Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.11 Pretoria Nov. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i11.16578
RESEARCH
Incidence and follow-up of persistent lung perfusion abnormalities as a result of suspected air trapping or microthrombosis in non-hospitalised COVID-19 patients during the early half of the pandemic -experience in a tertiary institution in South Africa
O EvbuomwanI; W EndresII; T TebieiaII; G EngelbrechtI
IMBBS, MMed (Nuclear Med); Department of Nuclear Medicine, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIMBBS; Department of Nuclear Medicine, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
BACKGROUND: Available clinical data have revealed that COVID-19 is associated with a risk of pulmonary microthrombosis and small airway disease, especially in patients with severe disease. These patients present with persistent pulmonary symptoms after recovery, with ventilation and perfusion abnormalities present on several imaging modalities. Few data are available on the occurrence of this complication in patients who earlier presented with a milder form of COVID-19, and their long-term follow-up
OBJECTIVE: To assess the incidence of persistent lung perfusion abnormalities as a result of suspected air trapping or microthrombosis in non-hospitalised patients diagnosed with COVID-19. The long-term follow-up of these patients will also be investigated
METHODS: This was a retrospective study conducted at the nuclear medicine department of Universitas Academic Hospital, Bloemfontein. We reviewed the studies of 78 non-hospitalised patients with SARS-CoV-2 infection referred to our department from July 2020 to June 2021 for a perfusion-only single-photon emission computed tomography/computed tomography (SPECT/CT) study or a ventilation perfusion (VQ) SPECT/CT study. All 78 patients were suspected of having pulmonary embolism, and had raised D-dimer levels, with persistent, worsening or new onset of cardiopulmonary symptoms after the diagnosis of COVID-19
RESULTS: Seventy-eight patients were studied. The median (interquartile range) age was 45 (41 - 58) years and the majority (88.5%) were females. Twenty-two (28.2%) of these patients had matching VQ defects with mosaic attenuation on CT. All 9 of the patients who had follow-up studies had abnormalities that persisted, even after 1 year
CONCLUSION: We confirm that persistent ventilation and perfusion abnormalities suspicious of small airway disease and pulmonary microthrombosis can occur in non-hospitalised patients diagnosed with a milder form of COVID-19. Our study also shows that these complications remain present even 1 year after the initial diagnosis of COVID-19
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus responsible for the ongoing coronavirus disease 2019 (COVID-19) global pandemic. Since its discovery, millions of cases have been recorded worldwide, with hundreds of thousands of deaths. The pathophysiology of SARS-CoV-2 in the lungs involves viral interaction with cells expressing angiotensin-converting enzyme 2 (ACE2) in the lungs.[1] This interaction is thought to involve ACE2-mediated cellular viral entry, tissue damage and systemic release of cytokines.[2] This ACE2 receptor is also expressed throughout the airway tract, including the small airways.[3,4] Available clinical data have highlighted that COVID-19 is associated with a long-term risk of persistent small airway disease as a result of small airway trapping, manifesting as mosaic hypoattenuation on computed tomography (CT) images, especially in patients with severe disease.[5-8] This small airway disease could result from direct infection of the small airways by SARS-CoV-2. Being small in diameter, these small airways are susceptible to occlusion by inhaled toxins or pathogens, or by inflammatory damage.[9,10] Small airway disease has also been known to be associated as a complication with the previous Middle East respiratory syndrome (MERS) and SARS respiratory infections.[7]
Available clinical data have also highlighted that COVID-19 is associated with a significant risk of thrombotic complications ranging from microvascular thrombosis, venous thromboembolic disease, and stroke.[11] The evidence to date is in keeping with the theory that the thrombotic manifestations of COVID-19 are due to the ability of SARS-CoV-2 to invade pulmonary capillary endothelial cells via ACE2 expressed on the endothelial cell surface.[11-13] This results in subsequent endothelial inflammation, complement activation, thrombin generation, and platelet and leukocyte recruitment, leading to immunothrombosis and ultimately causing thrombotic complications, including microthrombosis.[11] This prothrombotic state is said to be a risk factor for perfusion abnormalities at the pulmonary level.[14] Pulmonary microvascular thrombosis was reported as a complication of acute respiratory distress syndrome (ARDS) during previous coronavirus outbreaks.[15,16] However, this feature appears to be more pronounced in severe SARS-CoV-2 infection.[11]
At our nuclear medicine department, we have performed lung ventilation and perfusion imaging in a number of non-hospitalised patients diagnosed with COVID-19, for the diagnosis of pulmonary embolism (PE) with a ventilation perfusion (VQ) single-photon emission computed tomography/computed tomography (SPECT/ CT) scan. These patients presented with persistent or new-onset cardiopulmonary symptoms and raised D-dimer levels after de-isolation. Published data from our facility showed that lung perfusion abnormalities were not uncommon in these patients during the first wave of the pandemic.[17,18] These perfusion abnormalities could be associated with PE, mosaic perfusion, perfusion shunting and pulmonary infiltrates.[17] However, we observed that a subset of these patients had lung perfusion abnormalities that persisted, despite the fact that they had been on long-term anticoagulation therapy. This subset of patients also had their perfusion defects matched with a ventilation defect and mosaic hypoattenuation on CT. It has been observed in the literature that there are some post-COVID-19 patients with persistent respiratory symptoms who present with hypoattenuated areas in their lungs on CT.[6,7] These findings, based on their CT characteristics, have been suggested to be in keeping with air trapping from small airway disease and a secondary reflex vasoconstriction of the pulmonary capillaries.[6,7] We suspect that this is part of the process we are seeing on functional imaging, presenting as persistently matched ventilation and perfusion defects. We are also not sure if it is solely due to small airway trapping, pulmonary microthrombosis or a combination of both.
Our aim in the present study is to demonstrate with VQ scans that especially during the earlier phase of the pandemic, this process also occurred in non-hospitalised patients with a milder form of COVID-19, and is persistent over a long period of time. We are worried that the persistence of these perfusion defects might lead to more severe complications later on in life.
Methods
Study design and location
The study was a retrospective cohort study conducted in a tertiary institution.
Study population
We reviewed all the perfusion-only SPECT/CT and VQ SPECT/ CT studies of 412 patients who were investigated for PE between July 2020 and June 2021. Seventy-eight of these patients who were being investigated for PE as a complication of COVID-19 were included in the study and had their VQ scans evaluated. All baseline scans were performed within 30 days of the diagnosis of COVID-19. Nine patients who had at least one follow-up scan also had those scans reviewed in this study.
Inclusion criteria
• de-isolated non-hospitalised patients diagnosed with COVID-19 being investigated for PE
• age >18 years
• raised D-dimer levels
. had had a VQ SPECT/CT study
Exclusion criteria
• all patients without a diagnosis of COVID-19
• patients who had a VQ SPECT study only, without a CT component
• hospitalised patients or patients with severe disease.
Equipment
Ventilation studies were performed with 20-25 mCi of technetium-99 metastable diethylenetriamine pentacetate (99mTc DTPA), using the SmartVent radioaerosol delivery system (Diagnostic Imaging Ltd UK). Appropriate precautions were taken by the radiographers during ventilation of these patients and none of the staff members or patients, to the best of our knowledge, contracted COVID-19 from this ventilation procedure. Perfusion studies were performed with 3-5 mCi of 99mTc macro-aggregated albumin (MAA). Images were acquired with either a 16-slice SPECT/CT camera (Siemens Symbia T16 TruePoint; Siemens Medical Solutions, USA) or a 2-slice SPECT/CT camera (Siemens Symbia T2 TruePoint; Siemens Medical Solutions, USA). Both cameras are dual-headed gamma cameras, with similar workstations and processing units.
Acquisition protocol
Both gamma cameras were equipped with a low-energy high-resolution collimator. SPECT CT/CT imaging was acquired immediately after ventilation of the radioaerosol at 15 s/stop, with 3° steps, in a 128 x 128 matrix. Then perfusion SPECT imaging was acquired after injection of the perfusion tracer at 12 s/stop, with 3° steps, in a 128 x 128 matrix. This was followed by a low-dose non-contrast chest CT scan, with the patient remaining in the same position.
Image processing
Images were processed using the Syngo (Siemens, USA) workstations for both gamma cameras. SPECT/CT images were reconstructed using an iterative algorithm, and SPECT/CT fusion images were obtained using the multimodality Syngo imaging software on the workstation.
Data analysis
Data from each patient were collected using Excel 2019 (Microsoft Corp., USA) and analysed using the statistical package Stata version 16 (StataCorp, USA).
Ethical approval
Ethics approval was obtained from the Health Sciences Research Ethics Committee at the University of the Free State (ref. no. UFS-HSD2021/1575).
Results
Seventy-eight patients were enrolled during the study period. The median (interquartile range) age was 45 (41 - 58) years, and the majority (88.5%) were females. Twenty-two (28.2%) of these patients had matching segmental VQ defects with mosaic attenuation on CT. All 22 patients had a baseline VQ SPECT/CT study performed within 1 month of the diagnosis of COVID-19. Nine of the 22 patients (41%) had at least one follow-up VQ SPECT/CT performed after the baseline. These 9 patients initially had a non-diagnostic study for PE and were referred for follow-up studies by the referring doctor. Of the 9 patients who had at least one follow-up study, 100% had persistent matching perfusion defects on their follow-up studies. Six of these patients (54.5%) were on long-term therapeutic anticoagulation as they were also diagnosed with PE during the same period of the study. Ten (45.5%) of the patients had a single matched perfusion defect, 9 (40.9%) had 2 matched perfusion defects, 2 (9.1%) had 4 matched perfusion defects, while only 1 (4.5%) had 3 matched perfusion defects.
Discussion
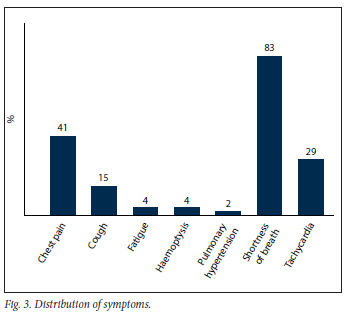
Our data suggest that during the early phase of the pandemic, and with a very high index of suspicion based on our scan findings, either pulmonary microthrombosis, air trapping from alveolar damage or both could occur in non-hospitalised patients with a milder form of COVID-19. In our study, 28.2% of non-hospitalised de-isolated COVID-19 patients who presented for a VQ scan in our facility to rule out PE presented with scan findings associated with matching ventilation and perfusion abnormalities, with mosaic hypoattenuation on CT. This is a finding we would like to refer to as COVID-19 mosaic hypoperfusion (Figs 1 and 2). To our knowledge, this is the first African study reporting on ventilation and perfusion follow-up outcomes after SARS-CoV-2 infection, up to a year after initial presentation. Just as in our patient population, it is well known that some patients who have recovered from COVID-19 infection later present with sequelae. It has been reported that >80% of patients report the persistence of at least one symptom after recovery from COVID-19, with dyspnoea being the most commonly reported symptom.[5] In our study population, 83% of the patients presented with persistent shortness of breath after de-isolation (Fig. 3).
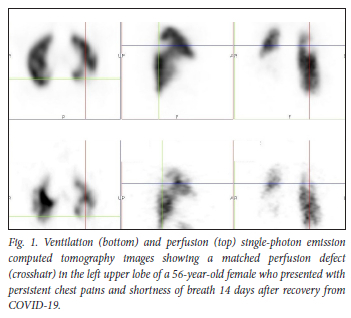
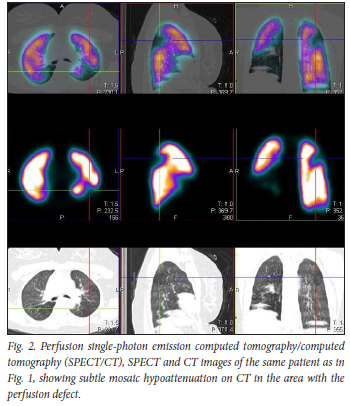
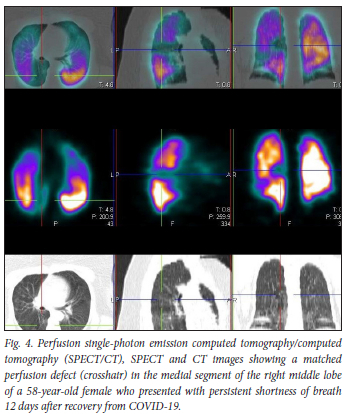
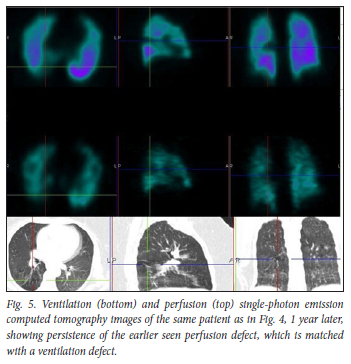
We had an average follow-up period of 9 months. All the follow-up patients, including all 6 patients followed up after 1 year, had persistent matching VQ defects, with mosaic hypoattenuation on their follow-up studies (Figs 4 and 5). A Swiss COVID-19 multicentre prospective study published in 2021[6] reported mosaic hypoattenuation in known COVID-19 patients 4 months after recovery. In their study, these findings, which were likely due to small airway disease, were more common in those patients who presented with severe disease. Our study has shown that these findings remain persistent even after 1 year, and that they can also occur in patients diagnosed with a milder form of the disease. Ebner et al.,[7] in a letter to the editor, confirmed the presence of areas of mosaic hypoattenuation in the lungs of patients who had recovered from COVID-19, 3 months later. They also concluded that these CT findings are likely due to small airway disease. Due to the limitation of a CT-only scan, these studies do not have adequate and clear information on the state of lung perfusion in these patients. In our study, the VQ scan was able to demonstrate that these patients also have matching perfusion abnormalities. This leads us to believe that pulmonary microthrombosis, due to the ability of SARS-CoV-2 to invade pulmonary capillary endothelial cells, could also be an added pathological finding in these patients.
Autopsy findings have shown that, in addition to the features of diffuse alveolar damage found in severe COVID-19 patients, platelet-fibrin thrombi are a common microscopic finding in the small pulmonary vasculature, occurring in up to 80% of lungs examined at autopsy.[19,20] Most of the imaging diagnosis of pulmonary microthrombosis has been done on dual-energy CT, where the presence of lung parenchymal hypoperfusion in the absence of PE is strongly indicative of pulmonary microthrombosis.[21-23] However, this modality does not give sufficient information on lung ventilation, and is not usually used for follow-up because of the high radiation dose associated with it. Hence it has been easier for us to demonstrate in one study the ventilation and perfusion abnormalities faced by these patients.
Another important finding in our study was that therapeutic anticoagulation had no effect in improving the perfusion defects, as seen in all the patients (63.6%) who were on therapeutic anticoagulation. This is in keeping with the literature, where a multicentre autopsy study confirmed the presence of microthrombi in the pulmonary capillaries despite the fact that patients were on anticoagulation therapy.[24]
Due to the retrospective nature of our study, a very important limitation is that our patient population did not have a baseline CT or VQ scan performed before COVID-19, hence we cannot completely rule out pre-existing ventilation and perfusion defects with mosaic hypoattenuation. However, we had not seen this pattern in our facility prior to the pandemic. We also do not have the benefit of good clinical data for each patient.
Conclusion
The findings on our initial and follow-up scans add to the growing body of evidence on post-COVID-19 trajectories. Matched perfusion defects, with corresponding mosaic hypoattenuation, were not an uncommon occurrence in non-hospitalised patients diagnosed with COVID-19 infection during the first wave of the pandemic in our facility. We strongly suspect that these findings are in keeping with a combination of pulmonary microthrombosis and small airway disease, and they are persistent for at least a year. There are likely more COVID-19 recovered patients all over the world who might not have been diagnosed with this complication. Although the long-term complications are not known, longstanding perfusion defects such as these could lead to pulmonary hypertension and right heart failure in the future, posing a serious public health issue. Future research is needed to determine the long-term persistence and effects of these matched perfusion defects after COVID-19 and its impact on patients, and methods to either prevent or treat it.
Declaration. None.
Acknowledgements. With their permission, the authors acknowledge all the radiographers, nurses and other staff in the Department of Nuclear Medicine at Universitas Academic Hospital.
Author contributions. OE was the major contributor in writing the article and interpreting the data. GE was a contributor in writing and proofreading the article. WE and TT played major roles in data collection.
All authors read and approved the final manuscript and have given consent for publication of this article.
Funding. None.
Conflicts of interest. None.
References
1. Patelli G, Paganoni S, Besana F, et al. Detection of lung hypoperfusion in COVID-19 patients during recovery by digital imaging quantification. medRxiv 2020. https://www.medrxiv.org/content/early/2020/06/03/2020.05.29.20117143 (accessed 14 May 2022). [ Links ]
2. Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res 2020;1(195):95-99. https://doi.org/10.1016%2Fj.thromres.2020.07.025 [ Links ]
3. Ortiz ME, Thurman A, Pezzulo AA, et al. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract E Bio Med 2020;60:102976. https://doi.org/10.1016/j.ebiom.2020.102976 [ Links ]
4. Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients postinfection by COVID-19. A systematic review and meta-analysis. J Pulmonol 2020;27(4):328-337. https://doi.org/10.1016/j.pulmoe.2020.10.013 [ Links ]
5. Pednekar P, Amoah K, Homer R Ryu C, Lutchmansingh DD. Case report. Bullous lungdisease following COVID-19. Front Med 2021;8(November):17-20. https://doi.org/10.1016/j.chest.2021.07.358 [ Links ]
6. Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19. First results from the national prospective observational Swiss COVID-19 lung study Eur Respir J 2021;57(4):2003690. https://doi.org/10.1183/13993003.03690-2020 [ Links ]
7. Ebner L, Funke-Chambour M,von Gamier C, Ferretti G, Ghaye B, Beigelman-Aubry C. Imaging in the aftermath of COVID-19. What to expect. Eur Radiol 2021;31(6):4390-4392. https://doi.org/10.1007/S00330-020-07465-6 [ Links ]
8. Huang R, Zhu J, Zhou J, et al. Inspiratory and expiratory chest high-resolution CT. Small-airway disease evaluation in patients with COVID-19. Curr Med Imaging 2021;17:1299-1307. https://doi.org/10.2174/1573405617999210112194621 [ Links ]
9. Garg A, Nagpal P, Goyal S, Cornelias AP. Small airway disease as long-term sequela of COVID-19. Use of expiratory CT despite improvement in pulmonary function test. medRxiv 2021;2021.10.19.21265028 http://medrxiv.org/content/early/2021/10/22/2021.10.19.21265028.abstract (accessed 12 May 2022). [ Links ]
10. Burgel PR Bergeron A, de Blic J, et al. Small airways diseases, excluding asthma and COPD. An overview. Eur Respir Rev 2013;22(128):131-147. https://doi.org/10.1183/09059180.00001313 [ Links ]
11. McFadyen JD, Stevens H, Peter K. The emerging threat of (micro)thrombosis in COVID-19 and its therapeuticimplications. CircRes2020;127(4):571-587. https://doi.org/10.1161/circresaha.l20.317447 [ Links ]
12. Patel BV, Arachchillage DJ, Ridge CA, et al. Pulmonary angiopathy in severe COVID-19. Physiologic, imaging and hematologic observations. Am J Respir Crit Care Med 2020;202(5):690-699. https://doi.org/10.1164/rccm.202004-1412oc [ Links ]
13. Sanfdippo F, la Rosa V, Astuto M. Micro-thrombosis, perfusion defects, and worsening oxygenation in COVID-19 patients. A word of caution on the use of convalescent plasma. Mayo Clin Proc 2021;6(1):259. https://doi.Org/10.1016/j.mayocp.2020.10.035 [ Links ]
14. Tomashefski JF, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983;112(1):112-126. [ Links ]
15. Lang ZW, Zhang LJ, Zhang SJ, et al. A clinicopathological study of three cases of severe acute respirator-syndrome (SARS). Pathology 2003;35(6):526-531. https://doi.org/10.1080/00313020310001619118 [ Links ]
16. Li K, Wohlford-Lenane C, Perlman S, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 2015;212(11):712-722. https://doi.org/10.1093/infdis/jiv499 [ Links ]
17. Evbuomwan O, Engelbrecht G, Bergman MV, Mokwena S, Ayeni OA. Lung perfusion findings on perfusion SPECT/CTimagingin non-hospitalised de-isolated patients diagnosed with mild COVID-19 infection. Egypt J Radiol Nucl Med 2021;52(1):1-12. https://doi.org/10.1186%2Fs43055-021-00521-1 [ Links ]
18. Evbuomwan O, Engelbrecht G, Bergman MV, Mokwena S, Ayeni OA. The prevalence of pulmonary embolism in non-hospitalised de-isolated patients diagnosed with mild COVID-19 disease. S Afr Med J 2021;111(8):741-746. https://doi.org/10.7196/samj.2021.vllli8.15657 [ Links ]
19. Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 2020;18(6):1517-1519. [ Links ]
20. Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med 2020; 8(5):518-526. https://doi.org/10.1016/S22132600(20)30121-1 [ Links ]
21. Grillet F, Busse-Coté A, Caíame P, Behr J, Delabrousse E, Aubry S. COVID-19 pneumonia. Microvascular disease revealed on pulmonary dual-energy computed tomography angiography. Quant Imaging Med Surg 2020;10(9):1852-1862. https://doi.org/10.21037%2Fqims-20-708 [ Links ]
22. Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19. Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020;20(12):1365-1366. https://doi.org/10.1016/S1473-3099(20)30367-4 [ Links ]
23. Santamarina MG, Boisier D, Contreras R, Baque M, Volpacchio M, Beddings I. COVID-19. A hypothesis regarding the ventilation-perfusion mismatch. Crit Care 2020;24(1):395. https://doi.org/10.1186/sl3054-020-03125-92020 [ Links ]
24. Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology. A multi-institutional autopsy cohort from Italy and New York City. Mod Pathol 2020;33(11):2156-2168. https://doi.org/10.1038/s41379-020-00661-1 [ Links ]
 Correspondence:
Correspondence:
O Evbuomwan
moreli14@yahoo.com
Accepted 8 June 2022














