Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.11b Pretoria Nov. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i11b.16840
CASE REPORT
Primary neuroendocrine invasive breast carcinoma: A case report and literature review
J G MthembuI; M M Z U BhuiyanII
IMB ChB; Department of General Surgery, Mankweng Hospital, Limpopo Academic Health Complex, and School of Medicine, University of Limpopo, Polokwane, South Africa
IIPRCS (Glasg), MMed (Surg); Department of General Surgery, Mankweng Hospital, Limpopo Academic Health Complex, and School of Medicine, University of Limpopo, Polokwane, South Africa
ABSTRACT
Primary neuroendocrine breast cancer (NEBC), also known as breast cancer with neuroendocrine differentiation, comprises a heterogeneous group of rare invasive breast carcinomas. The diagnosis depends on excluding metastasis from other sites, because the two entities require different management approaches. In this case report, we seek to explore and share our experience of primary NEBC, and provide a literature review.
Primary neuroendocrine breast cancer (NEBC) is currently considered rare, being thought to account for ~0.3 - 0.5% of all breast cancers, and for this reason there are no data from prospective clinical trials on its optimal management.[1,2] Also known as breast carcinoma with neuroendocrine differentiation, NEBC comprises a heterogeneous group of rare invasive breast carcinomas[2] that have historically been poorly defined owing to different definitions of what constitutes a neuroendocrine carcinoma.[3] Several theories on the histogenesis of primary NEBC have been proposed in the literature, the most widely recognised suggesting that it is derived from the divergent differentiation of a neoplastic stem cell in both epithelial and neuroendocrine cells.[3] Another theory hypothesises a derivation from neural crest cells that migrate to the mammary glands, or an origin from neuroendocrine cells present in breast tissue.[3] The observation that NEBCs often resemble breast tumours histologically supports the hypothesis that NEBCs derive from the differentiation of an epithelial progenitor cell.[3] Furthermore, the term neuroendocrine carcinoma of the breast was revised to carcinomas with neuroendocrine features in the 2012 WHO Classification of Tumours of the Breast.[4] The diagnosis is based on morphological features similar to those of lung and gastrointestinal neuroendocrine tumours, and neuroendocrine markers.[2] The World Health Organization (WHO) has stratified neuroendocrine neoplasms based on their histological differentiation into low, intermediate and high grade, with majority of NEBCs being hormone receptor positive and human epithelial growth factor receptor 2 (HER2) negative.[5-7] In 2019, the WHO classified NEBCs with neuroendocrine tumours of other organ systems into differentiated neuroendocrine tumours (low Nottingham grade) and poorly differentiated neuroendocrine carcinomas (high Nottingham grade). The group previously named intermediate is excluded, only two groups being recognised.[8-Further classifications and new findings in the future may shed more light on tumours of this histological type. Neuroendocrine tumours commonly occur in the gastrointestinal system (70%) and bronchopulmonary system (20%), with the mammary glands accounting for <1%.[6] Early-stage tumours are usually treated with the same strategy used for other types of invasive breast cancer.[1, 2]
The main objective of this case report is to share our experience with NEBC as an addition to the existing literature. The patient gave written informed consent to publish. Ethical approval was obtained from Pietersburg-Mankweng Research Ethics Committee (ref. no. PMREC 25 AUGUST UL 2021/F).
Case report
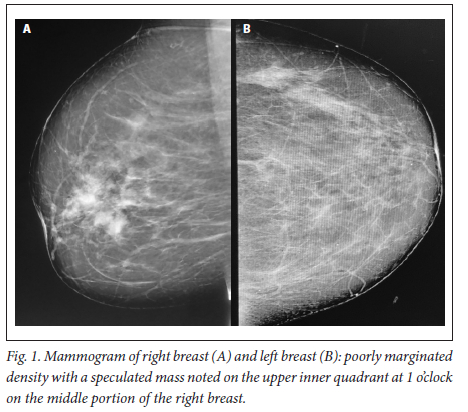
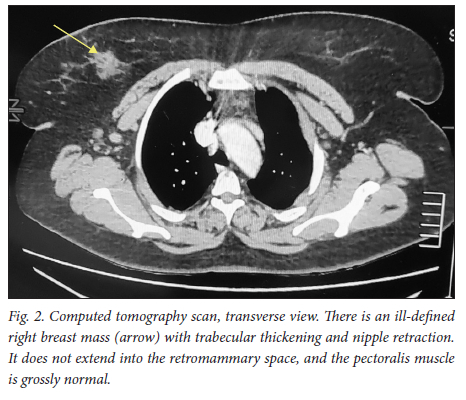
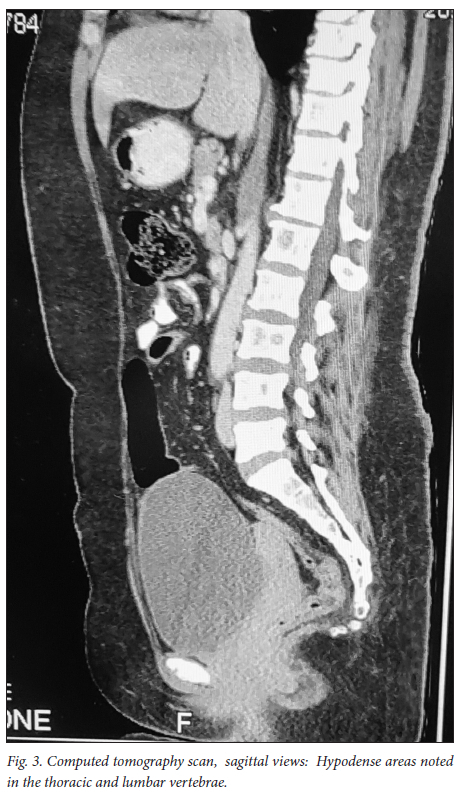
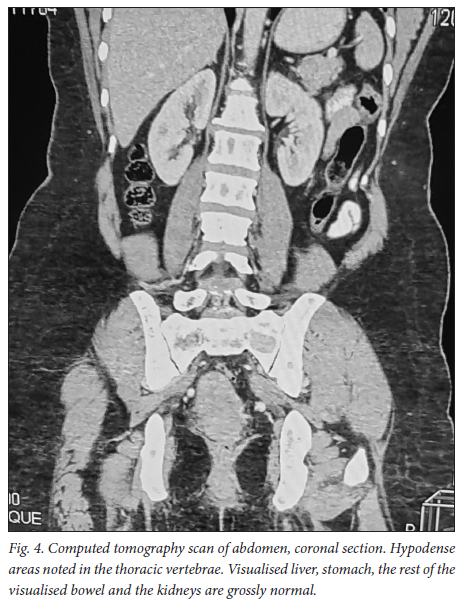
A 39-year-old obese woman had had a lump in the right breast for 18 months. She had gone to her local hospital when she noticed the lesion. A core biopsy was done and she was referred to the breast oncology clinic at Mankweng Hospital in Polokwane, Limpopo Province, South Africa, for further assessment and management. She had previously been healthy, with no known comorbidities and no family history of malignancy. On enquiry about her gynaecological history, she stated that her menarche had been at age 12, and she was still having normal periods and on contraception. She had a 15-year-old child, born when she was 24. After the birth of this child she had two more pregnancies, both resulting in unexplained stillbirths at term. She had no history of pregnancy-induced hypertension or any medical conditions diagnosed during pregnancy. After her discovery of the breast lump she was diagnosed with young-onset hypertension. There were no associated weight changes, and she was HIV negative. On examination she had a scar on the right upper quadrant, no palpable axillary lymph nodes, an elevated blood pressure (150/93 mmHg, pulse rate 88 bpm) and a high body mass index (37.7 kg/m2). The rest of the vital signs and the findings on systemic examination were unremarkable. On examination of the breast, the tumour was clinically staged as T3N0M0, making it stage IIB. Histological evaluation showed nests of cells infiltrating extensively throughout the tissue. The cells were hormone receptor (oestrogen and progesterone) positive, the HER2 score was 3+ and the Ki-67 proliferation index was 50 - 60%. Synaptophysin (SYP) and chromogranin (CGA) cytoplasmic staining was positive within the tumour cells, features in keeping with grade 3 invasive carcinoma showing neuroendocrine differentiation. A mammogram (Fig. 1) revealed poorly marginated density with a speculated mass noted on the upper inner quadrant at 1 o'clock on the middle portion of the right breast. The mass expended anteriorly to the retro-areolar space causing minimal traction of the peri-areolar skin and nipple. There were associated architectural distortions and trabecular thickening. The retromammary space was grossly normal bilaterally. There was no thickening of overlying skin, and no dilated ducts bilaterally. Regional benign calcifications were noted in the left breast. No axillary lymph nodes were visualised bilaterally. For further evaluation, a computed tomography (CT) scan (Figs 2, 3 and 4) was done and revealed multiple enlarged lymph nodes in the right axillary region. The right breast was ill defined with trabecular thickening and nipple retraction. The breast mass did not extend into the retromammary space, and the pectoralis muscle was grossly normal. Hypodense areas were noted in the thoracic and lumbar vertebrae, with involvement of the posterior elements. The right scapular blade and spine also had multiple hypodense areas. The lung parenchyma, the visualised liver, the stomach and the rest of the visualised bowel and the kidneys were grossly normal. With this evidence, the patient was diagnosed as having primary NEBC, with features suggestive of metastasis to the bones. A bone scan revealed widespread metastases in the skull and throughout the spine, pelvic bone, proximal femur and sternum. In view of this finding, the patient was re-assessed as having clinical stage IV disease. The management approach changed to focus on palliative care. There was no surgical intervention, and the patient was referred to the medical oncology clinic for further management, to come back if the need for surgical palliation arose.
Discussion
Primary NEBCs are rare and the diagnosis therefore depends on excluding metastasis from other sites, because the two entities require different management approaches.[9] Diagnostic tools include the use of tumour markers such as CGA and SYP immune reactivity, which is significantly associated with a neuroendocrine neoplasm.[1,10] Importantly, elevated CGA levels are also associated with hypertension, obesity and heart failure,[11] among other conditions. On breast imaging with mammography and/or ultrasound, secondary NEBCs are oval in shape, with circumscribed or microlobulated margins.[12] Triple assessment is mandatory; however, histopathological assessment and immunohistochemistry staining are the mainstay of diagnosis. [1,13,14] Clinically, the presentation of NEBCs cannot be distinguished from other types of breast cancer. It has also been postulated that, unlike other breast cancers, NEBCs can present with clinical features related to hormonal secretion because of ectopic production of adrenocorticotrophic hormone, norepinephrine or calcitonin.[15] Our patient was obese and hypertensive, with SYP- and CGA-positive cytoplasmic staining within the tumour cells. More knowledge and research on the possible association between the tumour markers and clinical syndromes could play a vital role in patient management, so further evidence on this association is of crucial importance. So far, there are no available research data specifying radiological pathognomic features in the diagnosis of NEBCs. As in our patient, mammographic and CT scan findings are similar to those in other histologically different types of breast cancer, and imaging therefore cannot be used alone. It is vital to distinguish between primary NEBCs and metastatic neuroendocrine tumours from another site, because treatment of the two is different.[15] The CT scan findings in our case suggested that the patient had advanced breast cancer with metastasis to the thoracic vertebrae, right scapular blade and spine. With no specific management strategies for advanced neuroendocrine breast malignancies, the management principles are currently the same as for other types of breast cancers. A multimodality therapeutic strategy includes chemotherapy, endocrine therapy, peptide receptor radionuclide therapy, radiation therapy or surgery, or combinations of the above. It is hoped that better knowledge of the biology of these tumours will provide new therapeutic targets for personalised treatment in the near future.
Conclusion
Owing to the rarity of NEBCs, there is not enough evidence to guide specific management, so the treatment is the same as that for invasive breast carcinoma of no special type. Further studies are indicated to elucidate the clinical behaviour of the tumour.
Declaration. None.
Acknowledgements. None.
Author contributions. Equal contributions (concept, acquisition of data, analysis of data, drafting of the manuscript and critical revision for important intellectual content).
Funding. None.
Conflicts of interest. None.
References
1. Rosen LE, Gattuso P. Neuroendocrine tumors of the breast. Arch Pathol Lab Med 2017;141(11):1577-1581. https://doi.org/10.5858/arpa.2016-0364-rs [ Links ]
2. Trevisi E, la Salvia A, Daniele L, et al. Neuroendocrine breast carcinoma: A rare but challenging entity. Med Oncol 2020;37(8):70. https://doi.org/10.1007/s12032-020-01396-4 [ Links ]
3. Pareja F, D'Alfonso TM. Neuroendocrine neoplasms of the breast: A review focused on the updated World Health Organization (WHO) 5th edition morphologic classification. Breast J 2020;26(6):1160-1167. https://doi.org/10.1111/tbj.13863 [ Links ]
4. Bussolati G, Badve S. Carcinomas with neuroendocrine features. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van der Vijver MJ, eds. WHO Classification of Tumours of the Breast. Lyon: IARC Press, 2012:62-63. [ Links ]
5. Irelli A, Sirufo MM, Morelli L, D'Ugo C, Ginaldi L, de Martinis M. Neuroendocrine cancer of the breast: A rare entity. J Clin Med 2020;9(5):1452. https://doi.org/10.3390/jcm9051452 [ Links ]
6. Özdirik B, Kayser A, Ullrich A, et al. Primary neuroendocrine neoplasms of the breast: Case series and literature review. Cancers (Basel) 2020;12(3):733. https://doi.org/10.3390/cancers12030733 [ Links ]
7. Lin X, Matsumoto Y, Nakakimura T, et al. Invasive solid papillary carcinoma with neuroendocrine differentiation of the breast: A case report and literature review. Surg Case Rep 2020;6(1):143. https://doi.org/10.1186/s40792-020-00905-x [ Links ]
8. Tsang JY, Tse GM. Breast cancer with neuroendocrine differentiation: An update based on the latest WHO classification. Mod Pathol 2021;34(6):1062-1073. https://doi.org/10.1038/s41379-021-00736-7 [ Links ]
9. Salemis NS. Primary neuroendocrine carcinoma of the breast: A rare presentation and review of the literature. Intractable Rare Dis Res 2020;9(4):233-246. https://doi.org/10.5582/irdr.2020.03046 [ Links ]
10. Juhlin CC, Zedenius J, Höög A. Clinical routine application of the second-generation neuroendocrine markers ISL1, INSM1, and secretagogin in neuroendocrine neoplasia: Staining outcomes and potential clues for determining tumor origin. Endocr Pathol 2020;31(4):401-410. https://doi.org/10.1007/s12022-020-09645-y [ Links ]
11. Goetze JP, Alehagen U, Flyvbjerg A, Rehfeld JF. Chromogranin A as a biomarker in cardiovascular disease. Biomark Med 2014;8(1):133-140. https://doi.org/10.2217/bmm.13.102 [ Links ]
12. Choudhery S, Xiao L, Zingula S. Review of nonmammary metastases to the breast: Imaging and clinical presentation. Curr Probl Diagn Radiol 2021;50(4):495-498. https://doi.org/10.1067/j.cpradiol.2020.04.010 [ Links ]
13. Chan KH, Lee CH, Sharif SZ, Hayati F, Sallapan S. Diagnostic challenge in diagnosing bilateral breast metastases from mediastinal neuroendocrine tumor: A case report. Ann Med Surg (Lond) 2020;60:438-441. https://doi.org/10.1016/j.amsu.2020.11.035 [ Links ]
14. Wachter DL, Hartmann A, Beckmann MW, et al. Expression of neuroendocrine markers in different molecular subtypes of breast carcinoma. Biomed Res Int 2014;2014:408459. https://doi.org/10.1155/2014/408459 [ Links ]
15. Salemis NS. Primary neuroendocrine carcinoma of the breast: A rare presentation and review of the literature. Intractable Rare Dis Res 2020;9(4):233-246. https://doi.org/10.5582/irdr.2020.03046 [ Links ]
 Correspondence:
Correspondence:
M M Z U Bhuiyan
bhuiyanmirza@gmail.com
Accepted 25 September 2022














