Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.113 no.1 Pretoria ene. 2023
http://dx.doi.org/10.7196/SAMJ.2023.v113i1.16670
RESEARCH
Exploring a community's understanding of HIV vaccine-induced seropositivity in a South African research setting
M MalahlehaI, VIII; A DilrajII; J JeanIII; N S MorarIV; J J DietrichV; M RossI; E MbatsaneVI; MC KeeferVII; K AhmedIX, X
IMB ChB, MPH; Setshaba Research Centre, Pretoria, South Africa
IIPhD; Setshaba Research Centre, Pretoria, South Africa
IIIMD; Department of Medicine, School of Medicine and Dentistry, University of Rochester, New York, USA
IVM Med Sc; HIV and other Infectious Diseases Research Unit, South African Medical Research Council, Durban, South Africa
VPhD; Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIMA Psych; Setshaba Research Centre, Pretoria, South Africa
VIIMD; Department of Medicine, School of Medicine and Dentistry, University of Rochester, New York, USA
VIIIMB ChB, MPH; Synergy Biomed Research Institute, East London, South Africa
IXMB ChB Setshaba Research Centre, Pretoria, South Africa
XMB ChB Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
BACKGROUND. The high HIV prevalence and incidence in South Africa makes it suitable for recruitment of participants for large-scale HIV preventive vaccine trials. However, fear of vaccine-induced seropositivity (VISP) may be a barrier for community acceptability of the trial, for volunteers to participate in HIV preventive vaccine trials and for uptake of an efficacious vaccine. Prior to 2015, when the first phase 1 safety HIV vaccine trial was undertaken at Setshaba Research Centre, Soshanguve, the local community stakeholders and healthcare workers were naive about HIV vaccine research and HIV preventive vaccines.
OBJECTIVE. To explore knowledge and perceptions regarding VISP among community stakeholders and healthcare workers in peri-urban Soshanguve, Tshwane.
METHODS. Using a quantitative-qualitative mixed-methods study design, surveys (n=50) and in-depth interviews (n=18) were conducted during July - August 2015. Participants included community stakeholders, community advisory board members and healthcare workers, who were >18 years old and had attended community educational workshops during September 2014 - May 2015. Audio recordings of interviews were transcribed verbatim and coded using content thematic analysis. Data were further analysed by sex, age and educational level.
RESULTS. Of a maximum score of 2 on knowledge on VISP, the 50 survey participants (mean age 33.78 years; 45 females) obtained an average of 0.88 (44%). Of 17 in-depth interviewees (one interview could not be transcribed; mean age 30.9 years; 12 females), 8 (47%) displayed some knowledge about VISP, of whom only 5 defined VISP correctly. Women were more knowledgeable about VISP than men; 5 of 12 women (42%) came close to defining VISP correctly, while none of the 5 men did so. The main fear of trial participation expressed by most participants (n=6) was testing HIV-positive as a result of the vaccine. While some participants believed that the community's perceptions of VISP would negatively affect HIV vaccine trial support and recruitment efforts, others noted that if trial participants understand the concept of VISP and are part of support groups, then they would have the information to combat negative attitudes within their community.
CONCLUSION. Most participants had an inaccurate and incomplete understanding of VISP. Many feared testing HIV-positive at clinics; therefore, education on improving a basic understanding of how vaccines work and why VISP occurs is essential. In addition, assessing participant understanding of HIV testing, transmission and VISP is critical for recruitment of participants into HIV vaccine trials and may improve acceptability of an HIV preventive vaccine.
Currently, no preventive HIV vaccines have been approved by the US Food and Drug Administration. A preventive HIV vaccine is given to people who do not have HIV, with the goal of preventing HIV infection by inducing protective anti-HIV immune responses. However, this may cause a reactive result in routine HIV testing in the absence of HIV infection.[1] The detection of HIV vaccine-induced antibodies by serological tests is commonly referred to as vaccine-induced sero-reactivity or vaccine-induced seropositivity (VISP).[2-The induction of VISP in HIV-vaccinated participants is common, especially with vaccines containing both the HIV-1 envelope and gag proteins.[1] VISP can be transient, or may last years, as observed in several HIV vaccine trials.[2-5] VISP can be differentiated from acquired HIV infection by polymerase chain reaction tests that detect HIV RNA. However, these tests are costly and are not used routinely in developing countries. An HIV vaccine, even if partially effective, offers the best hope of decreasing the epidemic, particularly in South Africa (SA) where there is a high HIV prevalence and incidence.[6]
The incidence of HIV in SA in 2017 in the 15 - 49-year age group was 0.79%, translating to almost 200 000 new infections in that year.[6] This high HIV incidence suggested early on that recruitment and decisions on efficacy of vaccines based on HIV vaccine trials could be expeditious in SA compared with regions with low HIV incidence. However, fear of VISP is a contributing barrier to participating in HIV vaccine trials.[7-15] VISP discussions are also likely to arise early in the community engagement and recruitment activities within a community naive to HIV vaccine trials.
Since 2003, 21 HIV preventive vaccine trials have been conducted in SA.[16] However, there is limited information on the community's understanding and responses to VISP. In 2014, as part of community preparedness for HIV vaccine trials, community educational workshops were conducted in the Soshanguve community, a periurban area where people were naive about HIV vaccines and HIV vaccine research. The workshops were conducted by the research site's community engagement officer using PowerPoint (Microsoft, USA) presentations. The educational topics included an introduction to vaccines, HIV research, HIV vaccine trials and VISP. The topics were covered using 45 slides, with 13 of these slides devoted to VISP. The workshop took approximately an hour, with 45 minutes devoted to the actual presentation and 15 minutes to a question-and-answer session. Educational materials, which did not contain illustrations or images, were available in English only, and were handed to attendees. The attendees at the workshops served as a suitable population for this study, which aimed to explore and contextualise the community's knowledge, fears and perceptions regarding VISP.
Methods
Study design
To assess the community's understanding and perceptions of VISP, a mixed-methods study using in-depth interviews (qualitative) and surveys (quantitative) was conducted among community members from Soshanguve between July and August 2015.
Participants
Eligible participants were women and men >18 years old residing within the research catchment area in Soshanguve, City of Tshwane, SA. They had previously attended educational workshops on vaccines, HIV vaccine trials and VISP. Participants were recruited using attendance lists taken at these community educational workshops, which were conducted between September 2014 and May 2015. Eight workshops were conducted, and there were approximately 700 attendees in total. Community members attending these workshops included ward councillors, representatives from non-governmental organisations, healthcare workers, tertiary nursing college students and community advisory board (CAB) members. We envisaged that participants recruited from the workshop attendees would have some working knowledge around HIV vaccines, HIV research and VISP.
Procedures
Following the eight workshops, 50 attendees were contacted via telephone or in person to complete self-administered surveys, and were invited to participate in the in-depth interviews. Of the 50 survey participants, 18 were selected purposively for the in-depth interviews, such that there was some representation from the different types of participants who attended the workshops. Written informed consent was obtained after assessing voluntariness and eligibility to participate in the study. A brief demographic questionnaire was administered to participants post consent and prior to starting the in-depth interviews, which lasted between 20 and 60 minutes and were conducted in a private office by an experienced social science interviewer. Enrolled participants were reimbursed ZAR50 (surveys) and ZAR100 (interviews) in cash for their time and travel expenses.
Study measures
Demographic information collected using the survey included age, sex and educational level. The in-depth interviews and surveys explored the community's understanding and views of VISP, knowledge and source of information on VISP, their understanding of the community's perceptions of VISP and participation in HIV vaccine studies, and what further information they wanted about VISP.
Data analysis
Data were analysed using unpaired Student's f-tests and Welch's f-tests. Analysis of participants' knowledge on VISP included comparisons based on sex, age and highest level of education. All participants who omitted questions, chose more than one response to a question (with only one possible correct answer), or chose 'not sure' as a response in the survey did not receive a point for questions that assessed knowledge, and for the questions with 'select all that apply', individual points were given for each correct answer chosen. The two questions assessing knowledge on VISP were formatted as true/false questions, which were: (i) 'standard HIV tests search for antibodies to HIV, which is why someone who gets the vaccine may test positive', and (ii) 'if tested positive for VISP, I can pass on the antibodies to another person by kissing or through sexual contact'. A participant could achieve a maximum score of 2 for knowledge.
Audio-recordings of interviews were transcribed verbatim and then coded by two coders. First, a code book was developed a priori using content thematic analysis.[17] Thereafter, a meeting was held by the investigator team to review and refine the code book. The code book was refined to address six key themes (knowledge of VISP, sources of knowledge of participants, sources of knowledge of the community, fears, what information about VISP participants wanted to know, and community perceptions of participation). The theme on knowledge of VISP was specifically analysed by age, sex and educational level.
Ethical considerations
Study procedures were approved by Pharma-Ethics in SA (ref. no. 150611642) and the Institutional Review Board at the University of Rochester in the USA (ref. no. RSRB00057563).
Results
Participant characteristics
Of the 50 workshop attendees who completed the survey and were invited to participate in the in-depth interviews, 40 accepted. The remaining 10 declined due to work commitments. Eighteen participants were purposively selected and interviewed from the 40 participants who accepted the invitations. Of the 18 interviews recorded, one interview could not be transcribed due to poor quality of the recording. Therefore the results are based on analysis of data from 17 participants.
The mean ages of the in-depth interviewees and survey participants were 30.88 and 33.78 years, respectively. Of the 17 interviewees, 6 participants were <25 years old, while 9 were >25 years (missing data n=2). Women represented the largest proportion of respondents (90% in surveys (n=45), and 71% (n=12) in interviews). Twenty-one survey respondents (42%) and 14 interviewees (82%) had an educational level of grade 12 and above (9 participants had secondary level education, while 5 participants had post-secondary education (missing data n=3). Therefore a greater proportion of the interviewees had an educational level above grade 12.
Survey results: Perceptions and knowledge of VISP
The average score among all the participants was 0.88 (44%), with a standard deviation (SD) of 0.80. Thirty-eight percent of the participants obtained a score of 0, making this the mode score (Table 1). Only 26% of participants achieved a maximum score of 2.

The mean scores among females and males were 0.84 (42%; SD 0.80) and 1.20 (60%; SD 0.84), respectively (Table 2). However, the difference was not statistically significant.
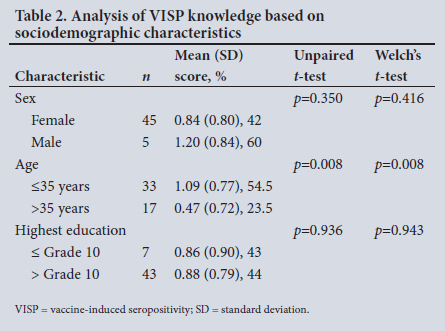
When comparing the mean scores by age, the mean among participants who were <35 years of age was 1.09 (54.5%; SD 0.77), compared with a mean of 0.47 (23.5%; SD 0.72) among those >35 years of age. Both unpaired and Welch's t-tests resulted in p-values much lower than 0.05, indicating that the difference in the means based on age was highly statistically significant (Table 2).
The mean score among those who had completed grade 10 or less was 0.86 (43%; SD 0.90), compared with a mean score of 0.88 (44%; SD 0.79) among those who had completed grade 11 or higher. However, the difference was not statistically significant (Table 2).
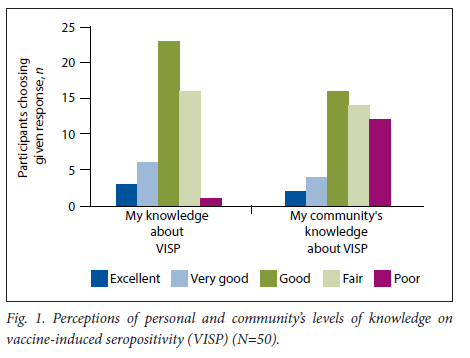
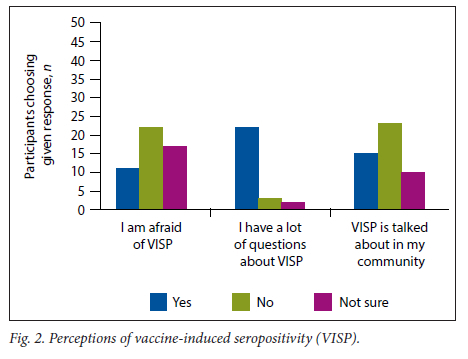
Participants' perceptions based on their personal levels of knowledge illustrate that most of them rated their knowledge as either good or fair. Few participants noted their personal knowledge as poor. In comparison, the majority of participants classified communal knowledge as good, fair, or poor (Fig. 1). Regarding perceptions of VISP, it was noted that most people had many questions about VISP. Although a large proportion of individuals noted that VISP is not talked about frequently, there was a substantial proportion of participants who believed that VISP was talked about frequently in their communities. Furthermore, the majority of participants indicated that they were either afraid of VISP or unsure whether or not they were afraid (Fig. 2).
In-depth interview results
Knowledge of VISP
Only 8 of the 17 participants were able to provide some information about VISP, of whom only five defined VISP correctly:
'It's a false positive … When you have that vaccine, when they test you, you will test positive, and then you are not positive. First time when they take your blood, you will test positive … Then when they check your viral load they will say that you are not positive. [Interviewer: 'Where are they going to check?'] The clinic … At the clinic. It's where they find out he is positive while he's negative where he was vaccinated [the research site].' [F, 33]
'I know that when actually they give you the vaccine and you went there [public health facility], you test and it clearly says that you are positive but you're not. [Interviewer: So false positive?] Yeahit's false positive' [F, 26].
'Then if you are vaccinated and then you go to the other clinics and then get tested for HIV, your status will be positive meanwhile before you are negative. If you come to Setshaba [the research site] and get tested, you'll be HIV negative because the instrument that they are using differ from the clinic's ones.' [F, 29]
'VISP is when you test positive but you are not because of the vaccine.' [F, 33]
Women (42%; 5/12) were more knowledgeable about VISP as they came close to defining VISP correctly, unlike the five men. Of the remaining women, five had no knowledge of VISP, while two women's responses were inconsistent with the definition of VISP. Forty-four percent (4/9) of participants aged ≥25 years had a good understanding of VISP, while none of the participants <25 years of age had a good understanding of VISP. Participants who had post-secondary education were better able to define VISP correctly (40%, 2/5 participants) compared with those with secondary-level education (22%, 2/9 participants); data on education for two participants were missing.
Sources of knowledge of participants
The eight participants who said something about VISP indicated that their VISP knowledge was acquired through the research centre.
Sources of knowledge of the community
The main source of information for community members to learn more about VISP was the research centre. Community members also heard about VISP at presentations held by research staff at hostels.
Fears
The main fear expressed by participants (n=6) for themselves or of community members was about testing HIV-positive as a result of the vaccine. One participant feared becoming HIV-positive if the vaccine did not work:
'What if you in future it says that you're positive, like you become more positive, like if they become stronger the virus in the vaccine becomes stronger and then it seems that you are positive. That's the only fear that people have. Let's say it's like this, it's a seed and then if you pour water we know the seed is going to grow, that people are afraid that it's going to grow, that it's in the blood; what if it becomes stronger; what if it grows.' [F, 26].
'They fear the vaccine because they think maybe if they are vaccinated then they are putting HIV on them.' [F, 33] 'Because they say VISP when you're injected, it produces the virus. They also fear that the virus will multiply in our blood. That's what they fear.' [M, 21]
'The fear is that if the vaccine cannot work, they will be HIV positive.' [F, 29]
Perceptions of VISP knowledge and what participants wanted to know more about
Two of the eight participants who knew something about VISP felt they had sufficient knowledge and did not require further information:
'So far I think I have enough knowledge. I know that knowledge is never enough but on my side so far I heard enough; I don't think there's anything more that.' [F, 26]
'I don't have a problem with VISP. I understand what it is.' [F, 33]
However, for three of the eight participants who had some knowledge about VISP, what they wanted to know more about stemmed from their fears, or lack of knowledge or the complexity of the information presented.
'What if the VISP cannot work? Am I going to be HIV infected?' [F, 29]
'How it works.' [M, 21]
'It was difficult for me to express it in a simpler language or rather in a simpler form that will enable me to share it with another person.' [F, 31]
VISP and effect on trial recruitment (community perceptions of research/trial participation)
The majority of participants believed that their community's perceptions of VISP would negatively affect HIV vaccine trial recruitment efforts. They indicated that if knowledge and confidence in one's reasons for participating in the study are not strong, one's participation in a trial may be adversely affected by negative perceptions of community members:
'If people think that you are positive, the community and you are known to them, is a problem because even if you pass, when you go to the shop and you find people talking, you think they are talking about you even if they are not because of your participation in the study. Some are affected but some are ... You know you are not positive, then you are not affected. Some, they know they are not positive but it's affecting them.' [F, 33]
On the other hand, some noted that if trial participants truly understand the concept of VISP, come in for their study visits and are part of the participant support group, then they would have tools to combat the negative attitudes within their community.
'If already you are confident as a participant that you understand everything that entails the study, I don't think you will have a setback . If you always attend your visits in the clinic as required, and if they do maybe have like buddy clubs and stuff so that they share their experiences, that would assist them also in overcoming maybe fears that might be there that are caused by the community members. If I am confident and well informed about the decision that I've taken, because I understand that there consent forms that are being filled. If I understand that, I think it would have been seen through the process when they were recruiting me.' [F, 31]
Discussion
Joint efforts by sponsors, researchers and community stakeholders including CABs involved in HIV vaccine trials play an important role in educating stakeholders, healthcare workers and potential trial participants about HIV, vaccine research and VISP. VISP can result in undesirable social impacts on stakeholders such as CABs, healthcare workers and trial participants. These include stigma, denial of job opportunities, relationship issues, insurance challenges and inappropriate medical care, and exclusion from blood or organ donation.
In our study, while a small minority of the participants understood and could define the term VISP, the majority of them either had no understanding or had incorrect and incomplete information on VISP. Many of the participants defined VISP as a separate entity rather than an outcome of vaccination. The four participants who had an understanding of VISP were able to explain that with VISP, one would have a false-positive test at an external HIV testing facility (where antibody tests are conducted), while the tests at the research site would be able to differentiate between VISP and true infection.
Interestingly, female participants were more knowledgeable about VISP than men, which can be attributed to the general interest by women in communities with high HIV incidence in finding preventive options, such as HIV vaccines. Although the age groups were different for the survey and in-depth interviews (cut-offs determined arbitrarily based on the data: 35 years for the survey and 25 years for the in-depth interviews), both the survey and interviews showed that age was associated with knowledge of VISP, with those in younger age groups being more knowledgeable. While survey participants did not demonstrate a significant difference in knowledge of VISP based on their educational level, those participants in the in-depth interviews who had post-secondary education were more knowledgeable about VISP.
One of the important themes that emerged from the participants' framing of VISP as either an independent entity or the vaccine itself was the fact that these definitions illustrated an understanding that the body is affected by the vaccine, but a lack of understanding in the ways in which the body and vaccines interact with each other. Another challenge highlighted was the inability of trial participants to always express themselves about VISP in English, and they would at times revert to a local language. Therefore important elements of educating the community are to provide a basic understanding of how vaccines work in the body, and to explain the reason why VISP occurs. Additionally, it is important to find innovative ways to explain VISP using audiovisual/graphical materials, and to translate VISP educational materials into local languages and concepts that participants are familiar with. The effect of not doing so, or more accurately, not doing so in a way that is easily understood, is reflected in the ways in which a great number of the interviewees were trying to negotiate the unintelligibility of their definitions of VISP. Based on the study results, it seems more important to address not what VISP is per se, but the reason why VISP occurs and how that phenomenon relates to other vaccines. By framing VISP in the context of other vaccines, this added perspective may address the fears and misunderstanding expressed by the participants of testing HIV-positive at a facility not connected with the HIV vaccine study, but testing negative at the research centre where they were vaccinated. Doing so also removes the potential for distrust of the research centre.
It was evident that even though many of the participants could not define VISP, their perceptions of the fears within their community about the HIV vaccine and research being tied to getting HIV, either directly through the research centre or through the parts of the vaccine becoming real HIV, creates a need for a greater understanding of VISP. The fears around the HIV vaccine/research and expressed dissonance around testing positive for HIV in clinics where they were not vaccinated, but not testing HIV-positive at the research site where they received the vaccine, illustrate how the idea of VISP is a part of communal discourses, despite an inability of individuals to formally define it. The most important aspect of this reality is the fact that only a very small number of individuals addressed the ways in which, even in the presence of VISP, individuals can get infected with HIV based on their sexual behaviours.
Even among those who could formally define the concept of VISP, the majority framed their understanding as 'although you may test positive in a local clinic, you know you are HIV negative because of your status at the beginning of the study.' While study participants will not inherently become HIV positive, the absence of the understanding of that possibility accounting for some of the positive tests in local clinics, even in the context of VISP, is particularly detrimental. The absence of such discourse or understanding is detrimental because it creates a platform where individuals who become HIV-positive can claim and/or rationalise that reality in terms of the research centre and the vaccine itself, rather than in voluntary behaviours they engaged in. Furthermore, there is the potential for them to introduce that belief into their community. This is not an indicator of inherently malicious behaviour, but rather a product of incomplete understanding of how vaccines work, the concept of VISP and the ability to get infected with HIV in the context of a research study on vaccines. An important theme that resonated is that negative perceptions and stigma associated with VISP can have detrimental impacts on recruitment and retention in HIV vaccine trials.[8,18]
Furthermore, it can lead to trial participants experiencing social harms. Participants also shared insights on suitable ways to engage communities around VISP, and these included using research sites as a source of information, holding regular community events and encouraging the use of participant social event 'clubs' where participants could share their experiences about trial participation and interact on topics such as VISP. There is a need to incorporate VISP education into healthcare worker engagement forums.[19]
One limitation of this study is the long period that elapsed between the workshops and the interviews for some participants, resulting in recall bias. Additionally, the interviewers noted a difficulty in participants expressing themselves and defining VISP purely in English, and they often reverted to express themselves in a local language.
Conclusion
This research study highlighted that while VISP awareness existed, there was limited knowledge and understanding about VISP within this local community. Members had diverse understanding and perceptions of VISP, but through continued education efforts, the misconceptions and myths about HIV vaccines and VISP may be addressed. The knowledge variability that exists within communities should be used to reflect the ways in which educational workshops on VISP could be adapted to fit the multitude of frameworks individuals use to engage in these discussions. As the efforts to find safe and efficacious HIV preventive vaccines intensify, there is a need to frame community-based educational strategies and engage community members about HIV vaccine research and VISP. Research centres undertaking HIV vaccine research need to prioritise evaluation of their educational efforts.
Declaration. None.
Acknowledgements. The authors acknowledge the HIV Vaccine Trials Network for its financial support and continued commitment to engaging and mentoring local researchers and international scholars. We extend our gratitude to the participants and the staff at Setshaba Research Centre, Soshanguve, who provided support in conducting the research.
Author contributions. All authors contributed to writing of the manuscript. MM and KA conceived the study, contributed to the development of the proposal and provided mentorship. JJ, MM and AD conducted the analysis, RM recruited the participants and EM conducted the interviews. MCK contributed to the development of the proposal and provided mentorship.
Funding. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) US Public Health Service Grant UM1 AI068614 (LOC: HIV Vaccine Trials Network (HVTN)) as part of the HVTN Research and Mentorship Program (RAMP).
Conflicts of interest. None.
References
1. Cooper CJ, Metch B, Dragavon J, Coombs RW, Baden LR, NIAID HIV Vaccine Trials Network (HVTN) Vaccine-Induced Seropositivity (VISP) Task Force. Vaccine-induced HIV seropositivity/ reactivity in noninfected HIV vaccine recipients. JAMA 2010;304(3):275-283. https://doi.org/10.1001/jama.2010.926 [ Links ]
2. Voronin Y, Zinszner H, Karg C, et al. HIV vaccine-induced sero-reactivity: A challenge for trial participants, researchers, and physicians. Vaccine 2015;33(10):1243-1249. https://doi.org/10.1016/j.vaccine.2014.10.040 [ Links ]
3. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med 2009;361:2209-2220. https://doi.org/10.1056/NEJMoa0908492 [ Links ]
4. Penezina O, Krueger NX, Rodriguez-Chavez IR, et al. Performance of a redesigned HIV Selectest enzyme-linked immunosorbent assay optimised to minimise vaccine-induced seropositivity in HIV vaccine trial participants. Clin Vaccin Immunol 2014;21(3):391-398. https://doi.org/10.1128/CVI.00748-13 [ Links ]
5. Lavreys L, Vingerhoets J, Colfer A, et al. Vaccine induced seropositivity in participants of the APPROACH study. Madrid: HIV Research for Prevention HIVR4P 2018 Conference, 2018. [ Links ]
6. Simbayi LC, Zuma K, SABSSM V Team, et al. South African National HIV Prevalence, Incidence, Behaviour and Communications Survey, 2017. Cape Town: HSRC Press, 2019. [ Links ]
7. Allen MA, La Salvia T, Tjugum B, Gulakowski RJ, Murguía M. Assessing the attitudes, knowledge, and awareness of HIV vaccine research among adults in the United States. J Acquir Immune Defic Syndr 2005;40(5):617-624. https://doi.org/10.1097/01.qai.0000174655.63653.38 [ Links ]
8. Buchbinder SP, Metch B, Holte SE, Scheer S, Vittinghoff CA. Determinants of enrollment in a preventive HIV vaccine trial: Hypothetical versus actual willingness and barriers to participation. J Acquir Immune Defic Syndr 2004:36(1):604-612. https://doi.org/10.1097/00126334-200405010-00009 [ Links ]
9. Jackson DJ, Martin HL Jr, Bwayo JJ, et al. Acceptability of HIV vaccine trials in high-risk heterosexual cohorts in Mombasa, Kenya. AIDS 1995;9(11):1279-1284. https://doi.org/10.1097/00002030-199511000-00010 [ Links ]
10. Kakinami L, Newman PA, Lee SJ, Duand N. Differences in HIV vaccine acceptability between genders. AIDS Care 2008:20(5):542-546. https://doi.org/10.1080/09540120701867180 [ Links ]
11. Koblin BA, Heagerty P, Sheon A, et al. Readiness of high-risk populations in the HIV Network for Prevention Trials to participate in HIV vaccine efficacy trials in the United States. Aids 1998;12(7):785-793. https://doi.org/10.1097/00002030-199807000-00015 [ Links ]
12. Koblin BA, Holte S, Lenderking B, Heagerty P. Readiness for HIV vaccine trials: Changes in willingness and knowledge among high-risk populations in the HIV Network for Prevention Trials. J Acquir Immune Defic Syndr 2000;24(5):451-457. https://doi.org/10.1097/00126334-200008150-00010 [ Links ]
13. Lesch A, Kafaar Z, Kagee A, Swartz L. Community members' perceptions of enablers and inhibitors to participation in HIV vaccine trials. S Afr J Psychol 2006;36(4):734-761. https://doi.org/10.1177/008124630603600406 [ Links ]
14. Newman PA, Duan N, Rudy ET, Roberts KJ, Swendeman D. Posttrial HIV vaccine adoption: Concerns, motivators, and intentions among persons at risk for HIV. J Acquir Immune Defic Syndr 2004;37(3):1393-1403. https://doi.org/10.1097/01.qai.0000127064.84325.ad [ Links ]
15. Dhalla S. An update on human immunodeficiency virus vaccine preparedness studies. J Med Microbiol 2015;64(7):731-738. https://doi.org/10.1099/jmm.0.000073 [ Links ]
16. Laher F, Bekker L, Garrett N, Lazarus EM, Gray GE. Review of preventative HIV vaccine clinical trials in South Africa. Arch Virol 2020;165(11):2439-2452. https://doi.org/10.1007%2Fs00705-020-04777-2 [ Links ]
17. Vindrola-Padros C, Johnson GA. Rapid techniques in qualitative research: A critical review of the literature. Qual Health Res 2020;30(10):1596-1604. https://doi.org/10.1177/1049732320921835 [ Links ]
18. Strauss RP, Sengupta S, Kegeles S, et al. Willingness to volunteer in future preventive HIV vaccine trials: Issues and perspectives from three US communities. J Acquir Immune Defic Syndr 1999;26(1):63-71. [ Links ]
19. Karg C, Wecker M. VISP and the HVTN's commitment to poststudy HIV testing. HVTNews, 2 March 2012. http://hvtnews.wordpress.com/2012/03/02/visp-and-the-hvtns-commitment-to-poststudy-hiv-testing/#more-560/ (accessed 1 December 2022). [ Links ]
 Correspondence:
Correspondence:
A Dilraj
ADilraj@setshaba.org.za
Accepted 12 September 2022














