Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.11 Pretoria nov. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i11.16453
RESEARCH
The characteristics and costs of severe theophylline toxicity in a tertiary critical care unit in Eastern Cape Province, South Africa
J V Oxley-OxlandI; R FreercksII; D BakerIII; E van der MerweIV
IMB ChB; Department of Medicine, Livingstone Tertiary Hospital and Faculty of Health Sciences, Walter Sisulu University, Gqeberha, South Africa
IIMB ChB, MPhil (Med); Division of Nephrology and Hypertension, Livingstone Tertiary Hospital, Gqeberha, and Department of Medicine, Faculty of Health Sciences., University of Cape Town, South Africa
IIIMB ChB; Department of Anaesthesia and Critical Care, Livingstone Tertiary Hospital and Faculty of Health Sciences, Walter Sisulu University, Gqeberha., South Africa
IVMB ChB, MMed (Int Med); Department of Anaesthesia and Critical Care, Livingstone Tertiary Hospital and Faculty of Health Sciences, Walter Sisulu University, Gqeberha., South Africa
ABSTRACT
BACKGROUND: Severe theophylline toxicity requiring haemodialysis accounts for approximately one-third of drug toxicity cases admitted to the Livingstone Tertiary Hospital (LTH) intensive care unit (ICU) in Gqeberha, South Africa, imposing a significant resource burden
OBJECTIVES: To investigate the characteristics and burden of severe theophylline toxicity in an Eastern Cape Province tertiary hospital adult ICU
METHODS: A retrospective review of all severe theophylline toxicity admissions to the ICU from 1 January 2013 to 31 December 2018 was conducted. Demographic and clinical data were captured and analysed. The National Department of Health 2019 fees schedule was used to calculate costs based on duration of ICU stay and number of haemodialysis sessions received
RESULTS: Of the 57 patients included in the study, 84% were cases of deliberate self-harm. The majority were aged <40 years (77%) and female (79%). The mean (standard deviation (SD)) initial serum theophylline level was 612 (269) umol/L. Complications included convulsions (n=12; 21%), arrhythmias (n=9; 16%), need for mechanical ventilation (n=7; 12%) and death (n=4; 7%). The main risk factors for these complications were age >30 years, an inappropriately normal or elevated initial serum potassium level, an elevated serum creatinine kinase level and an elevated initial serum theophylline level. Receiver operator characteristic curve analysis assessing the initial serum theophylline level as a discriminator for life-threatening complications produced an area under the curve of 0.71 for serum theophylline >400 umol/L (sensitivity 88%, specificity 12%). All the 4 patients who died had an initial serum theophylline level >1 000 umol/L. The mean (SD) cost per admission amounted to ZAR16 897 (10 718), with a mean of one 4-hour dialysis session per admission
CONCLUSION: Severe theophylline toxicity, usually in the context of deliberate self-harm, is a preventable yet life-threatening toxicity encountered at LTH. Demographic risk factors include young females from certain areas in and around Gqeberha. Risk factors for complications include older age, paradoxically normal or elevated serum potassium levels, elevated serum creatinine kinase levels and an initial serum theophylline level >400 umol/L. Patients with these clinical features should be closely monitored and treated timeously at an appropriate level of care. The need for ICU admission and dialysis, both limited resources, makes the treatment of severe theophylline toxicity costly. Further studies of the underlying psychosocial drivers, local prescribing practices and preventive interventions related to severe theophylline toxicity are required
Theophylline has been used to treat obstructive airway diseases since the 1920s.[1] In view of toxicity risk (particularly in the elderly), drug interactions, variable metabolism and inferior efficacy compared with long-acting beta-agonists, theophylline is no longer being recommended as a first- or second-line treatment in local guidelines for asthma[2] and chronic obstructive pulmonary disease (COPD).[3] In spite of this, its use is still widespread in the communities served by Livingstone Tertiary Hospital (LTH) in Eastern Cape Province, South Africa (SA). Severe theophylline toxicity is a common presentation of drug toxicity requiring admission to the LTH intensive care unit (ICU). An internal audit of admissions to this ICU over a 3-year period (2017 - 2019) found that 100 patients were admitted for drug toxicities. Of these, 29 were admitted for severe theophylline toxicity, making it the most common cause of admission for drug toxicity to the ICU.
Theophylline exerts its bronchodilator effect through the inhibition of phosphodiesterase 3, while its anti-inflammatory effects are thought to be mediated through the inhibition of phosphodiesterase 4 and activation of histone deacetylase 2.[1] The complications of toxicity may include refractory seizures,[4] cardiac arrhythmias,[5,6] rhabdomyolysis with acute kidney injury[7] and death.[6] Although levels above the therapeutic range of 28 - 83 µmol/L are considered toxic, current guidelines recommend extracorporeal treatment for levels >555 µmol/L in the acute setting and >333 µmol/L in the chronic setting, as these markedly elevated levels are commonly associated with life-threatening complications.[8] The standard of care for effective treatment of severe theophylline toxicity includes both ICU admission and intermittent haemodialysis.[8] Both are costly and scarce resources in low- and middle-income countries.[9]
The objectives of this study were to assess the characteristics (demographics, clinical features and biochemical parameters) and estimated ICU-related costs of severe theophylline toxicity in an Eastern Cape tertiary ICU.
Methods
Design, setting and population
This study was a retrospective folder review of patients with severe theophylline toxicity admitted to LTH's 16-bed ICU between 1 January 2013 and 31 December 2018. The hospital serves -1.7 million people[10] from an area of 60 000 km2.[1l] The ICU is a multidisciplinary, closed unit and admits patients aged >12 years. There is full-time on-site medical officer or registrar cover, full-time specialist cover and a registered nurse-to-patient ratio of 3:4. All modalities of emergency dialysis are offered, including after-hours dialysis.
Inclusion criteria
All patients aged >12 years with severe theophylline toxicity admitted to the LTH ICU between 1 January 2013 and 31 December 2018 were included. Admission criteria were based on the latest guidelines available at the time of study commencement in 2013.[12] Although the Extracorporeal Treatment in Poisoning (EXTRIP) workgroup guidelines were subsequently updated in 2015,[8] the original guidelines were used for the duration of the study period. Patients were admitted to the ICU if they required dialysis because of either life-threatening complications (e.g. seizures, shock or cardiac arrhythmias) or elevated peak serum theophylline levels >448 µmol/L or >168 µmol/L in the context of acute or chronic toxicity, respectively. In addition to the above, patients were admitted if their peak serum theophylline level was >336 umol/L in the context of any of the following scenarios: inability to tolerate multiple-dose oral activated charcoal (e.g. vomiting, ileus, obstruction); impaired theophylline metabolism (e.g. liver disease, heart failure); high risk of seizures or history of epilepsy; age >65 years; or chronic lung or heart disease.[12]
Instrument
Relevant epidemiological, clinical, biochemical and cost data were collected. Epidemiological data included gender, age, self-reported ethnicity, area of residence, and a history of a deliberate self-harm attempt. Clinical data included the presence of arrhythmias other than sinus tachycardia, the presence of convulsions, respiratory compromise requiring ventilation, and vital status at discharge from the ICU. Biochemical data included the initial serum theophylline level, serum creatinine kinase level and serum potassium level at admission to the ICU. Cost data included estimated cost per ICU admission and were based on standardised national costing estimates.[13] This figure was calculated by the sum of costs per ICU admission (daily costs multiplied by the number of days spent in the ICU) and haemodialysis costs (cost per haemodialysis session multiplied by the number of sessions required).
Statistical analysis
Excel version 16.0 (Microsoft Corp., USA) was used for data handling, while data analysis was carried out using Stata version 15.1 (StataCorp, USA). Normally distributed data are reported as means (standard deviation (SD)) and skewed data as medians (interquartile range (IQR)). Discrete data are presented as numbers (percentages). Multivariate analysis was applied using Student's t-test and Mood's median test for continuous data and the x2 test for categorical data Mood's median test was chosen over the Mann-Whitney test because of concern over outliers. The level of significance was set as p<0.05. A receiver operating characteristic (ROC) curve was obtained for initial theophylline level as a discriminator for the likelihood of one or more complications occurring.
Ethical review
The study was approved by the Walter Sisulu University Human Research Ethics Committee (ref. no. 121/2018).
Results
The demographic characteristics of the 57 patients included in the study are outlined in Table 1. The majority of the patients were aged <40 years (77%), female (79%) and from the suburbs of Gqeberha (64%). Areas outside Gqeberha with significant burdens of severe theophylline toxicity were Makhanda (11%) and Uitenhage (9%).
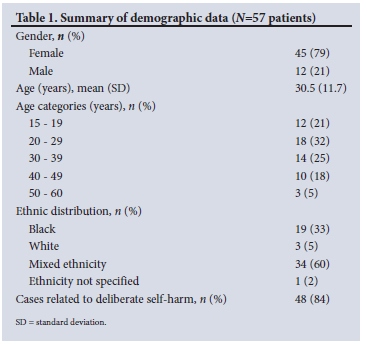
The mean (SD) serum theophylline level on presentation was 612 (269) µmol/L (therapeutic range 56 - 111 µmol/L). Seventy-seven percent of patients had theophylline levels in the dialysis range (>448 µmol/L), with the highest level being 1 398 µmol/L. The median (IQR) serum creatinine kinase level was 1 130 (357 - 2 677) U/L. Sixty-eight percent of the cohort presented with a potassium level below the normal range (<3.4 mmol/L).
Thirty-two serious complications (in 17 patients) were reported in the cohort of 57 patients. Arrhythmias occurred in 9 patients, 12 had convulsions, 7 required ventilation, and 4 deaths occurred. Fig. 1 highlights the specific complication events by patient age and peak theophylline level. All 4 patients who died had an initial theophylline level >1 000 µmol/L, while only 3 of the 7 patients (43%) with theophylline levels >1 000 µmol/L survived. Positive associations with complications were determined. The mean (SD) serum potassium level in patients who were ventilated or who died was 4.1 (1.3) mmol/L, compared with 3.1 (0.7) mmol/L in those who did not experience these complications (p=0.03). The median (IQR) creatinine kinase level in patients with convulsions was 3 945 (1 444 - 16 034) U/L, compared with 731 (270 - 1 799) U/L in those without convulsions (p=0.05). Arrhythmias and convulsions were more frequent in patients aged >30 years compared with those aged <30 (26% v. 7% for arrhythmias, p=0.046; 33% v. 10% for convulsions, p=0.031). The median (IQR) creatinine kinase level in patients requiring more than one 4-hour dialysis session was 2 907 (1 545 - 15 397) U/L, compared with 737 (286 - 1 650) U/L in those who required only one 4-hour dialysis session (p=0.02).
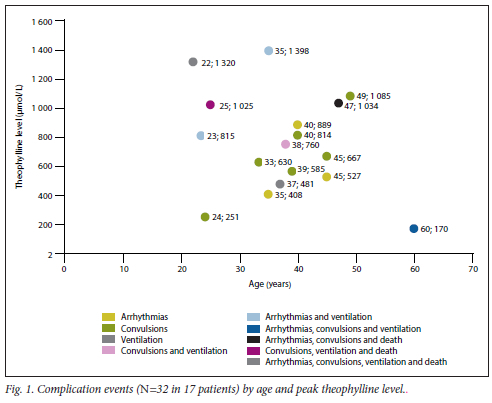
Fig. 2 shows the ROC curve obtained when the initial theophylline level was used as a discriminator for the likelihood of one or more complications occurring. The area under the ROC curve was 0.71. An initial theophylline level of 404.5 µmol/L correlated with a sensitivity of 88.2% for detecting patients with one or more complications. However, the specificity was low at 12.5%.
The cost per day for an ICU admission amounted to ZAR9 963, while the cost per dialysis session amounted to ZAR2 407. The majority of patients with severe theophylline toxicity required only one full day in the ICU (n=44; 77%) and one 4-hour dialysis session (n=46; 81%), with a mean (SD) ICU cost of ZAR16 897 (10 718) per patient admission. The mean (SD) cost for patients who were ventilated was ZAR32 248 (23 994), compared with ZAR14 748 (4 735) for those who did not require ventilation (p=0.001). The mean (SD) cost for patients who died was ZAR31 008 (29 611), compared with ZAR15 832 (7 527) for those who survived (p=0.005).
Discussion
A key principle in the management of theophylline toxicity is appreciation of the spectrum of risk for the development of life-threatening complications. This study outlines the profile of patients at highest risk. These include patients with markedly elevated serum theophylline levels, especially those with levels >1 000 umol/L, older patients, those with a paradoxically normal or elevated initial potassium level (in the context of severe poisoning), and those with significantly elevated creatinine kinase levels. These patients require expedited admission to an ICU and prompt initiation of intermittent haemodialysis.
The role of an initial serum theophylline level is key in risk-stratifying patients with theophylline toxicity, and it is a useful predictor of morbidity and mortality[12] The predictive value of the initial serum theophylline level was highlighted by our finding that 4 of 7 patients (57%) with levels > 1 000 umol/L died. The ROC curve analysis, with an area under the curve of 0.71, confirms the role of the initial serum theophylline level as a moderate or 'fair' tool for predicting the likelihood of one or more complications. Based on this analysis, the threshold of 448 umol/L used in this study correlated with a sensitivity of 82% for the occurrence of serious complications. In light of the potentially life-threatening consequences of severe toxicity, we consider a threshold of 400 umol/L more appropriate for ICU admission and dialysis. This threshold has a sensitivity of -88% for the occurrence of a serious complication, despite the trade-off in specificity (-88% false-positive rate). This suggested threshold is somewhat lower than the current recommendation of 555 umol/L proposed by the EXTRIP workgroup.[8] If the initial serum theophylline levels are between 444 and 555 umol/L, the EXTRIP guidelines recommend repeating measurement. Owing to significant delays in laboratory turnaround time in many SA public sector hospitals,[14] levels may rise above dialysis thresholds before repeat results become available and are acted upon. It is therefore our opinion that a somewhat more conservative threshold for dialysis, particularly in symptomatic patients, may be justified in the SA context. If the initial levels are below the threshold for dialysis, but in the toxic range, we recommend that follow-up serum theophylline levels be measured, bearing in mind that the use of sustained-release theophylline preparations may result in delayed or erratic absorption with potentially life-threatening consequences.[15]
Patient age is an important aspect of risk-stratifying patients with theophylline toxicity. In our cohort, arrhythmias and convulsions were more likely to occur in patients aged >30 years compared with younger patients. This predictor is in keeping with previous findings[16] and may be due to a higher incidence of comorbidities and concomitant use of other medications in older patients.[17]
Clinically significant hypokalemia is well described in the context of theophylline toxicity.[18] Counter-intuitively, we found that patients who required ventilation or who died presented with higher initial potassium levels than those who survived or did not require ventilation. This finding is probably explained by concomitant rhabdomyolysis and the excessive release of intracellular potassium ions into the circulation. The absence of a statistically significant association between initial potassium level and convulsions suggests that the main mechanism for the development of inappropriately normal serum potassium levels was rhabdomyolysis due to the direct myotoxic effects of theophylline,[19] and not rhabdomyolysis due to prolonged convulsions. Higher creatinine kinase levels were associated with the presence of convulsions and the need for additional haemodialysis sessions. In view of these findings, we recommend the serial measurement of potassium and creatinine kinase levels.
Calculation of the ICU-related cost of severe theophylline toxicity comprises two main cost drivers, namely the number of haemodialysis sessions received and the duration of ICU admission. Most of our patients (n=44; 77%) required only 1 day in the ICU, in contrast to a US study of patients with theophylline toxicity admitted to an ICU, in which the average stay was 2 days.[20] This difference may reflect the previously described referral pressure on ICUs in SA.[9] While it was noted that an average of only one haemodialysis session of -4 hours was required for most patients (n=46; 81%), all acute dialysis takes place in the ICU and therefore contributes significantly to resource utilisation. Ventilated patients and those who died incurred significantly higher costs than those who did not have these complications. This higher cost was due to prolonged admissions and complicated clinical courses. The mean (SD) ICU-related admission cost calculated in this study for a case of severe theophylline toxicity was ZAR16 897 (10 718). This figure is in keeping with the results of a study at Cecilia Makiwane Hospital, published in 2013, in which the average cost for a deliberate self-harm admission was found to be ZAR15 966,[21] and would be ZAR21 601 when adjusted for inflation between 2013 and 2019 using the gross domestic price deflator method.[22]
A key demographic finding relevant to the local context of the study was that the majority of patients were young women, with a mean age of just over 30 years. Of concern was that the theophylline toxicity was usually in the context of deliberate self-harm (84% of cases). As women of this age are both economically active and well into childbearing age, the unquantified economic and psychosocial impact of severe theophylline toxicity on families is likely to be significant. It was also noted that specific problem areas and suburbs emerged from the data. Further investigation into the social stressors of young women, particularly in high-incidence communities, and the risk factors associated with deliberate self-harm should be undertaken in order to address the root problems underlying this presentation and enable targeted, culturally relevant prevention strategies to be put in place.
The prescribing patterns of local primary care practitioners in the high-incidence communities should be investigated further. Further studies should investigate whether treatment guidelines for asthma and COPD in these areas are adhered to, whether alternative drugs are available to prescribing doctors, and whether patients are instructed to keep theophylline safely stored away. Considering its limited efficacy, narrow therapeutic index and safety concerns, we advocate removal of theophylline from the Essential Medicines List, limiting its use to specialist clinics as a third- or fourth-line agent. This change is in line with the recommendations of the South African Thoracic Society, which sees a limited role for the use of theophylline.[2,3]
Study limitations
The data surrounding the findings on creatinine kinase are a limitation of this study. Serum creatinine kinase levels were only measured in 68% of patients during their ICU admission. In terms of estimation of costs, several factors related to ICU admission, such as consumables and salaries to non-clinical staff, were not accounted for. This undoubtedly resulted in an underestimation of the true costs of treating severe theophylline toxicity in the ICU. The magnitude of the underestimation is attested to by the 2019 tariff guide provided by a well-known SA private hospital group, which amounts to ZAR 18 178 per day in an ICU without acute dialysis.[23] The cost of acute haemodialysis is ZAR6 168 according to the 2019 SA compensation fund tariff guide,[24] raising the total estimated amount to ZAR 24 346. In addition, costs related to patient care before and after ICU admission were not included in this estimate.
Conclusion
This study presents the largest investigation of the characteristics and burden of severe theophylline toxicity in SA to date. Severe theophylline toxicity, which is a preventable disease, imposes a significant resource burden on this tertiary hospital's ICU and dialysis services. Furthermore, since the study included only severe toxicity cases, the true burden of theophylline toxicity in the community is likely to be far higher. Patients with an initial serum theophylline level >1 000 µmol/L, older patients, those with paradoxically normal or elevated initial potassium levels and those with elevated creatinine kinase levels are at significantly increased risk for serious complications and death. Young women engaging in deliberate self-harm are the demographic most affected. Further investigation into the psychosocial causes and development of preventive strategies is vital in order to curb this alarming local trend. Finally, regulatory authorities should consider whether theophylline should remain available outside specialised clinics, given the significant safety concerns and the limited evidence to support its efficacy as a treatment for obstructive lung diseases.
Declaration. None.
Acknowledgements. We acknowledge the staff of the Livingstone Hospital Critical Care Unit and Renal Unit who assisted in the recruitment of participants into the study.
Author contributions. JVO-O, RF, DB and EvdM conceptualised and planned the study and performed statistical analysis of the data used. JVO-O wrote the first draft of the article. RF, DB and EvdM reviewed and edited the article. All the authors approved the final version of the article.
Funding. We thank the Discovery Foundation, which provided funding through the granting of a Discovery Foundation award, and the Kidneys. Infectious Diseases and Critical Care Public Benefit Organisation, which provided funding for the publication fees.
Conflicts of interest. None.
References
1. Barnes PJ. Theophylline. Am J Respir Crit Care Med 2013;188(8):901-906. https://doi.org/10.1164/rccm.201302-0388PP [ Links ]
2. Lalloo UG, Kalla IS, Abdool-Gaffar S, et al. Guidelines for the management of asthma in adults and adolescents. Position statement of the South African Thoracic Society - 2021 update. Afr J Thorac Crit Care Med 2021;27(4). https://doi.org/10.7196/AJTCCM.2021.v27i4.189 [ Links ]
3. Abdool-Gaffar MS, Calligaro G, Wong ML, et al. Management of chronic obstructive pulmonary disease - a position statement of the South African Thoracic Society. 2019 update. J Thorac Dis 2019;11(11):4408-4427. https://doi.org/10.21037/jtd.2019.10.65 [ Links ]
4. Singer EP, Kolischenko A. Seizures due to theophylline overdose. Chest 1985;87(6):755-757. https://doi.org/10.1378/chest.87.6.755 [ Links ]
5. Derby LE, Jick SS, Langlois JC, Johnson LE, Jick H. Hospital admission for xanthine toxicity Pharmacotherapy 1990;10(2):112-114. [ Links ]
6. Shannon M. Life-threatening events after theophylline overdose. A 10-year prospective analysis. Arch Intern Med 1999;159(9):989-994. https://doi.org/10.1001/archinte.l59.9.989 [ Links ]
7. Rumpf K, Wagner H, Criee C, et al. Rhabdomyolysis after theophylline overdose. Lancet 1985;325(8443):1451-1452. https://doi.org/10.1016/s0140-6736(85)91882-3 [ Links ]
8. Ghannoum M, Wiegand TJ, Liu KD, et al. Extracorporeal treatment for theophylline poisoning Systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol 2015;53(4):215-229. https://doi.org/10.3109/15563650.2015.1014907 [ Links ]
9. Murthy S, Wunsch H. Clinical review. International comparisons in critical care - lessons learned. Crit Care 2012;16(2):218. https://doi.org/10.1186/cc11140 [ Links ]
10. Statistics South Africa. Provincial profile. Eastern Cape Community Survey 2016. Report 03-01-08. Pretoria. Stats SA, 2018. http://cs2016.statssa.gov.za/wp-content/uploads/2018/07/EasternCape.pdf (accessed 20 February 2020). [ Links ]
11. Wikipedia Contributors List of municipalities in the Eastern Cape. Wikipedia, 2019. https://en.wildpedia.org/wild/List_of_municipalities_in_the_Eastern_Cape (accessed 24 March 2020). [ Links ]
12. Perry H. Theophylline poisoning. UpToDate 2021,last updated 16 August 2022. https://www.uptodate.com/contents/theophylline-poisoning?search=theophylline%20poisoning&source=search_result&selectedTitle=l~26&usage_type=defauit&display_rank=l (accessed 30 September 2022). [ Links ]
13. National Department of Health, South Africa. Uniform patient fee schedule 2019. https://www.health.gov.za/uniform-patient-fee-schedule/ (accessed 30 September 2022). [ Links ]
14. Jacobsz LA, Zemlin AE, Roos MJ, Erasmus RT. Chemistry and haematology sample rejection and clinical impact in a tertiary laboratory in Cape Town. Clin Chem Lab Med 2011;49(12):2047-2050. https://doi.org/10.1515/CCLM.2011.743 [ Links ]
15. Bernstein G, Jehle D, Bernaski E, Braen GR. Failure of gastric emptying and charcoal administration in fatal sustained-release theophylline overdose. Pharmacobezoar formation. Ann Emerg Med 1992;21(11):1388-1390. https://doi.org/10.1016/S0196-0644(05)81907-9 [ Links ]
16. Shannon M. Predictors of major toxicity after theophylline overdose. Ann Intern Med 1993;119(12):1161-1167. https://doi.org/10.7326/0003-4819-119-12-199312150-00002 [ Links ]
17. Hamilton RA, Gordon T. Incidence and cost of hospital admissions secondary to drug interactions involving theophylline. Ann Pharmacother 1992;26(12):1507-1511. https://doi.org/10.1177/106002809202601202 [ Links ]
18. Hall KW, Dobson KE, Daiton JG, Ghignone MC, Penner SB. Metabolic abnormalities associated with intentional theophylline overdose. Ann Intern Med 1984;101(4):457-462. https://doi.org/10.7326/0003-4819-101-4-457 [ Links ]
19. Parr M, Willatts S. Fatal theophylline poisoning with rhabdomyolysis. A potential role for dantrolene treatment. Anaesthesia 1991;46(7):557-559. https://doi.org/10.1111/j.1365-2044.1991.tb09655.x [ Links ]
20. Hamilton RA. Economic costs of theophylline toxicity. Pharmacoeconomics 1994;5(3):177-179. https://doi.org/10.2165/00019053-199405030-00001 [ Links ]
21. Favara DM. The burden of deliberate self-harm on the critical care unit of a peri-urban referral hospital in the Eastern Cape. A 5-year review of 419 patients. S Afr Med J 2013;103(1):40-43. https://doi.org/10.7196/SAMJ.6231 [ Links ]
22. Turner HC,Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health 2019;22(9):1026-1032. https://doi.org/10.1016/j.jval.2019.03.021 [ Links ]
23. Netcare Hospital Group. Private paying patients - Netcare tariffs January 2019. https://www.netcarehospitals.co.za/Portals/3/Images/Content-images/PDF/2019-Private-Paying-Patients.pdf (accessed 2 June 2021). [ Links ]
24. Government Gazette. Compensation fund guide fees for renal care 2019. http://www.coidlink.co.za/Downloads/Tariffs/2019/Renal%20care%2001%20April%202019.pdf (accessed 2 June 2021). [ Links ]
 Correspondence:
Correspondence:
J V Oxley-Oxland
vereoxland@gmail.com
Accepted 20 June 2022














