Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.11 Pretoria Nov. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i11.16671
RESEARCH
Usability study of a sleeve attachment device for enhancing ease of use of metered dose inhalers in children
K B MapondelaI; R DeyII, III; M LevinIV
IMD, MMed (ENT); Division of Paediatric Allergology, Department of Paediatrics and Adolescent Health, Faculty of Health Sciences, University of Cape Town, South Africa
IIPhD; Division of Biomedical Engineering, Department of Human Biology, Faculty of Health Sciences, University of Cape Town, South Africa
IIIPhD; Division of Orthopaedic Surgery, Department of Surgery, Faculty of Health Sciences, University of Cape Town, South Africa
IVMB ChB, PhD; Division of Paediatric Allergology, Department of Paediatrics and Adolescent Health, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Children with asthma often lack the strength to activate their pressurised metered dose inhaler (pMDI). A recently developed sleeve device that attaches to a pMDI reduces the activation force of pMDIs from 36 - 39 Newtons (N) to 12.6 N and monitors the remaining doses with a built-in counter
OBJECTIVES: To examine the usability and patient experience of the Easy Squeezy (ES) sleeve attachment device in the paediatric patient population
METHODS: This cross-over study included 40 participants aged 5-10 years, half of whom had previous experience in using a pMDI. The experienced participants had used a pMDI for at least 1 year, and the inexperienced participants had no experience of using a pMDI. Participants and their parents recorded their responses on the ease of use, perceptions and satisfaction with using the pMDI alone and the pMDI with the ES
RESULTS: The participants felt that it was easier for them to activate the pMDI using the ES. They liked the ES device more than the pMDI and felt happier using the ES device. The parents reported that their children would be happier using the ES and would find it easier to activate the pMDI using the ES, that the built-in counter in the ES would make it easier to keep track of the doses, and that their children would be more likely to take the ES to school and use it there compared with the pMDI. They would recommend the ES to other parents and were willing to buy the device with their own money
CONCLUSION: The paediatric participants and their parents reported that the ES made it easier for children to activate the pMDI, that the counter made it easier to keep count of the doses, and that the aesthetics of the ES could potentially remove the stigma attached to use of a pMDI
Asthma is a heterogeneous disease characterised by chronic airway inflammation resulting in airway hyper-responsiveness and recurrent symptoms of wheezing, shortness of breath, chest tightness and cough that vary over time and in intensity. These episodes are usually associated with widespread but variable airflow obstruction within the lungs that is often reversible, either spontaneously or with treatment.[1]
According to the World Health Organization, 262 million people were affected by asthma in 2019.[1] Many individuals with severe asthma have persistent symptoms and experience frequent life-threatening exacerbations despite standard care and treatment. In southern Africa, South Africa (SA) has the highest prevalence of asthma. Asthma is one of the most common respiratory diseases in children in SA, and its prevalence is rising.[2-5] One in five schoolchildren in SA suffers from asthma, and it is also the highest cause of hospitalisations in children. According to the Global Asthma Report 2018,[6] SA is ranked 25th worldwide with regard to asthma prevalence and 5th for asthma mortality, with an estimated 18.5 deaths per 100 000 asthma cases. Despite substantial reductions in mortality over the past decade, the death rates in SA therefore remain among the highest in the world.[6-8]
Treatment and effective management of asthma saves lives. Medication delivered directly to the lungs, rather than systemically, is the most effective and common controller and reliever medication for asthma. Medication can be delivered via pressurised metered dose inhalers (pMDIs), either via a spacer or in the mouth directly, or by dry powder inhalers.[9,10] Typically, dry powder inhalers are only used in older children, adolescents and adults, whereas pMDIs are used at any age range, including in children, provided that a spacer is used.[11]
The pMDI is not always easy to use, as the device requires 36 - 39 Newtons (N) of downward force for activation.[9] Studies have found that it is difficult for children to apply such a high activation force.[12] Dry powder inhalers require a very low force for priming, but a large inspiratory flow to activate the device. Breath-activated MDIs are not suitable to be used in a spacer and are often more costly than hand-actuated pMDIs.
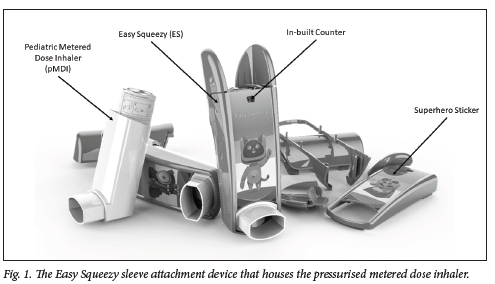
In order to allow a pMDI to be activated using less force, a sleeve attachment device, the Easy Squeezy (ES) (Fig. 1),[12] was developed by Impulse Biomedical (Pty) Ltd. With a novel dual-lever actuation mechanism, the ES reduces the activation force to 12.6 N.[12] The device has a built-in dose counter that activates every time the pMDI is actuated, enabling the patient caregiver and medical staff to keep track of the number of doses remaining in the pMDI canister and giving them an indication of when it will need to be replaced. To combat patient-reported social stigma, the ES has superhero characters attached to it as stickers. The usability and patient experience of this device have not previously been tested. The purpose of this study was to compare the user experience of a pMDI with the ES and a pMDI without the ES in the paediatric population.
Methods
After obtaining ethical approval from the University of Cape Town Human Research Ethics Committee (ref. no. 592/2021), 40 participants were recruited for this crossover usability testing. All were between 5 and 10 years old and were recruited at Red Cross War Memorial Children's Hospital, Cape Town. Twenty participants had prior experience of using a pMDI for at least 1 year and the rest had no experience of using a pMDI. The rationale for including inexperienced pMDI users was to remove experience bias of using a pMDI. Since this was a pilot study, no power calculation was performed to estimate the sample size. Our decision to have 20 participants in each group was based on the recommendations of the US Food and Drug Administration[13] and other literature discussing qualitative analysis sample size selection.[14,15] The study was performed in accordance with the Declaration of Helsinki, South African Good Clinical Practice (GCP) guidelines and all applicable laws. Each participant was accompanied by a parent/caregiver, and informed consent and assent were obtained before commencing the research. Participants with COVID-19 symptoms, hand injuries, or inability to follow instructions in English were excluded from the study.
Demographic and anthropometric information were obtained, after which each participant was randomly assigned to the order in which they rated the user experience and satisfaction with the pMDI alone or the pMDI with the ES. Participants and their parents activated the pMDI either with or without the ES and then completed the usability assessment questionnaire (supplementary file, https://www.samedical.org/file/1877). In the usability questionnaire, the first 3 questions were answered by the participant and the remaining 7 questions obtained the parent's experience using Likert scales of acceptability. The participant scale utilised graphics in the shape of happy neutral or sad faces indicating a positive response, a neutral response and a negative response, respectively. The parent Likert scale had five options of agreement with the question, ranging from 'strongly agree' to 'strongly disagree'. After completing the usability assessment questionnaire with the first device, participants waited for a crossover period of 15 minutes before using the second device and then completing the second usability assessment questionnaire.
Statistical analysis
The Shapiro-Wilk test was used to assess whether data were normally distributed. No data were found to be normally distributed so data are reported as medians and interquartile ranges. Mann-Whitney U-tests were used to compare the data between the devices (pMDI alone v. pMDI with ES). Data were entered into Excel version 2207 (Microsoft Corp., USA) and statistical tests were performed in SPSS version 28 (IBM Corp., USA). The condition for statistical significance was set at p<0.05.
Results
There were significant differences in height years of suffering from asthma, and years of pMDI use between the experienced and the inexperienced groups (Table 1). There was an equal distribution of gender (males 65%, females 35%) in the two groups. Inexperienced participants were non-asthmatic and had a lower number of siblings (15% v. 25%) and other family members (65% v. 90%) suffering from asthma compared with the experienced participants.
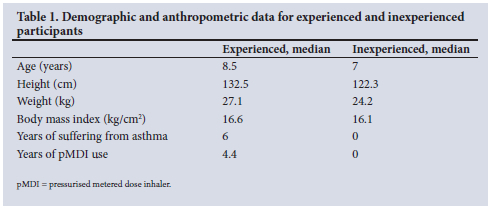
The paediatric participants found it significantly easier to activate the pMDI with the ES compared with activating the pMDI alone, and felt significantly happier activating it with the ES (Figs 2 and 3). Overall, they preferred activation using the ES compared with the pMDI alone (Fig. 4).
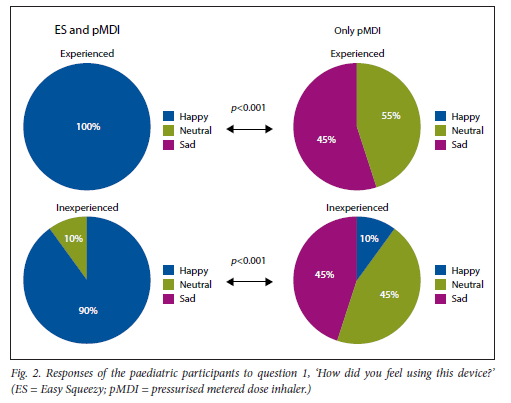
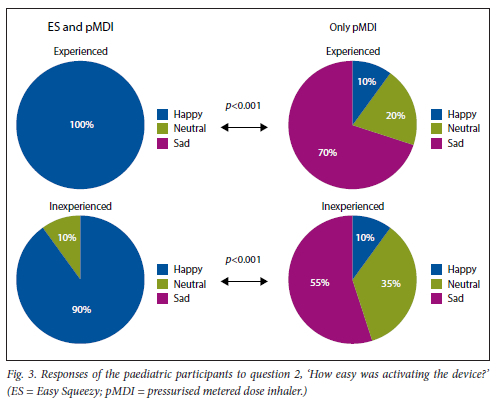
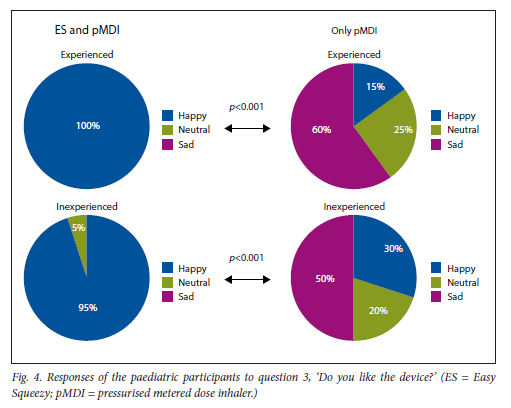
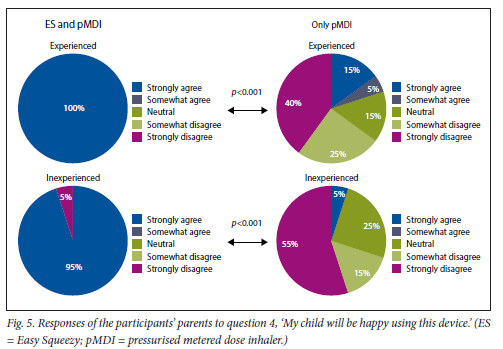
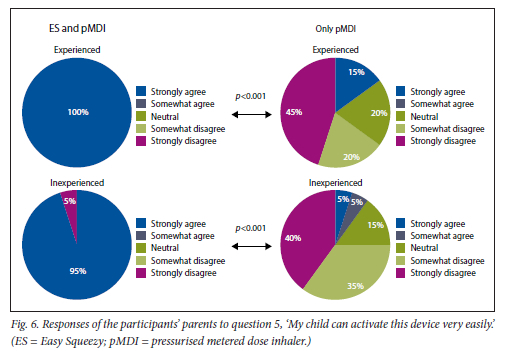
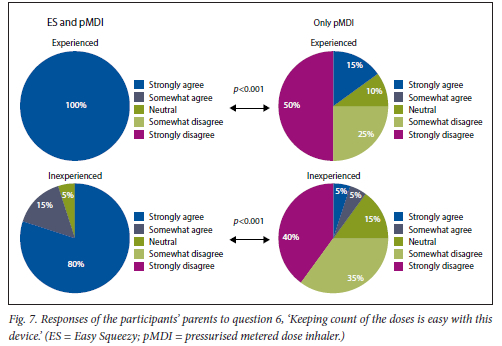
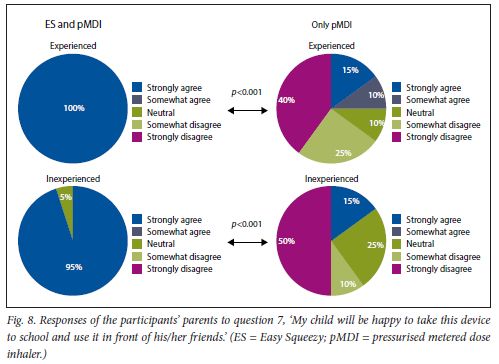
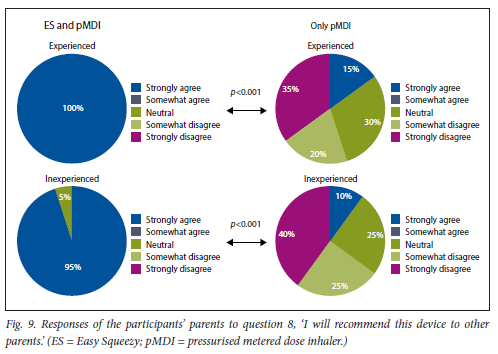
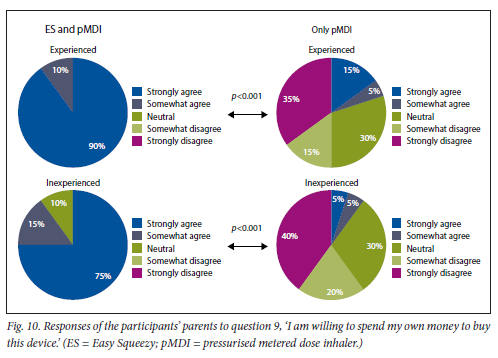
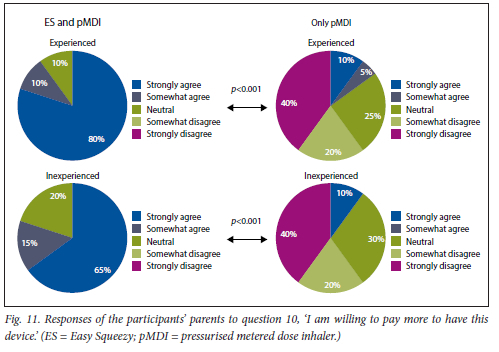
This was found for both experienced and inexperienced participants. Parents thought that their children would be happier using the ES compared with the pMDI alone (Fig. 5), and that the ES would make it easier for their children to activate the pMDI (Fig. 6). They strongly agreed that the ES would make it easy to keep count of medication doses (Fig. 7) and that their children would be happier to take the ES to school and use it around their friends compared with using the pMDI alone (Fig. 8). Parents reported that they would recommend the ES more to others compared with the pMDI (Fig. 9). There were no significant differences in these responses between the experienced and the inexperienced parents. All the experienced users' parents and 90% of inexperienced users' parents indicated their willingness to spend their own money to buy an ES, compared with just 20% of experienced and 10% of inexperienced users' parents being willing to spend their own money to purchase a pMDI alone, and this difference between the devices was statistically significant (Fig. 10). The vast majority of parents in both groups indicated that they would be willing to pay more to have an ES device rather than a pMDI alone (Fig. 11).
Discussion
Activating pMDIs may be difficult in both paediatric and geriatric age groups owing to the high forces required. This study was performed to test the usability of a sleeve attachment device, the Easy Squeezy, which reduces the activation force and also helps keep track of the remaining doses in a pMDI. Our observations suggest that paediatric patients and their parents prefer to activate the pMDI using the ES.
The force required to activate a pMDI ranges from 36 N to 39 N.[9,10] The average pinch strength of children aged 5-10 years is 21.4 N, with a range between 11.6 N and 31.6 N.[16] A large number of children are therefore unable to activate an inhaler on their own.[9] The geriatric population has been reported to generate similar pMDI activation forces to children, resulting in similar problems with activation of pMDIs.[9] In the present study, all the paediatric participants were able to activate both the pMDI and the pMDI with the ES, but they reported that activating the pMDI with the ES was easier than activating the pMDI alone. The present study strongly indicates that the ES makes the experience of using a pMDI more satisfactory, which may benefit both paediatric and elderly populations, and other vulnerable groups with reduced hand strength. The device would provide the paediatric and geriatric populations with independence in using a pMDI.
Experiences of social stigmas for young adults and children using pMDIs, including embarrassment' and exclusion, have been consistently reported.[17-21] These experiences may contribute to the high rates of absenteeism for schoolchildren due to asthma.[22,23] Our observations suggest that parents feel confident that their children would be happier with the ES device than the pMDI alone, and would be more likely to take the pMDI to school if the device is attached. This could be due to the superhero stickers attached to the body of the ES which conceivably make it less embarrassing for children to use their pMDI around their peers. These characters on the ES may make the pMDI look less like a medical device and more like a toy, which would be more acceptable around other children, without excluding the user or creating stigma. The pMDI (with or without the ES) needs to be attached to a spacer to ensure optimal lung deposition of the drug. The spacer device may add significantly to the embarrassment factor.
Counting the number of remaining doses in pMDI canisters that do not have built-in dose counters is a challenge for patients and can lead to under-use, over-use, and non-adherence with pMDIs.[24] Many subjective techniques such as shaking the canister, spray testing and floatation methods have been reported in the literature, but all are unreliable.[24,27] Previous studies have reported an increase in treatment compliance, disease control and patient satisfaction when inhalers with dose counters were used.[24,28-30] In the present study we found that the children's parents felt that the dose counter in the ES would make it easy to keep track of the remaining doses in the canister. This would remove the stress involved in constantly checking the pMDI for remaining doses.
Conclusion
Children found it easier to activate the pMDI using ES, and the ES improved the user experience for both the children and their parents. Future studies with the ES should focus on 'real-world' long-term use of the device and its clinical effectiveness, including assessment of adherence and disease control.
Declaration. The research for this study was done in partial fulfilment of the requirements for KBM's MSc Med (Paediatric Allergy) degree at the University of Cape Town.
Acknowledgements. None.
Author contributions. KBM and ML designed the study KBM performed the study and collected the data, KBM and RD performed the data analysis and wrote the first draft, and ML provided constructive changes towards the final manuscript.
Funding. None.
Conflicts of interest. RD and ML consult for Impulse Biomedical.
References
1. World Health Organization. Chronic respiratory diseases. Asthma. 15 May 2020, updated in English 3 May 2021. https://www.who.mt/news-room/quesuons-and-answers/item/chronic-respiratory-diseases-asthma (accessed 30 May 2021). [ Links ]
2. Lion-Cachet HC, Musonda JMM, Omole OB. Severe asthma in South Africa. A literature review and management approach for primary care. S Afr Fam Pract 2021;63(1):a5179. https://doi.org/10.4102ysafp.v63il.5179 [ Links ]
3. Baard CB, Franckling-Smith Z, Munro J, Workman L, Zar HJ. Asthma in South African adolescents; A time trend and risk factor analysis over two decades. ERJ Open Res 2021;79(2):00576-02020. https://doi.org/10.1183/23120541.00576-2020 [ Links ]
4. Masekela R Gray CL, Green RJ, et al., on behalf of the South African Childhood Asthma Working Group (SACAWG). The increasing burden of asthma in South African children. A call to action. S Afr Med J 2018;108(7):537-539. https://doi.org/10.7196/SAMJ.2018.V108I7.13162 [ Links ]
5. Manjra AI, van Niekerk A, White DA, et al., on behalf of the South African Childhood Asthma Working Group (SACAWG). Summary of childhood asthma guidelines, 2021. A consensus document S Afr Med J 2021;111(5):395-399. https://doi.org/10.7196/SAMJ.2021.VlllI5.15703 [ Links ]
6. Global Asthma Network. The Global Asthma Report 2018. Global Asthma Network 2018. http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf (accessed 25 June 2020). [ Links ]
7. CDC (Centers for Disease Control and Prevention). Global health - South Africa, https://wwwcdc.gov/globalhealth/countries/southafrica/default.htm (accessed 30 May 2021). [ Links ]
8. CDC (Centers for Disease Control and Prevention). 2019 National Health Interview Survey (NHIS) data, https://www.cdc.gov/asthma/nhis/2019/data.htm (accessed 30 May 2021). [ Links ]
9. Ciciliani AM, Langguth P, Wachtel H. Handling forces for the use of different inhaler devices. Int J Pharm2019;560:315-321. https://doi.org/10.1016/j.ijpharm.2019.01.053 [ Links ]
10. Young PM, Price R. Comparative measurements of pressurised metered dose inhaler (pMDI) stem displacement. Drug Devlnd Pharm 2008;34(l):90-94. https://doi.org/10.1080/03639040701484205 [ Links ]
11. Levin M. Optimal aerosol delivery. Review article. Curr Allergy Clin Immunol 2011;24(1):27-30. https://journals.co.za/doi/pdf/10.10520/EJC21701 (accessed 3 September 2022). [ Links ]
12. Beukes GL, Levin M, Sivarasu S. The paediatric metered dosage inhaler (PMDI) sleeve attachment. In. Proceedings of the 2017 Design of Medical Devices Conference. Minneapolis, Minn., USA, 10-13 April 2017. https://doi.org/10.1115/DMD2017-3459 [ Links ]
13. Food and Drug Administration. Public workshop on patient-focused drug development. Guidance 1 - collecting comprehensive and representative input. 2017. https://www.fda.gov/drugs/news-events-human-drugs/public-workshop-patient-focused-drug-development-guidance-l-collecting-comprehensive-and (accessed 2 September 2022). [ Links ]
14. Francis JJ, Johnston M, Robertson C, et al What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25(10):1229-1245. https://doi.org/10.1080/08870440903194015 [ Links ]
15. Dworkin SL. Sample size policy for qualitative studies using in-depth interviews. Arch Sex Behav 2012;41(6):1319-1320. https://doi.org/10.1007/sl0508-012-0016-6 [ Links ]
16. Ager CL, Olivett BL, Johnson CL. Grasp and pinch strength in children 5 to 12 years old. Am J Occup Ther 1984;38(2):107-113. https://doi.org/10.5014/ajot.38.2.107 [ Links ]
17. Guleria R Korukonda K, DUSS Investigators. Clinical impact of a digital dose counter pressurized metered-dose inhaler on uncontrolled asthma. Cross-sectional, observational, surveillance study. Interact J Med Res 2019;8(2):e13530. https://doi.org/10.2196/13530 [ Links ]
18. Cole S, Seale C, Griffiths C. 'The blue one takes a battering1 why do young adults with asthma overuse bronchodilator inhalers? A qualitative study. BMJ Open 2013;3(2):e002247. https://doi.org/10.1136/bmjopen-2012-002247 [ Links ]
19. Price D, David-Wang A, Cho S-H, et al. Time for a new language for asthma control. Results from REALISE Asia. J Asthma Allergy 2015;8:93-103. https://doi.org/10.2147/JAA.S82633 [ Links ]
20. Partridge MR, van der Molen T, Myrseth S-E, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy. The INSPIRE study. BMC Pulm Med 2006;,6:13. https://doi.org/10.1186/1471-2466-6-13 [ Links ]
21. Braido F, Chrystyn H, Baiardini I, et al. 'Trying but failing1 - the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract 2016;4(5):823-832. https://doi.org/10.1016/J.JAIP.2016.03.002 [ Links ]
22. Fitzgerald JM, Boulet L-P, Mclvor RA, Zimmerman S, Chapman KR. Asthma control in Canada remains suboptimal. The Reality of Asthma Control (TRAC) study. Can Respir J 2006;13(5):253-259. https://doi.org/10.1155/2006/753083 [ Links ]
23. Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults. The global asthma insights and reality surveys. J Allergy Clin Immunol 2004;114(1):40-47. https://doi.org/10.1016/J.JACI.2004.04.042 [ Links ]
24. Kaur I, Aggarwal B, Gogtay J. Integration of dose counters in pressurized metered-dose inhalers for patients with asthma and chronic obstructive pulmonary disease. Review of evidence. Expert Opin Drug Deliv 2015;12(8):1301-1310. https://doi.org/10.1517/17425247.2015.1000858 [ Links ]
25. Sander N, Fusco-Walker SJ, Harder JM, Chipps BE. Dose counting and the use of pressurized metered-dose inhalers. Running on empty. Ann Allergy Asthma Immunol 2006;97(1):34-38. https://doi.org/10.1016/S1081-1206(10)61366-X [ Links ]
26. Sangnimitchaikul W, Srisatidnarakul B, Ladores S. Perspectives on managing asthma and facilitators in asthma self-management among Thai school-age children. A qualitative study. J Health Res 2019;35(3):214-225. https://doi.org/10.1108/JHR-09-2019-0207/FULL/HTML [ Links ]
27. Rubin BK, Durotoye L. How do patients determine that their metered-dose inhaler is empty? Chest 2004; 126(4):1134-1137. https://doi.org/10.1378/CHEST.126.4.1134 [ Links ]
28. Wasserman RL, Sheth K, Lincourt WR Locantore NW, Rosenzweig JC,Crim C. Real-world assessment of a metered-dose inhaler with integrated dose counter. Allergy Asthma Proc 2006;27(6):486-492. https://doi.org/10.2500/AAP.2006.27.2921 [ Links ]
29. Laforce C, Weinstein C, Nathan RA, Weinstein SF, Staudinger H, Meltzer EO. Patient satisfaction with a pressurized metered-dose inhaler with an integrated dose counter containing a fixed-dose mometasone furoate/formoterol combination. J Asthma 2011;48(6):625-631. https://doi.org/10.3109/02770903.2011.587579 [ Links ]
30. Given J, Taveras H, Iverson H. Prospective, open-label evaluation of a new albuterol multidose dry powder inhaler with integrated dose counter. Allergy Asthma Proc 2016;37(3):199-206. https://doi.org/10.2500/AAP.2016.37.3938 [ Links ]
 Correspondence:
Correspondence:
M Levin
michael.levin@uct.ac.za
Accepted 31 August 2022














