Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.12 Pretoria Dez. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i12.16586
RESEARCH
HLA class I and class II antigens in sarcoidosis
R MorarI; R DuarteII; A A WadeeIII; C FeldmanIV
IMMed (Int Med), PhD; Division of Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIPhD; Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIPhD; Department of Immunology, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVPhD, DSc; Department of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Sarcoidosis is a multisystem granulomatous disorder. Its exact cause is unknown, but it is believed that an external agent may cause the characteristic immune reaction in genetically susceptible individuals. There is therefore general recognition that genetic vulnerability to sarcoidosis is one of the potential risk factors. HLA is encoded by genes in the major histocompatibility complex on chromosome 6. These surface cells are important in presentation of antigen and play a key part in the body's immune response to external antigens. Various HLA subtypes are more common in people with sarcoidosis than in those without. Variances in vulnerability, presentation, progression and prognosis have been related to different HLA phenotypes. HLA genes offer information into the factors driving sarcoidosis and prognosticating tools. However, in Africa, including South Africa (SA), there are no data on HLA types in relation to sarcoidosis
OBJECTIVES: To determine HLA class I and II associations in SA sarcoidosis patients
METHODS: Phenotype frequencies of HLA-A, B and C and DQB1 and DRB1 were calculated for 51 consecutive patients with biopsy-proven sarcoidosis attending the respiratory clinic at Charlotte Maxeke Johannesburg Academic Hospital and 63 controls, who were potential organ donors. The frequencies of the tested HLA loci were determined by direct counting. The significance of the associations between the various loci tested for and the presence or absence of sarcoidosis was estimated from 2 x 2 tables using the x2 test
RESULTS: Of the 51 patients, 70.6% were female. The mean age was 44.6 years. Analysis of HLA class I and class II phenotypes in sarcoidosis patients revealed a significant association with HLA-B15, C4, C7, C12, C15, C16, C17, DQ3, DR8 and DR11. In addition, a significant negative (protective) association with HLA A9, A28, B12, B17 and DR2 was observed
CONCLUSION: This HLA study in SA patients suggests that genetic factors play a role in the causation of sarcoidosis. Some HLA subtypes have a significant association with sarcoidosis in SA patients, while other subtypes may be protective. The study supported the association of HLA antigens with sarcoidosis and implies that there is a genetic predisposition to sarcoidosis in the SA population
Sarcoidosis is a multisystem disease characterised by development of granulomas, with the lungs the most frequent site.[1-3] The precise cause of sarcoidosis is not known, but several genetic, environmental, occupational and socioeconomic risk factors are implicated in its pathogenesis. It has been suggested that the disorder arises in people who are genetically predisposed to react to certain as yet unknown environmental agents, which leads to an exaggerated inflammatory immune response resulting in granulomas developing. The theory that genetic factors play a role in the pathogenesis of sarcoidosis is based on family groupings of sarcoidosis cases, increased monozygotic twin incidences in relation to non-twin siblings, and racial differences in disease expression, frequency and vulnerability.[4,5]
Sarcoidosis predisposition in different populations has been documented to be associated with various HLA antigens, and the interaction of sarcoidosis with HLA antigens has been studied.[6-13] Certain subtypes of HLA have an increased prevalence in sarcoidosis patients and can affect susceptibility to and the presentation, progression and prognosis of disease. Reports from various countries have often generated contradictory findings, with a few HLA alleles showing an increased risk while other alleles are related to disease protection.[14] There has been no consensus regarding a particular HLA locus that is directly involved in sarcoidosis pathogenesis. Various authors have suggested the presence of different loci within the HLA region, and several studies have concentrated mainly on the genes of class II HLA subtypes. However, in Africa, particularly South Africa (SA), there are no details about HLA types in relation to sarcoidosis. The objective of this study was therefore to determine and evaluate the association between HLA class I and II typing and sarcoidosis in SA patients.
Methods
A total of 51 patients with sarcoidosis and 63 controls were enrolled in the study. Demographic and clinical characteristics, laboratory data and chest X-ray stage of the patients at the time of diagnosis were noted. The diagnosis of sarcoidosis was based on the presence of clinical symptoms, radiological features compatible with sarcoidosis, and histological evidence of non-caseating epithelioid cell granulomas after exclusion of other known causes of granulomatosis, as defined by the joint statement of the American Thoracic Society, the European Respiratory Society and the World Association of Sarcoidosis and other Granulomatous Disorders.[1]
HLA-A, B and C and HLA-DR and DQ antigen typing was performed on DNA extracted from peripheral blood samples of 51 consecutive patients with sarcoidosis attending the respiratory clinic at Charlotte Maxeke Johannesburg Academic Hospital. Peripheral blood was collected and kept at -20°C until utilised. The 63 controls were potential organ donors who had had HLA typing performed previously. Phenotype frequencies of HLA class I antigens (A, B and C) and HLA class II alleles (DR and DQ) were calculated for the patient and control groups. Approval to conduct the study was granted by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (ref. no. M110750).
Typing for HLA class I and II antigens was performed with the standard low-resolution molecular technique. Briefly, genomic DNA was extracted from whole blood using the QIAmp DNA blood mini kit (Qiagen GmbH, Germany), according to the manufacturer's instructions. Following extraction, the DNA concentrations were determined spectrophotometrically and samples were diluted to a concentration of 50 ng/(µL. HLA-A, B, C, DR and DQ were analysed with the polymerase chain reaction (PCR) sequence-specific primer (SSP) technique (Invitrogen Dynal; Fisher Scientific Inc., USA), according to the manufacturer's specifications. Essentially, an appropriate amount of master mix was aliquoted into an Eppendorf tube to which sample DNA (at 50 ng/(µL) and AmpliTaq DNA polymerase (Applied Biosystems Corp., USA) at a final concentration of 1.25 U per reaction was added. The PCR mix was dispensed into each well of a Dynal Allset SSP tray (Fisher Scientific Inc., USA). After sealing securely, the Allset tray was placed into an MJ Mini Thermal Cycler (BioRad Inc., USA) and the amplifications were performed. The PCR conditions were as follows: 1 cycle of 96oC for 2 minutes (denaturation), followed by 10 cycles at 96oC for 15 seconds (denaturation) and 65oC for 1 minute (annealing and extension), and finally 20 cycles at 96oC for 10 seconds (denaturation), 61oC for 50 seconds (annealing), and 72oC for 30 seconds (extension). PCR products were detected using agarose gel electrophoresis run on 2% agarose gel containing ethidium bromide, after which gels were examined under ultraviolet light and products documented by photography. Gels were analysed according to specific tables provided by Invitrogen Dynal (Fisher Scientific Inc., USA), and alleles were scored using the SSP tool software supplied and manually. Alleles for each HLA subtype for each patient were recorded on the data sheets supplied.
Each individual was tested for the HLA-A, B, C, DR and DQ antigens (see Tables 4 - 8). Gene frequencies (gf) were calculated from the antigen frequency (af) using Bernstein's formula: gf = 1 - V(1 - af). The frequency of the 'blank' gene(s) was obtained by subtracting the sum total of defined gene frequencies from 1. The gene frequencies in the different groups were compared using the X2 test. The odds ratio (OR), statistical significance and 95% confidence interval of the antigen frequencies in the patients were compared with those in the controls.
Results
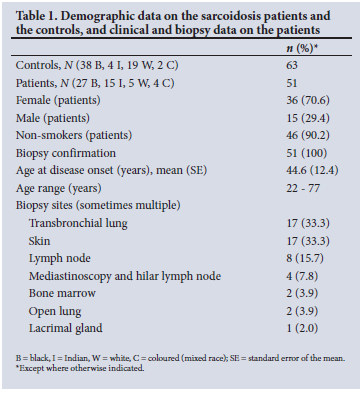
Demographic data on the sarcoidosis patients and the controls, and the clinical data, biopsy confirmation and biopsy sites of the patients, are shown in Table 1. A total of 51 patients with a diagnosis of sarcoidosis were consecutively enrolled in the HLA study. There were 27 black, 15 Indian, 5 white and 4 coloured patients, of whom 36 (70.6%) were female and 90.2% were non-smokers. The mean (standard error (SE)) age of disease onset was 44.6 (12.4) years, with a range of 22 - 77 years. All patients had a biopsy performed for histological confirmation of sarcoidosis, most commonly transbronchial lung biopsy by fibreoptic bronchoscopy (33.3%) and cutaneous biopsy (33.3%).
The laboratory (serum angiotensin-converting enzyme (sACE)), radiographic, lung function and therapeutic data of the patients are shown in Table 2. The mean (SE) sACE level was 139.4 (61.6) IU/L, and the level was elevated in 63.4% of the patients. The majority of the patients (45.1%) had stage II chest X-ray findings, 29.4% had a low forced expiratory volume in 1 second and 29.4 a low forced vital capacity, and 15.7% had an obstructive defect. Of the patients, 84.3% were treated with corticosteroids.

The HLA antigen frequencies of the class I (A, B and C) and class II (DR and DQ) loci of both the patients and the controls are presented in Tables 3 - 8. The 63 controls were potential organ donors who had had HLA typing performed previously, and were matched racially as closely to the patients as possible.
The antigen frequencies of the class IA locus antigens are shown in Table 3. HLA-A9 and A28 occurred more frequently in the control group and were both significantly raised in the control group compared with the patients, conferring a significant relative protective effect with ORs of 0.04 and 0.05, respectively, as shown in the table.
The antigen frequencies of the class IB locus antigens are shown in Table 4. HLA-B15 was significantly more frequent in the sarcoidosis patients compared with the controls. The relative risk for the development of sarcoidosis associated with having the locus B15 was found to be increased 10-fold. Of note, HLA-B12 and B17 occurred more frequently in the control group than in the patients with sarcoidosis and were both significantly higher in the control group, conferring a significant relative protective effect with ORs of 0.02 and 0.05, respectively, as shown in the table.
Table 5 shows the frequencies of HLA-C locus antigens. The frequencies of C4, C7, C12, C15, C16 and C17 were significantly higher in the patients than in the controls. These HLA-C locus antigens conferred a significantly increased relative risk of developing sarcoidosis. The increase in the relative risk for the development of sarcoidosis for these HLA-C locus antigens ranged from 3-fold to 36-fold, as shown in the table.
Antigen frequencies of various class II antigens were raised in the patient group. Table 6 shows the antigen frequencies of the class II antigens, representing those expressed by the DR (B1) locus. HLA-DR (B1) DR8 and DR11 were both significantly raised in the patients with sarcoidosis. The increase in the relative risk for the development of sarcoidosis associated with DR8 and DR11 was 15.0fold and 5.7-fold, respectively. Of note was the fact that the HLA locus antigen DR2 occurred significantly more frequently in the control group compared with the patients, conferring a significant relative protective effect (OR 0.06), as shown in the table.
Table 7 shows the antigen frequencies of the class II antigens, representing those expressed by the DQ locus. The frequency of DQ3 was significantly higher in the patients compared with the controls. Expression of DQ3 appears to confer a 3-fold increase in the relative risk in the patient group.
Table 8 summarises the significant differences in the class I and class II locus antigen frequencies in the patients with sarcoidosis and in the control group.
Because the majority of patients with sarcoidosis in the HLA study were black, comprising 27 (52.9%) of the cohort, they were compared with race-matched controls (Table 9). HLA-A9 and A28 were both significantly raised in the control group compared with the patients, conferring a significant relative protective effect with an OR of 0.056. HLA-B15 was significantly higher in the black patients compared with the controls. The increase in the relative risk for the development of sarcoidosis associated with having the locus B15 was found to be 34-fold. Of note, HLA-B12 occurred significantly more frequently in the control group compared with the patients with sarcoidosis, conferring a significant relative protective effect with an OR of 0.038. The frequencies of C12, C16 and C17 were significantly higher in the patients than in the controls. These HLA-C locus antigens conferred a significantly increased 18-fold relative risk of developing sarcoidosis. DR11 was significantly raised in the patients with sarcoidosis, conferring a 4-fold increase in the relative risk for the development of sarcoidosis.
Discussion
The findings of the present study suggest the possible existence of an association between different HLA genotypes and sarcoidosis in patients in SA, although a relatively small number of patients were recruited. Variations in the vulnerability, presentation, progression and prognosis of sarcoidosis have been noted to be linked to various HLA phenotypes in different populations studied outside SA, but no previous studies have been conducted in SA. It would be interesting to explore the correlation of the clinical phenotypes with the HLA genotype in patients with sarcoidosis in SA.
Studies of the association between sarcoidosis and HLA are not new, and began over 40 years ago. The earliest recorded sarcoidosis-HLA gene relationship was HLA-A7.[15] Many studies were unable to repeat the initial recorded HLA-A7 association in the ensuing years.[16,17]
Some of the studies of HLA associations have shown that HLA-A1, B8 and DRB1*11 are associated with susceptibility to sarcoidosis.[5] However, DQB1, DRB1*3 and 4 and DRB3*15 have been associated with protection, good prognosis and Löfgren syndrome, while DRB3*1 has been associated with susceptibility, as well as disease progression.[5] In 1977, Brewerton et al.[18] first described an association of acute sarcoidosis with the HLA class I
antigen HLA-B8 that was confirmed later by other groups.[19,20] Hedfors and Lindström[19] reported that genes HLA-B8/DR3 appear to be inherited as a sarcoidosis risk haplotype in whites. The haplotype HLA-B8/DR3 in whites is associated with a wide range of autoimmune diseases.[21] These earlier studies concentrated on HLA antigens of the class I subtype. A report by Grunewald et al.[22] indicated that HLA class I and II genes function together with regard to the pathophysiology of sarcoidosis.
HLA-DRB1 was the sarcoidosis-related antigen most commonly tested in the class II HLA antigens. The mutation of the HLA-DRB1 sarcoidosis gene affects both vulnerability and prognosis.[23,24] The suspected population risk in the A Case Control Etiologic Study of Sarcoidosis (ACCESS) sample was 16% in black and 9% in white patients.[23] The HLA-DRB1*1101 allele was related to sarcoidosis in both the blacks and the whites (p<0.01). In addition, the ACCESS analysis showed that HLA class II alleles may be markers for various forms of sarcoidosis, such as DRB1*0401 in blacks and whites for eye involvement, DRB3 in blacks for bone marrow involvement, and DPB1*0101 in whites for hypercalcaemia.[23] Another strong finding across populations was the relationship of the HLA-DQB1*0201 allele with reduced risk of disease progression.[25] The fact that certain distinctive class II HLA genes appear to predispose to sarcoidosis is firmly endorsed by other reports.[25,26]
Linkage disequilibrium (LD) refers to the non-random association of alleles at two or more loci in a general population, which happens when alleles at two distinctive loci occur in gametes more frequently than expected.[5] LD within the major histocompatibility complex (MHC) area restricts the capacity to precisely identify the involved HLA genes.[5] HLA-DRB1*03 has been correlated with resolved disease and HLA-DRB1*15 with chronic disease, while Grunewald et al.[22] suggested that HLA-DQB1*0201 was associated with resolved disease and HLA-DQB1*0602 with chronic disease. Determining the impact of HLA-DQB1 on sarcoidosis risk can therefore be an insurmountable challenge in whites, as well as for the evaluation of other genetic effects from the closely associated haplotypes in the MHC region. In African American people, the HLA-DRB1/DQB1 LD is not as robust as it is in Caucasians.[27]
A host of other countries have shown various HLA types to be found with increased frequency in sarcoidosis patients. In Japan DRw52, in Italy/Czech Republic A1, B8 and DR3, in Denmark DRw6, in Scandinavia DR17, in the UK C7 and B8, in African Americans A23 and B15, and in Turkey A2, A9, A24, A25, A69, B12, B22, B38, B48, DR4 and R14 were found with increased frequency.[4-10] In countries where HLA frequencies were decreased or absent (protective), these included B12 and DR4 in Italian/ Czech patients, DR3 in Danish patients, and A30, A33 and B17 in African Americans. In comparison, in the present study, a negative correlation with HLA-A9, A28, B12 (B12 also found in Italian/Czech patients), B17 (also found in African American patients) and DR2 was observed.[4-6,12,18,28-34]
Overall, in the current study, the frequencies of HLA B15 (also found in African Americans), C4, C7 (also found in UK patients), C12, C15, C16, C17, DQ3, DR8 and DR11 were found to be significantly higher in the sarcoidosis patients than in the controls. In addition, a statistically significant negative (protective) correlation with HLA A9, A28, B12, B17 and DR2 was observed. In the black patients with sarcoidosis, the frequencies of HLA B15, C12, C16, C17, and DR11 were found to be significantly higher than in the controls. In addition, a statistically significant negative (protective) correlation with HLA A9, A28 and B12 was observed.
The onset of sarcoidosis depends on genetic predisposition, as well as an environmental or occupational exposure to an unknown agent or agents, and the disease has considerable clinical heterogeneity.[35,36] The difficulties in identifying an HLA association with sarcoidosis have been attributed to these factors. Further studies on larger patient numbers, with well-defined clinical phenotypes and well-matched controls, may assist in circumventing these issues. HLA alleles were correlated consistently with the disease course, indicating that HLA plays a significant role in deciding the disease phenotype. In addition, the contradictory results in HLA connection in sarcoidosis vulnerability analysis may be described through variation in the phenotype composition of the patient groups studied.
Conclusion
The results of the present study suggest that there is a connection between sarcoidosis and HLA in SA, although patient numbers were relatively small. Different HLA phenotypes have been associated with variations in disease susceptibility, presentation, progression and prognosis. It would be interesting to explore the correlation of the clinical phenotype with the HLA genotype in our patients. This could not be done in the present study because of relatively small patient numbers, and because there were different racial groups. Nonetheless, certain HLA antigens were higher in frequency compared with control groups. These results are consistent with other HLA correlation reports. As mentioned above, because HLA antigens differ between countries, each country should probably develop its own HLA continuum to draw a global map of HLA antigen-sarcoidosis associations.
Declaration. None.
Acknowledgements. None.
Author contributions. RM: conception, study design, execution, acquisition of data, drafted the article; RD: execution, analysis, data acquisition, review of the article; AAW: conception, study design, controls; CF: conception, design, substantially revised and critically reviewed the article.
Funding. This research was supported by a grant from the South African Thoracic Society via the AstraZeneca Pharmaceuticals Grant.
Conflicts of interest. None.
References
1. Hunninghake G, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis: American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16(2):149-173. [ Links ]
2. Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers 20195(1):45. https://doi.org/10.1038/s41572-019-0096-x [ Links ]
3. Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med 2018;6(5):389-402. https://doi.org/10.1016/S2213-2600(18)30064-X [ Links ]
4. Spagnolo P, du Bois RM. Genetics of sarcoidosis. Clin Dermatol 2007;25(3):242-249. https://doi.org/10.1016/j.clindermatol.2007.03.001 [ Links ]
5. Iannuzzi MC, Rybicki BA. Genetics of sarcoidosis: Candidate genes and genome scans. Proc Am Thorac Soc 2007;4(1):108-116. https://doi.org/10.1513/pats.200607-141JG [ Links ]
6. Berlin M, Fogdell-Hahn A, Olerup O, Eklund A, Grunewald J. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med 1997;156(5):1601-1605. https://doi.org/10.1164/ajrccm.156.5.9704069 [ Links ]
7. Abe S, Yamaguchi E, Makimura S, Okazaki N, Kunikane H, Kawakami Y. Association of HLA-DR with sarcoidosis: Correlation with clinical course. Chest 1987;92(3):488-490. https://doi.org/10.1378/chest.92.3.488 [ Links ]
8. Martinetti M, Tinelli C, Kolek V, et al. 'The sarcoidosis map': A joint survey of clinical and immunogenetic findings in two European countries. Am J Respir Crit Care Med 1995;152(2):557-564. https://doi.org/10.1164/ajrccm.152.2.7633707 [ Links ]
9. Celik G, Çen E, Ülger A, et al Human leukocyte antigens A and B in Turkish patients with sarcoidosis. Arch Bronconeumol 2004;40(10):449-452. https://doi.org/10.1016/s1579-2129(06)60354-6 [ Links ]
10. Lenhart K, Kolek V, Bartova A. HLA antigens associated with sarcoidosis. Dis Markers 1990;8(1):23-29. [ Links ]
11. Kunikane H, Abe S, Tsuneta Y, et al Role of HLA-DR antigens in Japanese patients with sarcoidosis. Am Rev Respir Dis1987;135(3):688-691. https://doi.org/10.1164/arrd.1987.1353.688 [ Links ]
12. Pasturenzi L, Martinetti M, Cuccia M, Cipriani A, Semenzato G, Luisetti M. HLA class I, II, and III polymorphism in Italian patients with sarcoidosis. Chest 1993;104(4):1170-1175. https://doi.org/10.1378/chest.104.4.1170 [ Links ]
13. Beijer E, Veltkamp M, Meek B, Moller DR. Etiology and immunopathogenesis of sarcoidosis: Novel insights. Semin Respir Crit Care Med 2017;38(4):404-416. https://doi.org/10.1055/s-0037-1603087 [ Links ]
14. Fiorillo MT, Paladini F, Tedeschi V, Sorrentino R. HLA class I or class II and disease association: Catch the difference if you can. Front Immunol 2017;8:1475. https://doi.org/10.3389/fimmu.2017.01475 [ Links ]
15. Hedfors E, Möller E. HL-A antigens in sarcoidosis. Tissue Antigens 1973;3(2):95-98. 10.1111/J.1399-0039.1973.tb00983.x [ Links ]
16. Möller E, Hedfors E, Wiman L-G. HL-A genotypes and MLR in familial sarcoidosis. Tissue Antigens 1974;4(3):299-305. https://doi.org/10.1111/j.1399-0039.1974.tb00256.x [ Links ]
17. Kueppers F, Mueller-Eckhardt C, Heinrich D, Schwab B, Brackertz D. HL-A antigens of patients with sarcoidosis. Tissue Antigens 1974;4(1):56-58. https://doi.org/10.1111/j.1399-0039.1973.tb00983.x [ Links ]
18. Brewerton D, Cockburn C, James DC, James DG, Neville E. HLA antigens in sarcoidosis. Clin Exp Immunol 1977;27(2):227-229. [ Links ]
19. Hedfors E, Lindström F. HLA-B8/DR3 in sarcoidosis: Correlation to acute onset disease with arthritis. Tissue Antigens 1983;22(3):200-203. https://doi.org/10.1111/j.1399-0039.1983.tb01192.x [ Links ]
20. Smith M, Turton C, Mitchell D, Turner-Warwick M, Morris L, Lawler S. Association of HLA B8 with spontaneous resolution in sarcoidosis. Thorax 1981;36(4):296-298. https://doi.org/10.1136/thx.36.4.296 [ Links ]
21. Lio D, Candore G, Romano GC, et al. Modification of cytokine patterns in subjects bearing the HLA-B8,DR3 phenotype: Implications for autoimmunity. Cytokines Cell Mol Ther 1997;3(4):217-224. [ Links ]
22. Grunewald J, Eklund A, Olerup O. Human leukocyte antigen class I alleles and the disease course in sarcoidosis patients. Am J Respir Crit Care Med 2004;169(6):696-702. https://doi.org/10.1164/rccm.200303-459OC [ Links ]
23. Rossman MD, Thompson B, Frederick M, et al HLA-DRB1*1101: A significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet 2003;73(4):720-735. https://doi.org/10.1086/378097 [ Links ]
24. Ishihara M, Ohno S, Ishida T, et al. Molecular genetic studies of HLA class II alleles in sarcoidosis. Tissue Antigens 1994;43(4):238-241. https://doi.org/10.1111/j.1399-0039.1994.tb02331.x [ Links ]
25. Iannuzzi MC, Maliarik MJ, Poisson LM, Rybicki BA. Sarcoidosis susceptibility and resistance HLA-DQB1 alleles in African Americans. Am J Respir Crit Care Med 2003;167(9):1225-1231. https://doi.org/10.1164/rccm.200209-1097OC [ Links ]
26. Rybicki BA, Maliarik MJ, Poisson LM, et al The major histocompatibility complex gene region and sarcoidosis susceptibility in African Americans. Am J Respir Crit Care Med 2003;167(3):444-449. https://doi.org/10.1164/rccm.2112060 [ Links ]
27. Zachary AA, Bias WB, Johnson A, Rose SM, Leffell MS. Antigen, allele, and haplotype frequencies report of the ASHI minority antigens workshops: Part 1, African-Americans. Hum Immunol 2001;62(10):1127-1136. https://doi.org/10.1016/s0198-8859(01)00305-6 [ Links ]
28. Moller DR, Chen ES. Genetic basis of remitting sarcoidosis: Triumph of the trimolecular complex? Am J Respir Cell Mol Biol 2002;27(4):391-395. https://doi.org/10.1165/rcmb.2002-0164PS [ Links ]
29. Odum N, Milman N, Jakobsen B, Georgsen J, Svejgaard A. HLA class II (DR, DQ, DP) in patients with sarcoidosis: Evidence of an increased frequency of DRw6. Exp Clin Immunogenet 1991;8(4):227-232. [ Links ]
30. Smith G, Brownell I, Sanchez M, Prystowsky S. Advances in the genetics of sarcoidosis. Clin Genet 2008;73(5):401-412. https://doi.org/10.1111/j.1399-0004.2008.00970.x [ Links ]
31. Sato H, Woodhead FA, Ahmad T, et al. Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum Mol Genet 2010;19(20):4100-4111. https://doi.org/10.1093/hmg/ddq325 [ Links ]
32. Akokan G, Celiköglu S, Göksel F, Demirci S. Antigens in Turkish patients with sarcoidosis. N Engl J Med 1977;296(13):759. [ Links ]
33. Ozyilmaz E, Ozturk OG, Durmaz A, et al. Early prediction of sarcoidosis prognosis with HLA typing: A 5 year follow-up study. Sarcoidosis Vasc Diffuse Lung Dis 2018;35(3):184-191. https://doi.org/10.36141/svdld.v35i3.6781 [ Links ]
34. Rybicki BA, Iannuzzi MC. Sarcoidosis and human leukocyte antigen class I and II genes: It takes two to tango? Am J Respir Crit Care Med 2004;169(6):665-666. https://doi.org/10.1164/rccm.2401005 [ Links ]
35. Starshinova AA, Malkova AM, Basantsova NY, et al. Sarcoidosis as an autoimmune disease. Front Immunol 2019;10:2933. https://doi.org/10.3389/fimmu.2019.02933 [ Links ]
36. Malkova A, Starshinova A, Zinchenko Y, et al. The opposite effect of human leukocyte antigen genotypes in sarcoidosis and tuberculosis: A narrative review of the literature. ERJ Open Res 2020;6(3):00155-2020. https://doi.org/10.1183/23120541.00155-2020 [ Links ]
 Correspondence:
Correspondence:
R Morar
rajenmorar@webmail.co.za; rajen.morar@wits.ac.za
Accepted 21 August 2022














