was performed to extract variables that may be predictive of admission to the ICU. The predictive variables were the Brixia score (odds ratio (OR) = 1.1; 95% CI 1.0 - 1.3; p=0.03) and CRP (OR 1.0; 95% CI 1.0 - 1.0; p=0.03). However, the predictive power of these factors was not particularly strong. When ROC-curve analysis was performed (not shown) for ICU admission as the end point, the AUCs for Brixia score and CRP were 0.69 and 0.62, respectively.
Discussion
Our local study population was much younger than in most similar studies performed in the European setting.[16,20,21, 43] In addition, whereas these international studies were performed with mostly (sometimes exclusively) white participants,[20-22] most patients in our study were black.
The most commonly encountered comorbidities listed are similar to what has been described in other local studies.[34] However, we noted a larger proportion of obese patients, which could be explained by 'missing' obesity data in the cohort described by Jassat et al.[34] The in-hospital death rate of our sample is comparable with national figures.[34]
Borghesi et al.[20] did not include obesity or CRP in their final predictive models for the Brixia score. Similar to other data, however, we identified both obesity and CRP as predictors of in-hospital mortality,[37,38,40,44] and CRP was successfully introduced into a risk stratification model with the Brixia score. Comparable with local and international publications,[40,43] LDH was also statistically significantly higher for participants who died, but could not be introduced into a multivariate prediction model for participant demise. In accord with Venturas et al.[45] we also concluded that being HIV-positive did not confer a higher risk of in-hospital fatality. However, subsequent work has suggested a higher fatality rate for HIV-positive patients, in particular those not on antiretroviral therapy (ART).[34] Although we could not accurately identify patients who were on ART, the median viral load for HIV-positive individuals in our sample was 0 (recorded for 80.5% of the HIV-positive participants), which suggests that most participants in our sample were likely receiving ART. However, the number of HIV-positive participants in our sample was small (41; 15.6%), and this may explain the differences in outcomes between our data and those described by Jassat et al.[34]
International publications using ICC to assess Brixia score inter-rater agreement have yielded excellent results.'161 Similarly, in our setting the inter-rater reliability between the two general radiologists was good (ICC 0.77) despite differing levels of experience and no prior exposure to the Brixia scoring system. Another local publication also concluded good inter-rater agreement (Kappa = 0.64) for Brixia scoring between a registrar with 2 years of experience and a radiologist with 10 years' experience and subspecialty training in thoracic imaging.[19]
The optimal Brixia cut-off value along the ROC curve of 8 points in our study population affirms the findings by Borghesi et al.,[20] whereas the optimal age cut-off was much younger in our study (43 years v. 71 years). This is accounted for by a much younger study population in our sample.
This study is the first local effort to develop risk stratification models to predict clinical outcomes for patients hospitalised with COVID-19 pneumonia, showing that a higher Brixia score, increasing age, male gender, obesity and CRP were risk factors conferring the highest risk of in-hospital mortality. After multiple models were considered, the two best models combined Brixia score, increasing age, male gender and obesity as the first model, and Brixia score combined with CRP as the second model. International papers evaluating the utility of the Brixia score have yielded similar results. The models from this pilot study require follow-up studies with validation in larger clinical data sets. Thereafter, a potential use of these models may be to consolidate the models into existing medical records of patients upon hospital admission with computational risk stratification of death. This may help direct therapeutic decisions such as early consideration for more aggressive care in a high care/ ICU setting (which is a limited resource in our local setting) and early consideration for immunomodulatory agents such as higherdose dexamethasone, baricitin and tociluzimab. We recommend validation studies of these models in the pre-vaccination era and follow-up study during the era of vaccination.
Study limitations
This was a single-centre retrospective study. No comparison was made with patients with other causes for radiographic lung changes such as non-COVID-19 pneumonia or heart failure. Two general radiologists with differing levels of experience allocated Brixia scores. The gold standard would have been to involve subspecialised thoracic radiologists; however, these are not widely available (if at all) in SA. It must, however, be argued that for the scoring system to have local applicability, the agreement between general radiologists, as the available resource, must be good, as was the case in our study. The study cohort is from early in the pandemic prior to the vaccination era.
Conclusion
These are the first predictive models using a CXR scoring system that incorporate comorbidities and laboratory markers, and which was developed in a SA setting. The Brixia scoring system has been validated as a reliable tool internationally. This study shows that when used in conjunction with age, male gender, obesity and CRP, it is also a promising risk stratification tool locally. This may help inform the clinical decision pathway in resource-limited settings like ours during future waves of COVID-19.
Declaration. This study was conducted as part of the fulfilment criteria to obtain HCL's MMed degree.
Acknowledgements. The authors would like to thank the staff of internal medicine, critical care and emergency medicine at Charlotte Maxeke Johannesburg Academic Hospital for granting access to the COVID-19 clinical database. We are thankful to the staff of diagnostic radiology and radiography at Charlotte Maxeke Johannesburg Academic Hospital for the acquisition of the study material. We express gratitude to Drs Cornells van der Merwe and Johan Abrahams for acting as the reading radiologists for the study.
Author contributions. Dr H C Labuschagne was the principal investigator and author involved in the study design, literature review, data collection and interpretation as well as manuscript writing, editing and review for submission. Dr H Moodley was the lead study supervisor responsible for the study conceptualisation and design, literature review, data interpretation, manuscript editing and review. Dr J Venturas acted as co-supervisor and was involved in the study design, data interpretation, manuscript editing and review.
Funding. None.
Conflicts of interest. None.
References
1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727-733. https://doi.org/10.1056/NEJMoa2001017 [ Links ]
2. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: Single-center study and comprehensive radiologic literature review. Eur J Radiol Open 2020;7:100231. https://doi.org/10.1016/J.EJRO.2020.100231 [ Links ]
3. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032 [ Links ]
4. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020;25(3):1-8. https://doi.org/10.2807/1560-7917.ES.2020.25.3.2000045 [ Links ]
5. Yang Q, Liu Q, Xu H, Lu H, Liu S, Li H. Imaging of coronavirus disease 2019: A Chinese expert consensus statement. Eur J Radiol 2020;127(April):109008. https://doi.org/10.1016/j.ejrad.2020.109008 [ Links ]
6. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS One 2020;15(12 December):1-33. https://doi.org/10.1371/journal.pone.0242958 [ Links ]
7. Seidu AA, Hagan JE, Ameyaw EK, Ahinkorah BO, Schack T. The role of testing in the fight against COVID-19: Current happenings in Africa and the way forward. Int J Infect Dis 2020;98:237-240. https://doi.org/10.1016/j.ijid.2020.06.089 [ Links ]
8. Ducray V, Vlachomitrou AS, Bouscambert-Duchamp M, et al. Chest CT for rapid triage of patients in multiple emergency departments during COVID-19 epidemic: Experience report from a large French university hospital Eur Radiol 2021;31(2):795-803. https://doi.org/10.1007/s00330-020-07154-4 [ Links ]
9. Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic. Chest 2020;158(1):106-116. https://doi.org/10.1016/j.chest.2020.04.003 [ Links ]
10. Toussie D, Voutsinas N, Finkelstein M, et aL Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID-19. Radiology 2020;297(1):E197-E206. https://doi.org/10.1148/radiol.2020201754 [ Links ]
11. Qin L, Yang Y, Cao Q, et al. A predictive model and scoring system combining clinical and CT characteristics for the diagnosis of COVID-19. Eur Radiol 2020;(30):6797-6807. https://doi.org/10.1007/s00330-020-07022-1 [ Links ]
12. Cozzi D, Albanesi M, Cavigli E, Moroni C, Bindi A, Luvarà S. Chest X-ray in new coronavirus disease 2019 (COVID-19) infection: Findings and correlation with clinical outcome. Radiol Med 2020;2019:0123456789. https://doi.org/10.1007/s11547-020-01232-9 [ Links ]
13. Fang Y, Zhange H, Xie J, et al. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020;296(2):E115-E117. https://doi.org/10.1148/radiol.2020200432 [ Links ]
14. Ippolito D, Pecorelli A, Maino C, et al. Diagnostic impact of bedside chest X-ray features of 2019 novel coronavirus in the routine admission at the emergency department: Case series from Lombardy region. Eur J Radiol 2020;129(May):109092. https://doi.org/10.1016/j.ejrad.2020.109092 [ Links ]
15. Cozzi A, Schiaffino S, Arpaia F, et al Chest X-ray in the COVID-19 pandemic: Radiologists' real-world reader performance. Eur J Radiol 2020;132(August):109272. https://doi.org/10.1016/j.ejrad.2020.109272 [ Links ]
16. Balbi M, Caroli A, Corsi A, et al. Chest X-ray for predicting mortality and the need for ventilatory support in COVID-19 patients presenting to the emergency department. Eur Radiol 2021;31(4):1999-2012. https://doi.org/10.1007/s00330-020-07270-1 [ Links ]
17. Jacobi A, Chung M, Bernheim A, Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin Imaging 2020;64(April):35-42. https://doi.org/10.1016/j.clinimag.2020.04.001 [ Links ]
18. Sadiq Z, Rana S, Mahfoud Z, Raoof A. Systematic review and meta-analysis of chest radiograph (CXR) findings in COVID-19. Clin Imaging 2021;80:229-238. https://doi.org/10.1016/j.clinimag.2021.06.039 [ Links ]
19. Moodley S, Sewchuran T. Chest radiography evaluation in patients admitted with confirmed COVID-19 infection, in a resource-limited South African isolation hospital. S Afr J Radiol 2022;1(1):1-7. https://doi.org/10.4102/sajr.v26i1.2262 [ Links ]
20. Borghesi A, Zigliani A, Golemi S, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019 : A study of 302 patients from Italy. Int J Infect Dis 2020;96:291-293. https://doi.org/10.1016/j.ijid.2020.05.021 [ Links ]
21. Borghesi A, Zigliani A, Masciullo R, et al. Radiographic severity index in COVID-19 pneumonia: Relationship to age and sex in 783 Italian patients. Chest Radiog 2020;125:461-464. https://doi.org/10.1007/s11547-020-01202-1 [ Links ]
22. Borghesi A, Maroldi R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med 2020;125:509-513. https://doi.org/10.1007/s11547-020-01200-3 [ Links ]
23. Wasilewski PG, Mruk B, Mazur S, Póltorak-Szymczak G, Sklinda K, Walecki J. COVID-19 severity scoring systems in radiological imaging - a review. Polish J Radiol 2020;85(1):e361-e368. https://doi.org/10.5114/pjr.2020.98009 [ Links ]
24. Reeves RA, Pomeranz C, Gomella AA, et al. Performance of a severity score on admission chest radiography in predicting clinical outcomes in hospitalised patients with coronavirus disease (COVID-19). Am J Roentgenol 2021;217(3):623-632. https://doi.org/10.2214/AJR.20.24801 [ Links ]
25. Yasin R, Gouda W Chest X-ray findings monitoring COVID-19 disease course and severity. Egypt J Radiol Nucl Med 2020;51:193. https://doi.org/10.1186/s43055-020-00296-x [ Links ]
26. Singh A, Lim YH, Annamalaisamy R, et al. Chest X-ray scoring as a predictor of COVID-19 disease; correlation with comorbidities and in-hospital mortality. Scott Med J 2021;66(3):101-107. https://doi.org/10.1177/00369330211027447 [ Links ]
27. Au-Yong I, Higashi Y, Giannotti E, et al. Erratum: Chest radiograph scoring alone or combined with other risk scores for predicting outcomes in COVID-19: A UK study. Radiology 2021;301(3):E444. https://doi.org/10.1148/radiol.2021219021 [ Links ]
28. Maroldi R, Rondi P, Agazzi GM, Ravanelli M, Borghesi A, Farina D. Which role for chest X-ray score in predicting the outcome in COVID-19 pneumonia? Eur Radiol 2021;31(6):4016-4022. https://doi.org/10.1007/s00330-020-07504-2 [ Links ]
29. Agrawal N, Chougale SD, Jedge P, Iyer S, Dsouza J. Brixia chest X-ray scoring system in critically ill patients with COVID-19 pneumonia for determining outcomes. J Clin Diagnostic Res 2021;15-17. https://doi.org/10.7860/jcdr/2021/48844.15197 [ Links ]
30. Abo-Hedibah SA, Tharwat N, Elmokadem AH. Is chest X-ray severity scoring for COVID-19 pneumonia reliable? Polish J Radiol 2021;86(1):e432-e439. https://doi.org/10.5114/pjr.2021.108172 [ Links ]
31. Warren MA, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018;73(9):840-846. https://doi.org/10.1136/thoraxjnl-2017-21128 [ Links ]
32. Joseph NP, Reid NJ, Som A, et al. Racial and ethnic disparities in disease severity on admission chest radiographs among patients admitted with confirmed coronavirus disease 2019: A retrospective cohort study. Radiology 2020;297(3):E303-E312. https://doi.org/10.1148/radiol.2020202602 [ Links ]
33. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. E Clin Med 2020;29-30:100630. https://doi.org/10.1016/j.eclinm.2020.100630 [ Links ]
34. Jassat W, Cohen C, Tempia S, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: A cohort study. Lancet HIV 2021;8(9):e554-e567. https://doi.org/10.1016/S2352-3018(21)00151-X [ Links ]
35. Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: A systematic review and meta-analysis. Eur J Med Res 2020;25(1):1-15. https://doi.org/10.1186/s40001-020-00464-9 [ Links ]
36. Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020;395(10236):1544-1545. https://doi.org/10.1016/S0140-6736(20)31024-2 [ Links ]
37. Zhang F, Xiong Y, Wei Y, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol 2020;92(11):2536-2542. https://doi.org/10.1002/jmv.26039 [ Links ]
38. Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS One 2021;16(3):1-30. https://doi.org/10.1371/journal.pone.0247461 [ Links ]
39. Pillay-van Wyk V, Bradshaw D, Groenewald P, et al. COVID-19 deaths in South Africa: 99 days since South Africa's first death. S Afr Med J 2020;110(11):1093-1099. https://doi.org/10.7196/samj.2020.v110i11.15249 [ Links ]
40. Mohamed F, Raal FJ, Mbelle M, et al. Glycaemic characteristics and outcomes of COVID-19 patients admitted to a tertiary hospital in Johannesburg. Wits J Clin Med 2020;2(3):123. https://doi.org/10.18772/26180197.2020.v2n3a1 [ Links ]
41. Prattichizzo F, de Candia P, Nicolucci A, Ceriello A. Elevated HbA1c levels in pre-COVID-19 infection increases the risk of mortality: A systematic review and meta-analysis. Diabetes Metab Res Rev 2021;(May):1-8. https://doi.org/10.1002/dmrr.3476 [ Links ]
42. Kim H, Tanser F, Tomita A, Vandormael A, Cuadros DF. Beyond HIV prevalence: Identifying people living with HIV within underserved areas in South Africa. BMJ Glob Heal 2021;6(4):1-10. https://doi.org/10.1136/bmjgh-2020-004089 [ Links ]
43. Schalekamp S, Huisman M, van Dijk RA, et al Model-based prediction of critical illness in hospitalised patients with COVID-19. Radiology 2020;298(1):e46-e54. https://doi.org/10.1148/RADIOL.2020202723 [ Links ]
44. Gatti M, Calandri M, Biondo A, et al. Emergency room comprehensive assessment of demographic, radiological, laboratory and clinical data of patients with COVID-19: Determination of its prognostic value for in-hospital mortality. Intern Emerg Med 2021;2019:0123456789. https://doi.org/10.1007/s11739-021-02669-0 [ Links ]
45. Venturas J, Zamparini J, Shaddock E, et al. Comparison of outcomes in HIV-positive and HIV-negative patients with COVID-19: HIV-positive and negative patients with COVID-19. J Infect 2021;83(2):217-227. https://doi.org/10.1016/j.jinf.2021.05.020 [ Links ]
 Correspondence:
Correspondence:
H C Labuschagne
chrisjanlab@live.com
Accepted 27 September 2022














