Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 n.11 Pretoria Nov. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i11.16541
RESEARCH
Recognition of infants at high risk for vertical HIV transmission at delivery in rural Western Cape Province, South Africa
T R RichardsonI; T M EsterhuizenII; A L EngelbrechtIII, IV; A L SlogroveV, VI
IMB BCh; Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIMSC; Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIMB BCh; Department of Paediatrics, Worcester Provincial Hospital, Worcester, South Africa
IVMB BCh; Ukwanda Centre for Rural Health, Department of Global Health, Stellenbosch University, Worcester, South Africa
VMB BCh; Ukwanda Centre for Rural Health, Department of Global Health, Stellenbosch University, Worcester, South Africa
VIMB BCh; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Worcester, South Africa
ABSTRACT
BACKGROUND: Despite South Africa's substantial reduction in vertical HIV transmission (VHT), national paediatric HIV elimination is not yet attained. National and Western Cape Province (WC) HIV guidelines recommend enhanced postnatal prophylaxis for infants at high risk for VHT, identified in the WC 2015/2016 guidelines by any single high-risk criterion (maternal antiretroviral therapy (ART) <12 weeks, absent/ unsuppressed maternal HIV viral load (HIV-VL) <12 weeks before/including delivery, spontaneous preterm labour, prolonged rupture of membranes, chorioamnionitis). Accuracy of high-risk infant identification is unknown
OBJECTIVES: Primarily, to determine the proportion of infants at high risk for VHT, the accuracy of labour-ward risk classification, the criteria determining high-risk statuses and the criteria missed among unrecognised high-risk infants; secondarily, to determine maternal factors associated with high-risk infants
METHODS: Infants born to women living with HIV at a rural regional hospital (May 2016 - April 2017) were retrospectively evaluated using data from the labour ward VHT register, standardised maternity case records, National Health Laboratory Service database and WC Provincial Health Data Centre. The study-derived risk status for each infant was determined using documented presence/absence of risk criteria and compared with labour ward assigned risk to determine accuracy. Proportions of high-risk and unrecognised high-risk infants with each high-risk criterion were determined. Maternal characteristics associated with having a high-risk infant were evaluated using multivariable logistic regression
RESULTS: For liveborn infants, labour ward assigned risk classifications were 40% (n=75/188) high risk, 50% (n=94/188) low risk and 10% (n=19/188) unclassified. Study-derived risk was high risk for 69% (n=129/188) and low risk for 31% (n=59/188), yielding a high-risk classification sensitivity of 51% (95% confidence interval (CI) 42 - 60) and specificity of 69% (95% CI 56 - 80). Absent/unsuppressed HIV-VL <12 weeks before delivery accounted for 83% (n=119/143) of study-derived high-risk exposures and 81% (n=60/74) of missed high-risk exposures. Fewer mothers of high-risk infants had >4 antenatal visits (38% v. 81%, p<0.01) and first antenatal visit <20 weeks' gestation (57% v. 77%, p=0.01). Only the number of antenatal visits remained associated with having a high-risk infant after adjusting for gestation at first visit and timing of HIV diagnosis and ART initiation: each additional antenatal visit conferred a 39% (95% CI 25 - 50) reduction in the odds of having a high-risk infant
CONCLUSION: Labour ward risk classification failed to recognise half of high-risk infants. Infant high-risk status as well as non-detection thereof were driven by suboptimal maternal HIV-VL monitoring. Reinforcing visit frequency later in pregnancy may improve antenatal HIV-VL monitoring, and point-of-care HIV-VL monitoring at delivery could improve recognition of virally unsuppressed mothers and their high-risk infants
Halting new HIV infections among children is a critical, unmet step toward the World Health Organization's global goal to end the AIDS epidemic by 2030.[1] Achieving elimination status requires a final vertical (perinatal and postnatal) HIV transmission (VHT) rate of <5% and an annual paediatric HIV incidence of <50 per 100 000 live births.[1-3] Despite attaining a VHT rate of 3.9% in 2021, South Africa (SA)'s paediatric HIV incidence exceeded 750 per 100 000 livebirths due to the high antenatal HIV prevalence of 30.7%.[1,3-6] Reducing transmission further requires strategic optimisation of VHT prevention interventions. One such intervention is risk-stratified prophylaxis for infants born to women living with HIV (WLHIV), including augmented and prolonged prophylaxis for infants at high risk of VHT. Although guidelines recommend such a risk-based approach, it is unknown whether this is being implemented successfully.
While early maternal viral suppression through antiretroviral therapy (ART) represents the foundation of preventing VHT, many WLHIV fail to receive sustained ART required to achieve HIV viral load (HIV-VL) suppression before and during pregnancy.[7-11] This is as a result of challenges in the uptake and implementation of the VHT prevention programme including ongoing barriers to ART access, the time lag between introduction and implementation of revised policies, suboptimal training and knowledge of these policies by providers as guidelines have rapidly evolved, incomplete programme uptake and progressive drop-out through the cascade.[7,9-14] Because labour and delivery represent a short peak of HIV exposure, this period of exposure is amenable to infant post-exposure prophylaxis (PEP).[7] If maternal HIV-VL is unsuppressed, infant prophylaxis plays an even more important role in reducing VHT.[7-11,15,16]
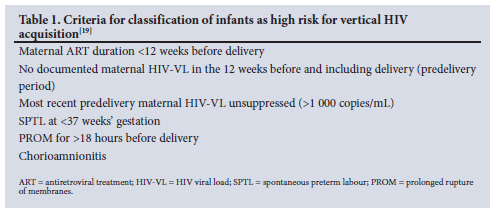
In 2015 the Western Cape HIV Guidelines introduced lifelong ART for all pregnant and breastfeeding WLHIV irrespective of CD4 count.[17,18] Non-pregnant WLHIV initiated ART once CD4 count fell <500 cells/mm3.[18,19] Prior to this, pregnant and breastfeeding WLHIV were eligible for ART until cessation of breastfeeding, and eligibility in non-pregnant people was based on a CD4 count threshold of 350 cells/mm3.[18-20] Additionally, these guidelines identified specific criteria that would place an infant at high risk for VHT.[19] As summarised in Table 1, infants were classified as high risk in the presence of >1 criterion, and low risk in the absence of all criteria. Low-risk infants received 6 weeks of nevirapine (NVP) prophylaxis and high-risk infants received dual prophylaxis with azidothymidine (AZT) and NVP for at least 6 weeks.[19] Breastfeeding infants continued extended NVP prophylaxis for at least 12 weeks, which could be extended further if required until maternal viral suppression was established.[11,16,18,19,21]
Objectives
To evaluate the process of infant VHT risk classification at delivery to determine if missed opportunities for VHT prevention existed in the context of a busy, rural regional hospital in the Western Cape Province during 2016/2017. The primary objectives were: (i) to estimate the proportion of infants at high risk for VHT; (ii) to assess the accuracy of VHT risk classification at delivery, overall and for high- and low-risk groups; and (iii) to describe the distribution of criteria responsible for high-risk infants overall and for unrecognised high-risk infants. The secondary objective was to assess maternal factors associated with having a high-risk infant.
Methods
This was a retrospective cohort study using routinely collected data for WLHIV who delivered their infants at a rural regional hospital in the Western Cape Province of SA between May 2016 and April 2017. The labour ward managed on average 140 secondary-level deliveries per month, approximately 12% to WLHIV. WLHIV were identified in the labour ward VHT register, and were included if they delivered an infant >500 g during the study period and for whom there was a maternity case record available. In the case of twins, the first born of twin deliveries was included. Four sources provided information characterising mothers and their infants: the labour ward VHT register, the mother's individual standardised maternity case records, the National Health Laboratory Service (NHLS) database and the Western Cape Provincial Health Data Centre (WCPHDC) database.[22] The labour ward VHT register provided the labour ward-assigned risk status and the PEP dispensed for each infant, documented by a labour ward nurse after risk assignment using information from the standardised maternal case records, obtained directly from the mother, and from the NHLS database if accessible and required for HIV-VL results. The WCPHDC serves as a central collection of routinely collected electronic health data from multiple sources using individual unique identifiers to electronically link individual-level data. Information was extracted regarding maternal HIV diagnosis and management, the presence or absence of high-risk criteria for calculating study-derived risk classification, and blood results for mother and infant. HIV polymerase chain reaction (HIV-PCR) results were extracted for liveborn infants up to at least 18 months of age. Day 0 - 2 infant HIV-PCRs were 'birth' PCRs (within 48 hours of birth as per guidelines) and week 8 - 12 HIV-PCRs were considered '10-week' HIV-PCRs. 'Predelivery HIV-VLs' were those performed in the 12 weeks before and including the date of delivery.
Analysis was conducted using Stata version 15.0 (StataCorp, USA). Descriptive statistics were calculated as proportions with 95% confidence intervals (CIs) for categorical variables, and measures of central tendency and variation for continuous variables. Maternal and infant characteristics were compared between study-derived high and low risk using x2 tests for categorical variables (Fisher's exact if assumptions not met), t-tests for normally distributed continuous variables and Mann-Whitney U tests for non-normally distributed continuous variables. The presence of each high-risk criterion (maternal ART duration <12 weeks before delivery; absent/unsuppressed predelivery HIV-VL, spontaneous preterm labour (SPTL), prolonged rupture of membranes (PROM) and chorioamnionitis) was used to assign each infant a study-derived VHT risk classification (high or low risk). The proportion of high-and low-risk infants was calculated. Thereafter, labour ward assigned and study-derived risks were compared to assess the accuracy and validity of labour ward risk assignment for liveborn infants (classification for provision of appropriate prophylaxis was not relevant for stillbirths). The proportion of liveborn study-derived high-risk infants with each high-risk criterion was calculated. For all unrecognised high-risk infants, the proportion resulting from non-detection of each high-risk criterion was calculated. Maternal factors associated with having a high-risk infant were evaluated through multivariable logistic regression. Maternal factors assumed a priori to be associated with high VHT risk were maternal age, parity, gestation at first antenatal visit, number of antenatal visits, timing of HIV diagnosis and ART initiation. Factors associated on univariable logistic regression analysis with p<0.2 were entered into a forward stepwise multivariable logistic regression, starting with the most statistically significant factor, and were retained in the regression model at p<0.05.
This study was approved by the Stellenbosch University Human Research Ethics Committee (ref. no. S17/01/021) and the Western Cape Government Health Impact Assessment Committee (ref. no. WC_2017RP13_50). Only routinely collected data was used, retrospectively, with no impact on the patient care. As such, a waiver for individual informed consent was granted. All precautions were taken to maintain patient confidentiality.
Results
Of 216 WLHIV identified in the VHT register, 14 were excluded due to unavailable maternity case records (Fig. 1). Table 2 provides maternal and infant characteristics compared by study-derived infant risk (high v. low). Mean (standard deviation (SD)) maternal age was 30 (6) years and 81% (n=163/202) were multiparous. Two-thirds (63%, 119/202) attended their first antenatal visit before 20 weeks' gestation and half (51%, n=103/202) attended >4 (recommended minimum) antenatal visits. Three-quarters (78%, n=157/202) had known HIV prior to the first antenatal visit, almost one-fifth (16%, 32/202) were diagnosed at the first antenatal visit, and later diagnoses occurred for 13 women (6 after testing negative at previous visits; 1 not previously tested; and 6 with first presentation at delivery). Median (interquartile range; IQR) pregnancy CD4 was 417 cells/mm3 (270 - 598). Sixty-two percent (n=126/202) were receiving ART prior to first antenatal visit, of whom an additional 22% (n=46/202) commenced ART. Of the remaining 30 women (n=30/202, 15%), 20 were initiated before delivery; 5 started at delivery, 2 after delivery and the 3 diagnosed at delivery had no documented ART initiation. A total of 82% (n=165/202) received ART for >12 weeks before delivery, however, only half (50%, n=102/202) had a documented predelivery HIV-VL and only 41% (n=83/202) had confirmed suppressed predelivery HIV-VL. HIV-VL monitoring was not documented in 22% (n=44/202). There was no significant difference in age, parity, timing of HIV diagnosis or timing of ART initiation between mothers of study-derived low- and high-risk infants. However, mothers of low-risk infants attended more antenatal visits (median (IQR) 5 (4 - 6) v. 3 (1 - 5) visits, p<0.01) and more often presented for antenatal care before 20 weeks' gestation (77% v. 57%, p=0.01) than mothers of high-risk infants.

Of 202 deliveries with available records, 14 were stillbirths and 188 were liveborn infants of whom 3 died within the first week. Infants were born at median (IQR) gestation 38 (34 - 39) weeks, weighing median (IQR) 2.8 (2.1 - 3.2) kg. The labour ward VHT register documented 40% of 188 liveborn (n=75/188) infants as high risk and 50% (n=94/188) as low risk, while 10% (n=19/188) were unclassified. By contrast, 69% (n=129/188) of livebirths were high risk and 31% (n=59/188) were low risk by study determination following review of all available clinical information. Study-derived high-risk infants were significantly more likely to be born preterm (55% v. 8%, p<0.01), with low birthweight (48% v. 17%, p<0.01), and have a non-viable birth outcome (12% v. 0%, p<0.01) than low-risk infants. They were also less likely to have a 10-week HIV-PCR done (69% v. 53%, p=0.01). HIV-PCR results were available for 181 liveborn infants, of whom 170 (94%) had birth HIV-PCRs, all of which were negative. There were 8 confirmed HIV transmissions, all following a negative birth HIV-PCR; 1 was confirmed at 11 weeks; 3 between 4 and 7 months of age and 4 between 12 and 24 months of age. All 8 vertical HIV acquisitions occurred in infants born to mothers starting ART before pregnancy or at first antenatal visit; however, 4 had no predelivery HIV-VL, 3 had unsuppressed predelivery HIV-VL and 1 had a suppressed predelivery HIV-VL. Seven transmissions were to study-derived high-risk infants and 1 to a low-risk infant. The resultant VHT rate was 4.3%.
Fig. 1 presents deliveries compared by labour ward and study-derived VHT risk. The labour ward correctly risk classified 57% (n=107/188) of liveborn infants. The labour ward sensitivity (ability to correctly identify high-risk infants) was 51% (n=66/129 infants, 95% CI 42 - 60) and specificity (ability to correctly identify low-risk infants) was 69% (n=41/59, 95% CI 56 - 80). Where PEP was documented, only one high- and two low-risk infants received inappropriate prophylaxis for labour ward assigned risk. However, only 52% (n=67/129) of actual high-risk infants and 81% (n=48/59) of actual low-risk infants received the correct prophylaxis for study-derived risk. Of the eight HIV transmissions, one was a correctly assigned low-risk infant and seven were high-risk infants (p=0.26), five of whom were correctly identified and two misclassified.
As detailed in Table 3, among 129 liveborn infants at high risk for VHT, 221 high-risk events were responsible: absent maternal predelivery HIV-VL occurred in 69% (n=89/129) of high-risk infants; unsuppressed maternal predelivery HIV-VL in 14% (n=18/129); maternal ART duration <12 weeks in 25% (n=32/129); SPTL in 43% (55/129); PROM in 17% (n=22/129); and chorioamnionitis in 3% (n=5/129). Lack of HIV-VL monitoring was not completely explained by short duration on ART as 69% percent of women without predelivery HIV-VLs had received at least 12 weeks of ART prior to delivery.
There were 63 unrecognised high-risk liveborn infants, exposed to n=92/221 (42%) high-risk events. Among these infants, absent maternal predelivery HIV-VL went unrecognised in 71% (n=45/63), unsuppressed maternal predelivery HIV-VL in 10% (n=6/63), maternal ART duration <12 weeks in 13%, (n=8/63), SPTL in 40% (n=25/63), PROM in 11% (n=7/63) and chorioamnionitis in 2% (n=1/63).
Infants may have more than one risk factor, therefore the sum of the percentages in the columns exceeds 100%; 'liveborn high-risk infants' are a sub-group of 'all high-risk deliveries' and 'unrecognised high-risk infants' are a sub-group of 'liveborn high-risk infants'.
Table 4 presents uni- and multivariable logistic regression analyses for maternal factors associated with having a high-risk infant. By univariable analysis, each additional week to the gestation at first antenatal visit was associated with 6% greater odds of having a high-risk infant (crude odds ratio (OR) 1.06 (95% CI 1.02 - 1.10), and each additional antenatal visit was associated with 41% lower odds (crude OR 0.59 (95% CI 0.50 - 0.71). After adjusting for maternal age, parity, timing of HIV diagnosis and ART initiation and gestation at first antenatal visit, it was only the number of antenatal visits that remained associated with having a high-risk infant (adjusted OR 0.61 (95% CI 0.50 - 0.75)). Each additional antenatal visit conferred a 39% (95% CI 25 - 50) reduction in the odds of having a high-risk infant.
Discussion
In this regional-level labour ward, 40% of 188 infants were classified as high risk for VHT at the time of delivery. However, 69% were actually high risk after applying criteria documented in clinical records. Low sensitivity for detecting high-risk infants meant that half of all liveborn high-risk infants went unrecognised and therefore were not considered for dual enhanced antiretroviral prophylaxis. Non-recognition of high-risk infants is a missed opportunity in the pursuit of VHT elimination. The infants' high-risk status was not related to lack of access to ART, as 83% of mothers received >12 weeks of ART before delivery. Instead, it was absence of HIV-VL monitoring that caused the majority of high-risk deliveries.
A study from a neighbouring HIV high-prevalence country, Zimbabwe, evaluated risk classification of infants at delivery based on information in routine registers alone.[23] While retrospective risk classification was impossible for 90% of the infants due to insufficient information, the authors determined that 10% of infants were high risk and received single prophylaxis instead of dual prophylaxis.[23] Due to the lack of HIV-VL monitoring information specifically, the authors questioned Zimbabwe's ability to base VHT risk classification on HIV-VL.[23] Similarly, in our study, non-adherence to antenatal HIV-VL monitoring guidelines resulted in 69% of high-risk deliveries. At delivery, challenges with retrieving HIV-VL results and providing monitoring for those without available results accounted for a total of 81% of high-risk infants going unrecognised. These difficulties in implementing HIV-VL monitoring in pregnant WLHIV hampered the provision of available enhanced dual antiretroviral prophylaxis for the infants, as well as identification of potentially more vulnerable high-risk mother-infant dyads in need of additional support.
Under the Western Cape guidelines at the time, HIV-VL monitoring was recommended 3-monthly during pregnancy, and at delivery if not documented in the previous 3 months. That 50% of mothers in our study lacked predelivery HIV-VL monitoring is not unique to the Western Cape or this rural setting, as suboptimal HIV-VL monitoring during pregnancy has been documented elsewhere in both urban and rural settings in SA.[24-28] A KwaZulu-Natal Province study found that among infants with vertical HIV acquisition, 70% of mothers did not have an HIV-VL during pregnancy.[27] The need for improved adherence to antenatal HIV-VL monitoring guidelines has previously been highlighted.[11,29] Lesosky et al.[29] found that among women initiating ART during pregnancy, HIV-VL monitoring according to gestation-specific clinic visit timepoints, rather than by duration between tests, integrated better with the existing antenatal programme and was more sensitive. In our study, each additional antenatal visit reduced the odds of having a high-risk infant by 39%, independent of gestational age at first antenatal visit, timing of HIV diagnosis and timing of ART initiation. Reinforcing antenatal visit frequency, particularly later in pregnancy, may improve outcomes by providing additional opportunities for adherence counselling, HIV-VL monitoring and documentation of results.
Current 2020 Western Cape HIV guidelines have simplified HIV-VL monitoring and now recommend that all women should receive an HIV-VL at delivery.[30] This substantially simplifies decision-making at delivery by making HIV-VL routine in all WLHIV. However, unless the HIV-VL result is available prior to the mother-infant dyad being discharged from the delivery unit, it is unlikely to prove very helpful for correct infant risk classification and provision of appropriate and timeous infant prophylaxis.[30] Formal HIV-VL testing has a variable turnaround time of ~4 days, and so the delivery HIV-VL result would need to be obtained at the routine postnatal clinic visit 3 - 6 days after delivery.[30,31] Point-of-care (POC) HIV-VL monitoring, under evaluation for use in SA but not yet implemented, would offer the advantage of prompt identification of unsuppressed HIV-VL, allowing for immediate clinical intervention both antenatally and at delivery.[32] Considering that unknown or unsuppressed predelivery HIV-VL was the driver of being at high risk for VHT in this study, POC HIV-VL monitoring implemented at delivery has the potential to substantially improve infant risk classification and ensure that appropriate early infant prophylaxis is prescribed before the mother-infant dyad leaves the delivery facility. HIV-VL monitoring is also critical during breastfeeding. In this study, all HIV transmissions occurred following negative birth HIV-PCRs, and all but one occurred in infants whose mothers had no predelivery HIV-VL (4/8 transmissions) or an unsuppressed HIV-VL (3/8). This suggests that prompt detection of unsuppressed HIV-VL at delivery or during breastfeeding could have assisted toward preventing vertical HIV acquisition.
Strengths of this study include the use of multiple data sources to ensure the completeness of data for infant risk classification, and the comprehensive laboratory result search strategy, which allowed near-complete information on maternal and infant HIV testing received. The study facility is in a non-metro area serving a semi-rural community, a setting that is seldom represented in perinatal HIV research. Although the generalisability of these findings may be limited, as this was a single-site study, findings from elsewhere indicate that similar challenges are experienced broadly across the southern African region. The largest limitation is that data were retrospectively recorded in the sources utilised, and not prospectively collected for this study's purposes. This resulted in some missing data. While many sources were used to counter this limitation, infant risk assessment and prophylaxis relied on documentation in the VHT register and could not be verified elsewhere.
Conclusion
Although 83% of pregnant WLHIV delivering at this rural regional labour ward were on ART for at least 12 weeks prior to delivery, the majority of infants were still at high risk for VHT and frequently went unrecognised owing to remediable gaps in maternal HIV-VL monitoring. Although this study was conducted under previous Western Cape guidelines, it does inform how current guidelines and their implementation in relation to VHT can be strengthened through reinforcing antenatal visit frequency, particularly later in pregnancy, by simplifying HIV-VL monitoring and by considering POC HIV-VL monitoring strategies.
Declaration. This study was submitted as a research project of TRR's MSc Clinical Epidemiology degree.
Acknowledgments. The authors thank Dr Charl Oettle for assistance with Obstetric and Gynaecology Department information and the Western Cape Provincial Health Data Centre for assisting with additional maternal HIV date.
Author contributions. Conceptualisation, TRR, ALE and ALS. Data curation, TRR. Formal analysis, TE and TRR. Investigation, TRR. Methodology, TRR, ALE and ALS. Project administration, TRR. Resources, TRR. Supervision, TE, ALE and ALS. Validation, TRR. Writing - original draft, TRR. Writing - review and editing, TRR, TE, ALE and ALS.
Funding. None.
Conflict of interest. None.
References
1. Joint United Nations Programme on HIV/AIDS. Start free, stay free, AIDS free: Final report on 2020 targets. Geneva: UNAIDS Joint United Nations Programme on HIV/AIDS, 2021. https://www.unaids.org/sites/default/files/media_asset/2021_start-free-stay-free-aids-free-final-report-on-2020-targets_en.pdf (accessed 10 March 2022). [ Links ]
2. Joint United Nations Programme on HIV/AIDS. Fast-track: Ending the AIDS epidemic by 2030. Geneva: UNAIDS Joint United Nations Programme on HIV/AIDS, 2014. https://www.unaids.org/sites/defaut/files/media_asset/JC2686_WAD2014report_en.pdf (accessed 10 March 2022). [ Links ]
3. World Health Organization. Global guidance on criteria and processes for validation: Elimination of mother-to-child transmission of HIV and syphilis. 2nd ed. Geneva: WHO, 2017. https://apps.who.int/iris/bitstream/handle/10665/259517/9789241513272-eng.pdf?sequence=1&isAllowed=y (accessed 10 March 2022). [ Links ]
4. World Health Organization. Global guidance on criteria and processes for validation: Elimination of mother-to-child transmission of HIV and syphilis. Geneva: WHO, 2014. https://apps.who.int/iris/bitstream/hande/10665/112858/9789241505888_engpdf?sequence=1&isAllowed=y (accessed 10 March 2022). [ Links ]
5. Joint United Nations Programme on HIV and AIDS. Country factsheets: South Africa 2020. Geneva: UNAIDS, 2020. https://www.unaids.org/en/regionscountries/countries/southafrica (accessed 10 March 2022). [ Links ]
6. Woldesenbet S, Lombard C, Kufa T, Manda S. The 2017 National Antenatal Sentinel HIV Survey Key Findings, South Africa. S Afr Med J 2016(7):1-100. https://doi.org/10.13140/RG.2.2.25252.01928 [ Links ]
7. Kroon M. Recognising and managing increased HIV transmission risk in newborns. S Afr J HIV Med 2015;16(1):1-7. https://doi.org/10.4102/sajhivmed.v16i1.371 [ Links ]
8. Hurst SA, Appelgren KE, Kourtis AP. Prevention of mother-to-child transmission of human immunodeficiency virus type 1 (HIV): The role of neonatal and infant prophylaxis. Expert Rev Anti Infect Ther 2015;13(2):169-81. https://doi.10.1586/14787210.2015.999667 [ Links ]
9. Azia IN, Mukumbang FC, van Wyk B. Barriers to adherence to antiretroviral treatment in a regional hospital in Vredenburg, Western Cape, South Africa. S Afr J HIV Med 2016;17(1)1-8. https://doi.org/10.4102/sajhivmed.v17i1.476 [ Links ]
10. Mnyani CN, McIntyre JA. Challenges to delivering quality care in a prevention of mother-to-child transmission of HIV programme in Soweto. S Afr J HIV Med 2013;14(2):64-69. https://doi.10.7196/sajhivmed.902 [ Links ]
11. Myer L, Essajee S, Broyles LN, et al. Pregnant and breastfeeding women: A priority population for HIV viral load monitoring. PLoS Med 2017;14:(8)1-7. https://doi.10.1371/journal.pmed.1002375 [ Links ]
12. Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359(21):2233-2244. https://doi.org/10.1056/nejmoa0800971 [ Links ]
13. Feucht UD, Meyer A, Thomas WN, et al. Early diagnosis is critical to ensure good outcomes in HIV-infected children: Outlining barriers to care. AIDS Care 2016;28(1):32-42. https://doi.http://doi.org/10.1108/17506200710779521 [ Links ]
14. National Committee for Confidential Enquiry into Maternal Deaths. Saving Mothers 2011 - 2013: Sixth report on the Confidential Enquiries into Maternal Deaths in South Africa, Short Report. South Africa: NCCEMD, 2013. http://www.health.gov.za/index.php/shortcodes/2015-03-29-10-42-47/2015-04-30-08-18-10/2015-04-30-08-24-27?download=885:saving-mothers-2011-2013-facts-sheet-front-and-back-page (accessed 10 March 2022). [ Links ]
15. Goga AE, Dinh TH, Essajee S, et al What will it take for the global plan priority countries in sub-Saharan Africa to eliminate mother-to-child transmission of HIV? BMC Infect Dis 2019;19(1):783-795. https://doi.10.1186/s12879-019-4393-5 [ Links ]
16. World Health Organization. HIV diagnosis and ARV use in HIV-exposed infants: A programmatic update. Geneva: WHO, 2018. https://apps.who.int/iris/bitstream/handle/10665/273155/WHO-CDS-HIV-18.17-eng.pdf (accessed 10 March 2022). [ Links ]
17. United Nations Children's Fund, National Department of Health, South Africa. A multi-pronged approach to the elimination of MTCT in South Africa. South Africa: UNICEF, 2015. https://www.knowledgehub.org.za/system/files/elibdownloads/2019-07/eMTCT-best-practices-document.pdf (accessed 10 March 2022). [ Links ]
18. National Department of Health, South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV. Pretoria: NDoH, 2015. https://sahivsoc.org/Files/ARTGuidelines15052015.pdf (accessed 10 March 2022). [ Links ]
19. Western Cape Department of Health. The Western Cape consolidated guidelines for HIV treatment: Prevention of mother-to-child transmission of HIV (PMTCT), children, adolescents and adults, 2015. Geneva: WHO, 2015. http://www.paediatrics.uct.ac.za/sites/default/files/image_tool/images/38/Western%20Cape%20Consolidated%20HIV%20guidelines%20November%202015.pdf (accessed 10 March 2022). [ Links ]
20. National Department of Health, South Africa. The South African antiretroviral treatment guidelines. Pretoria: NDoH, 2013. http://www.sahivsoc.org/Files/2013_ART_Guidelines-Short_Combined_FINAL_draft_guidelines_14_March_2013.pdf (accessed 10 March 2022). [ Links ]
21. Provincial Government of the Western Cape Department of Health. The Western Cape consolidated guidelines for HIV Treatment: Prevention of mother-to-child transmission of HIV (PMTCT), children, adolescents and adults. Western Cape: Western Cape Government, 2018. https://www.westerncape.gov.za/assets/departments/health/wc_hiv_consolidated_guidelines_march_2018_0.pdf (accessed 10 March 2022). [ Links ]
22. Boulle A, Heekes A, Tiffin N, et al. Data Centre Profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Popul Data Sci 2019;4(2):1-11. https://doi.org/10.23889/ijpds.v4i2.1143 [ Links ]
23. Komtenza B, Satyanarayana S, Takarinda KC, et al. Identifying high or low risk of mother to child transmission of HIV: How is Harare City, Zimbabwe doing? PLoS One 2019;14(3):1-10. http://doi.org/10.1371/journal.pone.0212848 [ Links ]
24. Goga AE, Dinh TH, Jackson DJ, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Comm Heal 2015;69(3):240-248. http://doi.org/10.1136/jech-2014-204535 [ Links ]
25. Ibeto M, Giddy J, Cox V. Closing the gaps: Steps towards elimination of mother-to-child transmission of HIV. SA J HIV Med 2014;15(3):107-109. https://doi.org/10.7196/SAJHIVMED.1047 [ Links ]
26. Goga A, Chirinda W, Ngandu NK, et al. Closing the gaps to eliminate mother-to-child transmission of HIV (MTCT) in South Africa: Understanding MTCT case rates, factors that hinder the monitoring and attainment of targets, and potential game changers. S Afr Med J 2018;108(3a):17. https://doi.org/10.7196/samj.2017.v108i3b.12817 [ Links ]
27. Moyo F, Haeri Mazanderani A, Bhardwaj S, et al. Near-real-time tracking of gaps in prevention of mother-to-child transmission of HIV in three districts of Kwazulu-Natal Province, South Africa. S Afr Med J 2018;108(4):319-324. https://doi.org/10.7196/SAMJ.2018.v108i4.12630 [ Links ]
28. Woldesenbet SA, Kufa T, Barron P, et al. Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa. AIDS 2020;34(4):589-597. https://doi.org/10.1097/QAD.0000000000002457 [ Links ]
29. Lesosky M, Glass T, Mukonda E, Hsiao NY, Abrams EJ, Myer L. Optimal timing of viral load monitoring during pregnancy to predict viraemia at delivery in HIV-infected women initiating ART in South Africa: A simulation study. J Int AIDS Soc 2017;20(57):26-31. https://doi.org/10.1002/jia2.25000 [ Links ]
30. Provincial Government of the Western Cape Department of Health. The Western Cape consolidated guidelines for HIV treatment: Prevention of mother-to-child transmission of HIV (PMTCT), children, adolescents and adults. Western Cape: Western Cape Government, 2020. http://www.paediatrics.uct.ac.za/sites/default/files/image_tool/images/38/Western_Cape_Consolidated_HIV_guidelines_November_2015.pdf (accessed 10 March 2022). [ Links ])
31. Lecher S, Williams J, Fonjungo PN, et al. Progress with scale-up of HIV viral load monitoring - seven sub-Saharan African countries, January 2015 - June 2016. Morb Mortal Wkly Rep 2016;65(47):1332-1335. https://doi.org/10.15585/mmwr.mm6547a2 [ Links ]
32. Ganesh P, Heller T, Chione B, et al. Near point-of-care HIV viral load: Targeted testing at large facilities. J Acquir Immune Defic Syndr 2021;86(2):258-263. https://doi.org/10.1097/qai.0000000000002555 [ Links ]
 Correspondence:
Correspondence:
T R Richardson
tracy@benilli.co.za
Accepted 21 July 2022














