Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.11 Pretoria Nov. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i11.16546
RESEARCH
Diabetes in the public healthcare sector of four South African provinces: A comparative analysis
N SahadewI; S PillayII; V S SingaramIII
IBTech (Podiatry), MMedSc; Clinical and Professional Practice, School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, PhD; Department of Internal Medicine, King Edward VIII Hospital, Durban, South Africa
IIIBMedSc, PhD; Clinical and Professional Practice, School of Clinical Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: The growing burden of diabetes has long been under the radar in developing countries such as South Africa (SA). In recent years, there has been an unprecedented and exponential increase in recorded and undiagnosed diabetes mellitus (DM) cases. Unreliable data collection, overburdened health systems and poor infrastructure have all proved to be barriers to achieving optimum disease management. The District Health Information System (DHIS) serves as the data collection tool for the SA public healthcare sector. It is used in all nine SA provinces to gather data without individual patient identifiers
OBJECTIVE: To analyse and compare the DM data collected by the DHIS in the Western Cape (WC), Eastern Cape (EC), KwaZulu-Natal (KZN) and Gauteng provinces of SA
METHODS: An audit of diabetes-related data from the DHIS for 2016 was conducted. The data were then analysed using Excel. Time-series and cross-sectional analyses were made possible using pivot tables. Graphics were designed using Thinkcell software
RESULTS: Of the four provinces surveyed, Gauteng recorded the highest incidence of DM, 67% higher than the reported global DM incidence estimate, while the WC had the lowest incidence. A similar pattern was also noted regarding the incidence of DM in people aged <18 years, with Gauteng having the highest and WC the lowest prevalence results. When comparing the number of DM-related consultations conducted in each province, the metropolitan districts were highlighted as hotspots of activity for DM care. This study found a moderate inversely proportional relationship between the incidence of DM in all provinces and education deprivation (p<0.05). Among the provinces that collected data on screening (excluding EC), KZN recorded the highest number of diabetic screenings
CONCLUSION: Metropolitan areas were highlighted as areas to be targeted, further reinforcing the current connection observed between urbanisation and DM in SA. The presence and recording of screening efforts is an excellent step in the right direction for the SA public healthcare sector and the DHIS. With improved interprovincial co-ordination regarding standardisation of the criteria and specifications of data collection fields, and enhanced training for data officers and primary collection agents, good quality and rich data is a very close possibility
Diabetes mellitus (DM) remains a common and costly disease in many countries.[1] According to the International Diabetes Federation (IDF), the prevalence of DM in Africa is predicted to increase to unprecedented levels by an estimated 156%.[1] This population will form part of the projected 700 million people living with diabetes (PLWD) worldwide by 2045.[1] In South Africa (SA), the increasing threat of DM grows alongside the high prevalence of communicable diseases (CDs) such as tuberculosis (TB), HIV and AIDS and, more recently, the COVID-19 pandemic.[2] For example, an HIV-positive patient is twice as likely to develop DM and is at triple the risk of developing TB. This vicious interaction between CDs and non-communicable diseases (NCDs) has manifested throughout the African continent, creating immense pressure for government welfare efforts, economic reforms and an already strained public health sector.[3] More than 80% of the SA population is currently dependent on public healthcare. The recent COVID-19 pandemic has reinforced the need to strengthen this sector to facilitate speedier responses and more effective healthcare outcomes for the large populations served by the public sector.[2]
The multifaceted management of DM can often pose a logistical challenge for under-resourced systems. One tool that may help overcome this challenge is a data management system that collects and holds updated and good-quality data.[4] This invaluable resource will result in efficient evidence-based treatment and management protocols and improved education and funding initiatives. This study aims to analyse and compare the incidence of DM across the public healthcare sector of SA using a well-established data collection system called the District Health Information System (DHIS). The DHIS operates as an extensive electronic database collecting information from the SA public healthcare sector.[3,4] By assessing an individual disease via this database, we hope to evaluate the strengths and limitations of this valuable national database. Hence, together with investigating the burden of DM in SA, this study aims to inform improvements to optimise data collection by the DHIS.
Methods
Context
The Republic of SA is situated on the southern tip of the African continent. The country is home to approximately 59 million citizens with 11 official languages. The 1 220 813 km2 area of SA is divided into nine provinces.[5] According to Statistics SA, >80% of the SA population falls outside private medical insurance schemes.[6] The National Department of Health (NDoH) is the custodian of the public healthcare sector and is meant to serve the majority of the population, equating to approximately 45 million South Africans.[6] Research shows that an essential tool in managing health services is the availability and use of reliable and up-to-date health information.[7] In SA, the DHIS is the primary health information system collecting data within the public healthcare sector at provincial and national levels.[7]
Study design
This quantitative study serves as a retrospective audit of the NDoH database, the DHIS. This database stores health information without patient identifiers and collects data on various diseases and medical markers. All diabetes-related data were requested for 2016 from each of the nine provinces in SA.
Study sample and data collection
Of the nine provincial health departments approached, four responded with favourable permission and provided the relevant data electronically. These provinces were the WC, EC, KZN and Gauteng. Data collection fields related to DM were requested (Table 1).
The public healthcare sector was identified by reducing each province's total population by its medical scheme coverage, resulting in a more focused analysis. Our study calculated the incidence of DM in patients within two categories, those patients <18 years old and those >18 years. Type 1 diabetes typically presents in patients during childhood or early adolescence, while type 2 is often diagnosed in adults, although its prevalence among younger patients is growing.[8] Considering this unofficial clinical distinction, we have assumed that the data collected on patients < 18 reflects the population of those with type 1 diabetes, and data collected on patients > 18 years represents those diagnosed with type 2 diabetes. Data on education deprivation were obtained directly from an author of the SA Index of Multiple Deprivation (SAIMD) study, Michael Noble, via personal correspondence. This study, released in 2007 by the SA Department of Social Development used different socioeconomic scores to determine various types of deprivation among the municipalities and provinces of SA.[9]
Data analysis
A quantitative data analysis was conducted using a combination of nominal, ordinal and ratio data measurements using Excel (Microsoft Corp., USA). Using pivot tables, the tabulation of data provided a comprehensive overview of data collected and any presented patterns.
Ethical considerations
Ethical approval was obtained from the University of KZN (ref. no. HSS/ 1835/017D) and the Department of Health of each province. Data detailing population statistics were requested from Statistics SA.
Results
The WC, EC, KZN and Gauteng provinces operate individual NDoH databases, with variations in collection fields. Fig. 1 displays and compares the incidence of DM in each province using data from 2016. Gauteng, the most populated of the provinces reviewed,[10] showed the highest incidence of DM (47 people in every 10 000), followed by KZN and EC, which had 27 and 20 people per 10 000, respectively. WC had the lowest incidence of DM with 14 people per 10 000.
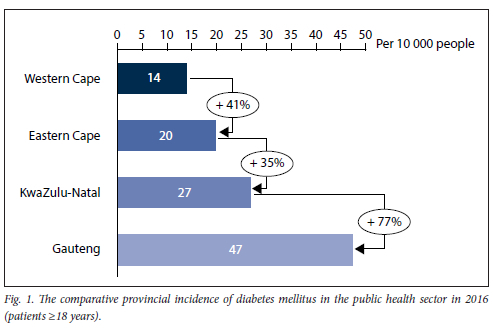
As seen in Fig. 2, when the arrays of incidence data and corresponding education deprivation data were correlated, a statistically significant moderate inversely proportional relationship was observed (p=-0.46, Pearson's correlation coefficient).
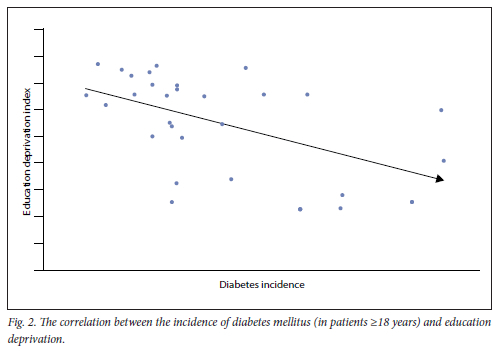
Fig. 3 displays the incidence of DM in people < 18 years of age for 2016. KZN was excluded as the data requested from the respective DHIS did not contain this collection field, as this specific collection field was discontinued from 2014. As highlighted in Fig. 3, Gauteng had the highest incidence of DM in patients <18 years, with WC having the lowest, of only 2 per 10 0000 population.
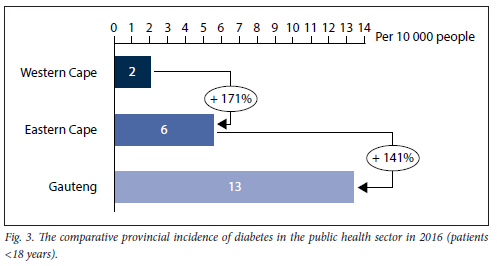
Fig. 4 shows a detailed comparison of the DM-related consultations. The collection field used for this analysis was 'diabetes patients on register'. This field collects the number of DM-related consultations conducted. Patient identifiers were not collected; one patient could be counted twice if they had more than one consultation in the year relating to their DM. Considering the nature of the disease, we assume this possibility is very likely. EC did not collect this data field. The metropolitan areas (defined as districts that execute local government functions for a city[11] of each province) were examined and highlighted in colours, while the other districts of the province are depicted in grey. The metropolitan regions in Gauteng and WC were shown to have the largest proportion of patients diagnosed with DM. In addition, over one-third of patients in KZN with DM were found in the metropolitan areas of KZN.
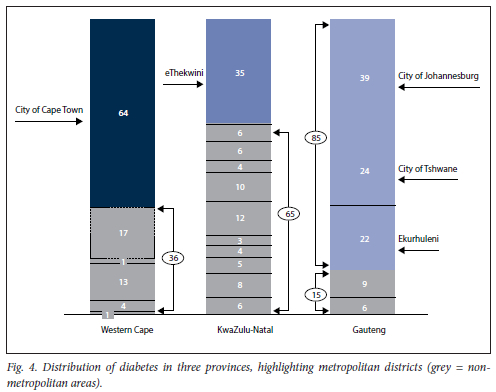
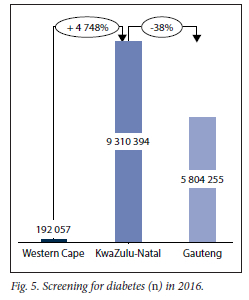
Fig. 5 depicts the number of diabetic screenings done in the provinces that collected like data, which demonstrates that the KZN is screening significantly more patients than other provinces. In contrast, EC had not at the time (2016) initiated the collection of data regarding DM screening.
Discussion
Incidence of DM
An incidence rate can be described as the change in the number of new cases of a specific disease over a specified period of time. Governments and researchers use the measure of incidence to track chronic diseases and their rate of change in a population over a period of time.[11] This information is often used to direct policy, inform treatment, and structure healthcare budgets to curb the spread of the disease and its effects.[11] In a recent study, the worldwide age-standardised incidence of DM was calculated to be 28.5 per 10 000 (285/100 000 persons).[12] In the past, SAs poor data quality and a sizeable undiagnosed population acted as barriers to determining the incidence of DM in the country[3,13]
Our comparative incidence of type 2 diabetes results ranged from 14 per 10 000, found in WC, to 47 in 10 000 people, located in Gauteng. The global incidence of type 2 diabetes calculated in 2017 was 27.9 per 10 000 people (279/100 000).[12] Our results reveal that the highly urbanised Gauteng province has an incidence rate of 69% higher than the estimated global incidence. The incidence rates of the other three provinces all fell below the worldwide estimate. The high prevalence of undiagnosed DM in low- to middle-income countries could explain this finding.[4] Another plausible explanation is that Gauteng is highly urbanised and is home to the most prominent city in SA. While there are known genetic risk factors for type 2 diabetes, extensive literature links the disease to other risk factors associated with an urban lifestyle that are considered modifiable. These include a lack of physical activity, high stress levels and obesity[13]
Type 2 DM accounts for >90% of global cases, which explains the vast difference between the number of patients registered within our study's two age classification groups.[8] The most likely reason for this distinction is that type 1 diabetes is primarily diagnosed in children and teens, as onset has been documented to peak at age 15 years in European populations.[14] However, data highlighting the African population have shown the peak onset of type 1 DM between the ages of 20 and 29 years.[15] For this reason, the recording of the specific type of DM rather than the age category would more accurately serve to predict the burden of type 1 diabetes in SA. This information could inform treatment requirements such as the supply of insulin to more rural or high-volume health facilities. One study estimated the incidence of type 1 DM in Africa to be 0.8 per 10 000 (8 per 100 000).[16] Each of the three provinces assessed in our study yielded an incidence rate higher than the known incidence estimate of 0.8. Gauteng was revealed as a high-risk area, with an incidence rate of 13 per 10 000. A margin of error should be considered when interpreting this result, as the data used for the calculation did not specify the type of diabetes. However, the finding is useful when considering possible modifications to the current data collection fields used by the DHIS. For example, a collection field distinguishing between the type of diabetes at the time of diagnosis would allow for more accurate incidence calculations.
This information could be used to prioritise and concentrate DM-related health interventions in high-risk provinces. Proactive interventions that aim to prevent or manage the unprecedented increase in the prevalence of DM have never been more critical when considering the competing and interacting burdens of infectious (TB, HIV/ AIDS and COVID-19) and non-infectious diseases in SA.[1,2,17]
DM and education poverty
Poverty and low socioeconomic status have a reported link with the incidence of DM.[18] Other studies have shown that this link is influenced by occupation, education, income and neighbourhood type.[18] People in resource-poor countries must often choose between DM care and basic needs such as food and safe and secure shelter.[18] In this study, a SA measure of poverty, the SAIMD, was used, and its relationship with the incidence of DM was investigated. The SAIMD ranks SA districts and categorises them into quintiles that communicate their overall level of deprivation or poverty. The data used to establish these ranks involve correlating individual socioeconomic domains, namely income, employment, education and living environment.[9] With additional data requested and obtained from Michael Noble,[9] we used the SAIMD study to correlate the socioeconomic domains with the corresponding districts' incidence of DM in those patients >18 years. According to the calculation, the only domain to yield a significant result was that of education. The level of education deprivation was seen to be inversely proportional to that of the incidence of DM, i.e. as the level of education decreases (increasing deprivation), the incidence of DM is seen to decrease.
This finding is in keeping with a recent study by Seiglie et al.[19] that examined data from 29 low- to middle-income countries, including SA. They found that higher education levels were associated with a higher risk of developing DM, explained by nutritional and obesity transitional frameworks. Their framework considered economic, demographic and epidemiological changes in low-income countries' diet and activity patterns that progress from a more active tribal lifestyle to industrialised societies with low levels of physical activity and increased activity consumption of energy-dense foods. According to the data, lower-income countries tend to see a higher DM prevalence in their more affluent and educated populations. However, the reverse is observed in higher-income countries, which find the prevalence of DM highest in their lowest socioeconomic populations, a trend driven mainly by obesity. Hence our results support the observation noted in the literature that education level is strongly related to DM risk.[19]
As a proactive approach to the rising levels of DM in our more industrialised communities, diabetic care should include education on obesity and its dangers, emphasising healthy eating practices. In addition, educational content should consider the urban food environment, food security and accessibility to allow easy lifestyle integration and understanding.
DM and urbanisation
As seen in Fig. 4, our data showed metropolitan areas as home to the most significant proportion of patients with DM. These areas are densely populated urban areas and often include large cities. One study cited a 200% - 500% increase in the risk of DM associated with living in a metropolitan area.[13] There are specific characteristics often associated with urbanisation that contribute to an increased risk of DM. These include sedentary lifestyles, changes in food security, obesity and an ageing population.[13]
Urbanisation on the continent of Africa is increasing at a rapid rate, with large populations experiencing the effect of the change to a more western, high-fat diet and processed diet, as well as a reduction in physical activity from using motorised transport.[13] Our study suggests a positive correlation between urbanising SA and the growing burden of DM in the country. While other countries in Africa are showing a closing gap between the urban and rural populations in their risk for DM,[13] SA, as informed by research, should concentrate on DM interventions in large metropolitan areas.
Diabetic screening
The issue of undiagnosed DM remains a solid barrier to appropriate care and complication avoidance. Worldwide, up to 50% of PLWD remain undiagnosed and untreated.[17] This challenge is most pronounced in Africa, where studies have cited a 62% undiagnosed rate.[3] Our study was able to show that screening for DM was done in three of the four provinces surveyed. KZN showed the most significant proportion of patients screened for DM in 2016. While the presence and subsequent recording of screening are encouraging, some concerns can be raised regarding data quality. A standardised method should be applied across all provinces to gain more accurate information regarding screening. Considering the variations in the collection fields, it is unlikely that this is currently the case. The IDF recommends that only patients with risk factors for the disease be screened. These include people aged >40, with an existing family history and increased waist circumference, and diagnosed with hypertension. Using these criteria in a simple pre-formatted and standardised screening tool to determine the appropriate patients for screening and intervention could save resources, increase awareness of the diseases and their comorbidities, and encourage compliance among healthcare workers working in an already over-burdened system.
Limitations
• As most data collection at this level is done by a nurse who is often additionally occupied with clinical duties, the limitation of human error must be considered.
• As patient identifiers are not currently collected on the DHIS system, there does exist the possibility that a patient could have been counted twice in error for specific collection fields.
• Calculating the correlation between education deprivation and the incidence of DM indicated a moderate relationship. However, the data set was not normally distributed when closely examined, showing little linear correlation.
Conclusion
The increase in the prevalence of DM is seen most prominently in African countries, which also remain home to the highest proportion of undiagnosed patients.[4,17] In addition, the disease is well known for its damaging complications that result in financial burdens for both the patient and the state.[12] In SA, competing burdens of HIV/AIDS and TB, together with a strained public health sector, are burdens to attaining optimal management of DM. This study has highlighted the highly urbanised province of Gauteng as one that should take more attentive care of DM. We recommend emphasising screening, education initiatives and the development of multidisciplinary treatment teams, which have shown great success in managing the inevitable complications of the sizeable undiagnosed population.[3] However, the availability of reliable, recent and sufficient amounts of data could assist in the growing DM epidemic despite the challenges faced by the country.[3] The DHIS has proven itself an essential data collection tool and has the potential to provide the data requirements needed to direct healthcare policy, initiatives and funding. After examining data collection fields on the isolated subject of DM across different provinces, this study found that the following could be used to amplify the effectiveness of the current DHIS:
• Co-ordination between the provinces regarding the collection criteria and establishing a set of standard collection fields would assist in interprovincial comparisons. Should provinces feel it necessary to collect additional or different data, an additional collection field can be constituted once redundancy has been discounted.
• Training at each level of data management such as collection, cleaning and compiling would reduce omitted and inconsistent data entries.
• Patient identifiers should be included as a collection field so that calculations such as prevalence can be determined. The DHIS collects data on patients who have already been diagnosed with the disease; however, as no unique patient identifiers are used, the same patient can be counted more than once. This technicality posed a challenge when calculating the prevalence of DM. Hence the focus of this study is on incidence calculations.
• Findings at facility, district and provincial levels should be shared and discussed with stakeholders at the primary and management level. This will encourage a shared understanding regarding the implications of the data being collected and how healthcare can be optimised at ground level, e.g. if screening numbers are low, nurses can encourage more patients within the high-risk categories to be screened.
Declaration. None.
Acknowledgements. We would like to thank the Gauteng, KZN, EC and WC health departments for their co-operation and assistance.
Author contributions. NS: researched the data, conducted the data analysis, generated the graphs and prepared the first draft of the manuscript; SP and VSS: reviewed, edited and contributed to the introduction and discussion of the article.
Funding. None.
Conflicts of interest. None.
References
1. Sahadew N, Singaram VS. Progress in diabetes care in the KwaZulu-Natal public health sector' A decade of analysis. J Endocrinol Metab Diabetes S Afr 2019;24(3):83-91. https://doi.org/10.1080/16089677.2019.1629080 [ Links ]
2. Kraef C, Juma P, Kallestrup P, Mucumbitsi J, Ramaiya K, Yonga G. The COVID-19 pandemic and non-communicable diseases-a wake-up call for primary health care system strengthening in sub-Saharan Africa. J Prim Care Community Health 2020;11:2150132720946948. https://doi.org/10.1177/2150132720946948 [ Links ]
3. Sahadew N, Singaram VS, Brown S. Distribution, incidence, prevalence and default of patients with diabetes mellitus accessing public healthcare in the 11 districts of KwaZulu-Natal, South Africa. S Afr Med J 2016;106(4):389-393. https://doi.org/10.7196%2FSAMJ.2016.vl06i4.10143 [ Links ]
4. Sahadew N, Singaram VS. A diabetes profile of the eight districts in the public health sector Eastern Cape Province, South Africa. S Afr Med J 2019;109(12):957-962. https://doi.org/10.7196/SAMJ.2019.v109il2.13972 [ Links ]
5. South African Government. Provinces breakdown. Pretoria. SA Government, 2021. https://www.gov.za/about-sa/south-africas-provinces (accessed 20 November 2021). [ Links ]
6. Statistics South Africa. Public healthcare. How much per person? Pretoria. StatsSA, 2017. http://www.statssa.gov.za/?p=10548 (accessed 20 December 2021) [ Links ]
7. Garrib A, Stoops N, McKenzie A, et al. An evaluation of the District Health Information System in rural South Africa. S Afr Med J 2008,98(7):549-552. [ Links ]
8. Amod A. The 2012 SEMDSA guideline for the management of type 2 diabetes. J Endocrinol Metab Diabetes S Afr 2012;17(1):61-62. https://doi.org/10.1080/22201009.2012.10872277 [ Links ]
9. Noble MHB, Wright G, Roberts B. Small area indices of multiple deprivation in South Africa. Soc Indicators Res 2010;95(2):281-297. https://doi.org/10.1007/s11205-009-9460-7 [ Links ]
10. Kamer L. Total population of South Africa 2019, by province. Hamburg. Statista, 2021 [ Links ]
11. Kenton W. Incidence rate. Definition, calculation, and examples. New York. Investopedia, 2021. https://www.investopedia.com/terms/i/incidence-rate.asp (accessed 20 December 2021). [ Links ]
12. Liu J, Ren Z-H, Qiang H, et al. Trends in the incidence of diabetes mellitus. Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020;20(1):1415. https://doi.org/10.1186/sl2889-020-09502-x [ Links ]
13. Peer N, Kengne A-P, Motala AA, Mbanya JC. Diabetes in the Africa region. An update. IDF Diabetes Atlas 2014;103(2):197-205. https://doi.org/10.1016/j.diabres.2013.11.006 [ Links ]
14. Weets I, De Leeuw IH, Du Caju MVL, et al. The incidence of type 1 diabetes in the age group 0-39 years has not increased in Antwerp (Belgium) between 1989 and 2000. Evidence for earlier disease manifestation. Diab Care 2002;25(5):840-846. https://doi.org/10.2337/diacare.25.5.840 [ Links ]
15. Padoa CJ. The epidemiology and pathogenesis of type 1 diabetes mellitus in Africa. Review article I Endocrinol Metabol Diabetes S Afr 2011;16(3):130-136. https://doi.org/10.1080/22201009.2011.10872264 [ Links ]
16. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world. A systematic review and meta-analysis. Health Promot Perspect 2020;10(2):98-115. https://doi.org/10.34172%2Fhpp.2020.18 [ Links ]
17. International Diabetes Federation. IDF Diabetes Atlas. Brussels. IDF, 2017 [ Links ]
18. Shaikh A, Kumar KVSH. Diabetes and poverty. A primer for resource-poor countries. J Soc Health Diabetes 2018;06(01):11-14. https://doi.org/10.4103/JSHD.JSHD_23_17 [ Links ]
19. Seiglie JA, Marcus M-E, Ebert C, et al. Diabetes prevalence and its relationship with education, wealth, and BMI in 29 low- and middle-income countries. Diab Care 2020;43(4):767-775. https://doi.org/10.2337/dcl9-1782 [ Links ]
 Correspondence:
Correspondence:
N Sahadew
nsahadew@gmail.com
Accepted 18 July 2022














