Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.10 Pretoria Out. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i10.16527
RESEARCH
Autologous whole blood clot and negative-pressure wound therapy in South Africa: A comparison of the cost and social considerations
L NaudeI; G BalendaII; A LombaardIII
IMCur, Cert Advanced Wound Care; Nurse Specialist and Educator in Advanced Wound Care, Eloquent Health & "Wellness, Pretoria, South Africa; Principal Lecturer in Wound Care., Eloquent Learning Health and Foundation for Professional Development, Pretoria, South Africa
IIMD, MMed (Gen Surg); General Surgeon and Clinical Manager, Tembisa Provincial Tertiary Hospital, Johannesburg, South Africa
IIIBCur, Cert Advanced Wound Care; Advanced Wound Care Practitioner, Eloquent Health & Wellness, Pretoria, South Africa
ABSTRACT
BACKGROUND: Advanced wound treatment modalities enhance healing of hard-to-heal wounds, decrease the risk of amputations, and improve the quality of life of patients. Modalities have different rates of efficacy and incur different social and financial costs to the individual and the healthcare system. Two such modalities, the autologous whole blood clot (WBC) and negative-pressure wound therapy (NPWT), were compared in the South African (SA) context. The comparison was conducted on hard-to-heal wounds, with a specific focus on diabetic foot ulcers (DFUs
OBJECTIVES: To compare the social considerations and financial costs of using autologous WBC v. NPWT in the treatment of DFUs in SA
METHODS: Data were obtained based on current supply costs from SA suppliers for the two modalities, the standard of care for both modalities, the number of applications required for each, and social considerations provided by SA wound management clinicians. Wound healing rates were obtained from the published literature. This information was used to calculate costs of two scenarios (scenario 1: low exudate v. scenario 2: high exudate), which were compared over two treatment durations (4 and 12 weeks) for each treatment modality. Calculations included weekly cost of supplies, total cost saved by a patient with a DFU managed with either of the wound therapies, and the difference in total cost saved between the two modalities. Key social considerations were assessed qualitatively from discussions with SA clinicians experienced in both autologous WBC and NPWT, and from published research
RESULTS: The cost of supplies per week was ZAR3 250 for autologous WBC and ZAR4 804 for NPWT in scenario 1, and ZAR3 332 and ZAR6 612 in scenario 2. With healing rates over 4 weeks' treatment duration of 19% for autologous WBC and 10% for NPWT, autologous WBC saved ZAR17 719.93, or 9% more than using NPWT, in scenario 1 and ZAR18 381.47, or 10% more, in scenario 2. At 12 weeks' treatment duration, healing rates for autologous WBC and NPWT were 75% and 43%, respectively. In scenario 1, results indicated a 43% cost difference between the two modalities. Autologous WBC had a total cost saving of ZAR61 874.40 compared with NPWT over a period of 12 weeks. In scenario 2, results indicated a 46% cost difference between the two modalities. Autologous WBC had a total cost saving of ZAR70 454.68 compared with NPWT over a period of 12 weeks. One of the identified social considerations is that NPWT needs a reliable supply of electricity to recharge the pump, while autologous WBC does not
CONCLUSION: Both modalities are safe and effective in treating hard-to-heal wounds of the lower extremities. Autologous WBC consistently demonstrated better outcomes than NPWT in terms of both healing rate and cost-effectiveness, as well as having some advantages in terms of social considerations in SA
Hard-to-heal wounds refer to all chronic or non-healing wounds that have not healed by at least 40 - 50% after 4 weeks of standard care treatment.[1-3] Hard-to-heal wounds may progress slowly, or healing may be interrupted or delayed, due to several intrinsic and extrinsic factors.[2-5] These factors include high levels of proteases, prolonged inflammation, reduced growth factors, recurrent infections, the inability of epidermal or dermal cells to respond to reparative stimuli, and formation of drug-resistant biofilms.[6,7] Hard-to-heal wounds benefit from alternative therapies and interventions managed by a wound care specialist in an interprofessional team.[1,2]
Hard-to-heal wounds affect the quality of life (QoL) of >40 million people worldwide,[8] and their incidence is expected to increase as populations age.[9] Hard-to-heal wounds have both social and economic costs at individual and societal levels.[10] Individuals with such wounds suffer from a range of physical, financial and social restrictions, and these are exacerbated in resource-limited settings where access to appropriate care and treatment may be limited or nonexistent.[2] Hard-to-heal wounds have a significant negative impact on various aspects of patients' QoL, influenced by low healing rates, pain, levels of exudate, alterations in body image due to wound malodour, prolonged hospital stays, self-care deficit, inability to sustain paid employment or perform household duties, social isolation, financial burden and chronic morbidity[11-13] Moreover, emotions such as depression, anxiety, shame, embarrassment and loneliness are common among individuals with hard-to-heal wounds.[14]
Hard-to-heal wounds place a considerable economic burden on the healthcare system and the affected individual's circle of care across the globe.[3,8] In high-income countries, wound treatment costs account for ~1 - 3% of health expenditure.[15,16] In the UK, the annual cost to the National Health Service of managing wounds was estimated to be GBP8.3 billion (ZAR157.7 billion) in 2017/2018,[17] while in the USA, chronic ulcers as a primary diagnosis are estimated to cost the healthcare system USD28 billion (ZAR532 billion) per year (conversion of all pounds/dollars to rands is based on an exchange rate of ZAR19 = USD1 = GBP1).[18] Ulcers precede 85% of all amputations, and 70% of lower limb amputations are attributed to diabetic foot ulcers (DFUs).[19]
Advanced wound treatment modalities such as growth factors, extracellular matrices (ECMs), autologous whole blood clot (WBC) products, skin substitutes, cell-based therapies and engineered skin and negative-pressure wound therapy (NPWT) are the preferred treatment for hard-to-heal wounds.[9,20-22] These advanced treatment modalities enhance wound healing, decrease the risk of amputation, and improve the QoL of the individual. However, each incurs different social and financial costs to the individual and the healthcare system, and they have different rates of efficacy. This article reports a comparison between autologous WBC and NPWT in SA with regard to financial costs and social considerations, performed by comparing two scenarios of hard-to-heal DFUs.
Autologous WBC
The autologous WBC is created using a small sample of the patient's own peripheral blood at point of care.[23] The clot creates a protective scaffold and acts as an ECM, initiating the healing properties of the wound and creating a natural healing environment.[23] A weekly application of the clot is recommended, and no capital equipment or special storage is required. Autologous WBC treatment is indicated for the management of exuding cutaneous wounds, such as leg ulcers, pressure injuries, DFUs and mechanically or surgically debrided wounds.[23] It has been found to be safe and efficacious in treating hard-to-heal wounds. Clinical evidence shows that autologous WBC treatment in patients with several comorbidities achieved 78% complete wound healing in wounds of different severities. Snyder et al.[21] reported a percentage area reduction of 61.6% and 67.1% at weeks 4 and 12, respectively, for the intention-to-treat population and 60.3% and 76.2% for the per protocol population. In an observational study conducted in SA and Israel,[22] the autologous WBC was found to achieve 65% wound reduction by week 4 with 55% complete closure by week 12 on hard-to-heal wounds in patients with multiple comorbidities, while the overall efficacy was found to be 94% at week 12. Autologous WBC treatment has been found to be cost-effective in acute to post-acute settings, with an estimated weekly cost of USD265 (ZAR3 710),[2] comprising USD250 (ZAR3 500) for supplies and USD15 (ZAR210) for staff time.[9] Lastly, compared with four other skin substitutes, Snyder and Ead[9] found that the autologous WBC has a cost advantage ranging from USD1 245 (ZAR17 430) to USD5 527 (ZAR77 378) over 12 weeks of treatment.
Negative-pressure wound therapy
NPWT involves the delivery of continuous subatmospheric pressure through a special pump connected to a resilient foam-surfaced dressing that collects wound exudate.[24] The benefits of this treatment include fast wound granulation, epithelialisation and contraction,[24,25] reduced dressing changes, reduced risk of infection and exudate control, while patients report improved tolerability. Priyatham et al.[26] compared the use of vacuum-assisted closure/ NPWT (n=60) and a conventional moist dressing (n=60) in the management of hard-to-heal wounds. They found that the rate of granulation tissue formation, graft survival and client compliance was better in the group treated with NPWT compared with those treated with a conventional dressing. Additionally, the NPWT group had increased wound contracture, better graft survival and a shorter hospital stay than the control group. Similarly, Siddha et al.[27] found that the rate of granulation and tissue formation and clearance of wound infection was better in the NPWT group than the control group (conventional Betadine dressing), which then decreased the duration of hospital stay and treatment costs. Randomised controlled studies by Armstrong et al.[28] and Blume et al[29] showed that NPWT had higher efficacy than other treatment modalities reviewed, and was safe to treat chronic wounds. In the study by Blume et al.,[29] NPWT had an overall efficacy of 43% by week 12. In the German DiaFu-RCT by Seidel et al.,[12] reported in 2020, the cumulative proportion of patients with open wounds was >70% after 16 weeks, but the researchers could not calculate the medians for the time of closure. The DiaFu study did not demonstrate significant superiority of NWPT in wound closure rate or time to complete closure compared with standard moist wound care.[30] Nonetheless, research has shown NPWT to be effective in treating DFUs.[31-33]
NPWT promotes a moist environment and reduces oedema.[24] It requires dressing changes every 48 - 72 hours.[24] Patients may experience discomfort and/or pain from the vacuum and during dressing changes,[34] and frequent dressing changes are costly and can increase the risk of contamination.[35] Researchers have examined the cost-effectiveness of NPWT, and a review by Searle and Milne[36] showed strong evidence of cost saving from the use of NPWT in comparison with conventional therapies. They found that the total weekly cost of NPWT was USD456 (ZAR6 384), which comprised USD375 (ZAR5 250) for supplies and USD81 (ZAR1 134) for staff time. A study in India found NPWT to be less costly in treating DFUs compared with conventional dressing (INR3 750 v. INR7 000, the equivalent of ZAR730 v. ZAR1 360 based on an exchange rate of ZAR0.10 = INR1).[37] Alipour et al.[31] compared the cost of NPWT with that of traditional wound care in the treatment of DFUs and found that NPWT was less costly and more effective.
Overall, both autologous WBC and NPWT are safe and efficacious in treating hard-to-heal wounds. The study conducted in South Africa (SA) showed that autologous WBC was able to achieve greater overall size reduction and, in some cases, complete wound closure in several wounds for which alternative therapies, including NPWT, had previously been used.[22] The cost comparison between these two products in the SA setting is unknown, and the differences in their application need to be considered in the SA context. The objective of the present study was to compare the social considerations and financial costs of using autologous WBC v. NPWT in the treatment of DFUs in SA. Although both products are commonly used on all hard-to-heal wounds, DFUs are extremely common and are therefore particularly suitable for comparing the two modalities in terms of wound size and the amount of product used.
Methods
Data and information were obtained from various primary and secondary sources. Healing rates used to complete the costing calculations were obtained from published literature on the two modalities. Costs were obtained from SA suppliers of each of the two modalities, while the number of applications for each modality was based on the standard of care for WBC and NPWT. Finally, social considerations were based on conversations with SA clinicians, based on their experience with hard-to-heal wounds.
For the costing calculations for both products, two data points were based on a DFU with a surface area <28.3 cm2, for comparability. Furthermore, two scenarios were provided for two different durations of treatment (4 and 12 weeks). The first scenario compared the cost of treating DFUs with a low amount of exudate using either autologous WBC or NPWT, while the second scenario compared the cost of treating DFUs with a high amount of exudate.
Cost of the supplies
The parameters used for the calculation of the weekly average cost of the supplies for both treatments were: (i) average cost of supplies for each treatment; and (ii) number of applications for each treatment. Furthermore, the total cost of supplies was calculated for both scenario 1 and scenario 2 over 4- and 12-week periods.
Total cost saved by a patient
We then calculated the total cost saved by a patient with a DFU receiving either of the advanced wound care therapies, as well as the difference in the total cost saved between autologous WBC and NPWT. The total cost savings for autologous WBC and NPWT were calculated based on the number of weeks of application (4 and 12 weeks). To calculate the total cost saving per treatment, the percentages of hard-to-heal wounds that were unhealed and healed at 4 weeks and 12 weeks were used based on studies assessing the efficacy and safety of both treatments. The percentages of those healed and unhealed at 4 and 12 weeks were extracted from a study assessing the efficacy and safety of autologous WBC by Naude et al.,[22] while the studies assessing the efficacy and safety of NPWT were by Armstrong et al.[28] and Blume et al.[29] The studies by Armstrong et al.[28] and Blume et al.[29] showed homogeneity in healing rates,[38] and they were the only published studies we found that provided an estimate of the time for complete healing, although they were not conducted in the SA context. The article by Naude et al.[22] reported healing rates for WBC in an observational pilot study recently conducted in SA and Israel. Healing rates in these studies were based on the following sample sizes: N=342 in the Blume et al.[29] study, N=162 in the Armstrong et al.[28] study and N=29 in the Naude et al.[22] study.
Efficacy rates for autologous WBC and NPWT at weeks 4 and 12 from each of these studies are shown in Table 1.
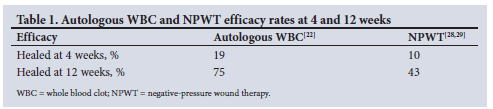
Furthermore, the average cost of an unhealed hard-to-heal wound was calculated using the information from McCall,[39] with an average cost of ZAR207 299.25 [5] for a patient whose hard-to-heal wound remains untreated. The following calculation is a weighted standard average cost of treatment, for both 4 and 12 weeks and for each modality (NPWT and autologous WBC):
[treatment cost healed x probability healed + [treatment cost healed x probability healed]
All costs shown in the results section are in rands.
Social considerations
Finally, social considerations in the application and requirements of each of the therapies have been included as a final section in the results. These social considerations are important in the SA context. Social considerations were assessed qualitatively from discussions with SA clinicians with experience working with both autologous WBC and NPWT.
Results
Costing results
We present the cost implications of treating DFUs with a surface area <28.3 cm2 in SA using either autologous WBC or NPWT treatment options. Direct costs measured for this study were supplies.
Table 2 shows the costs of supplies for 1 week of treatment for autologous WBC and NPWT. The total cost of supplies in scenario 1 (low exudate) is ZAR3 250 for autologous WBC and ZAR4 804 for NPWT. In scenario 2 (high exudate), the total cost of supplies is ZAR3 332 for autologous WBC and ZAR6 612 for NPWT.

Fig. 1 shows the total cost of supplies for the two treatment time periods (4 v. 12 weeks) for scenarios 1 and 2. In scenario 1, a 4-week treatment period would have a total cost of autologous WBC supplies of ZAR13 000 compared with ZAR19 216 for NPWT supplies over the same period. Over a 12-week period, scenario 1 would cost ZAR39 000 for autologous WBC supplies but ZAR57 648 for NPWT supplies. The difference in the cost of supplies between the two treatments increases in scenario 2, where 4 weeks of treatment with autologous WBC would cost ZAR13 328 in supplies v. ZAR26 448 for NPWT. Finally, if a patient required treatment over a 12-week period in scenario 2, the supplies for autologous WBC would cost ZAR39 984 v. ZAR79 344 for NPWT.

The cost-saving analysis is shown in Table 3. Again, results are shown over 4- and 12-week treatment periods for both scenario 1 (low exudate) and scenario 2 (high exudate). Considering 4-week healing rates from the published literature for both autologous WBC (19%) and NPWT (10%), and the average cost of an unhealed hard-to-heal wound for scenario 1 over a 4-week period, autologous WBC would save ZAR17 719.93, or 9% more than NPWT. Similarly, for scenario 2 there was a ZAR18 381.47 cost saving, or 10% more than NPWT.
Over a 12-week period, the results in Table 3 again show a noteworthy cost saving for autologous WBC in both scenarios, albeit less than over a 4-week period. At 12 weeks the rates of efficacy for autologous WBC and NPWT were 75% and 43%, respectively.
For scenario 1, the results indicate a 43% cost difference and a total cost saving of ZAR61 874.40 more for autologous WBC for 12 weeks of treatment. For scenario 2, the results indicate a 46% cost difference between autologous WBC and NWPT and a total cost saving of ZAR70 454.68 more for autologous WBC over 12 weeks of treatment.
Social considerations and challenges
Table 4 shows some of the social considerations highlighted in the literature and from discussions with SA clinicians. The description identifies the key theme or social consideration, and the two therapies are compared based on these social considerations.
Discussion
Financial costs of hard-to-heal wound care treatments
Hard-to-heal wounds place a considerable burden on patients, their families and the healthcare system.[2,3,8] It is important to find sustainable and cost-effective treatment solutions, while also considering their safety and efficacy. This article addresses the cost implications of using NPWT or autologous WBC to treat DFUs with a wound surface area <28.3 cm2, as well as some key considerations that need to be highlighted in the SA context.
The two scenarios in the study clearly outline the cost implications for two potential groups of patients, those with hard-to-heal wounds that have low exudate secretion (scenario 1) and those with wounds that have high exudate secretion (scenario 2). In both scenarios, analysis of the supplies for autologous WBC and NPWT, as well as the total cost over 4- and 12-week periods, showed that autologous WBC is less costly than NPWT. The cost of autologous WBC supplies over a 1-week period is ZAR3 250 in scenario 1 compared with ZAR4 804 for NPWT. In scenario 2, the respective costs over a 1-week period are ZAR3 332 v. ZAR6 612.
Furthermore, the total cost saving calculated using the cost of an unhealed hard-to-heal wound as well as the percentage healed and non-healed for both autologous WBC and NPWT at 4 and 12 weeks also showed differences between the two wound care therapies. Both autologous WBC and NPWT resulted in considerable cost savings for patients who receive either of these wound care therapies, compared with unhealed or untreated DFUs. Furthermore, previous studies have found that both these options are safe, although their rates of efficacy differ. Autologous WBC has an efficacy of 19% and 75% by week 4 and week 12, respectively,[22] compared with 10% and 43% for NPWT.[29] In both scenarios and over both the 4- and 12-week treatment periods, autologous WBC therefore consistently had a lower treatment cost than NPWT. For scenario 1 (low exudate), the total cost saving for autologous WBC over a 4-week period was ZAR170 770.99 compared with ZAR188 490.93 for NPWT. The cost savings for a 12-week period were ZAR81 074.81 and ZAR142 949.21, respectively. This translated to a cost saving of ZAR17 719.93 for patients receiving autologous WBC treatment instead of NPWT over a 4-week period and a saving of ZAR61 874.40 over a 12-week period. For scenario 2 (high exudate), results were similar. For a 4-week period of treatment, the total cost saving for autologous WBC was ZAR170 832.66 and that for NPWT ZAR189 214.13, with a total cost saving of ZAR18 381.47 more for patients receiving autologous WBC treatment rather than NPWT. Over a 12-week period, the total cost saving for autologous WBC and NPWT was ZAR81 812.81 and R152 278.49, respectively, which translated into a total cost saving of ZAR70 454.68 more for autologous WBC than NPWT. The cost saving found in this analysis was consistent with other studies that compared autologous WBC with four other advanced wound care therapies.[9] Furthermore, autologous WBC has been shown to be safe and effective when applied on appropriate chronic wounds in appropriate patients, as well as on wounds that had failed to heal using NPWT.[22] Previous studies have also shown that the duration of treatment with autologous WBC is shorter and the complete healing rate higher, and that it takes fewer days to achieve complete wound closure, compared with reports from NPWT studies in other contexts.[21,32,33,40,41] The shorter treatment duration is an added cost-saving benefit, as it would reduce the costs of repeatedly implementing treatments that are not as effective in completely healing hard-to-heal wounds. In addition, effectively treating hard-to-heal wounds may alleviate the pressure on resource-poor health facilities that already struggle to provide adequate care.[22]
Social considerations
It is not simply the financial costs of hard-to-heal wound care that may be burdensome to patients. Over and above these costs, some key considerations need to be highlighted when comparing the two treatment options of autologous WBC and NPWT in the SA context (Table 4). High unemployment, poor access to healthcare, lack of and cost of transportation, uncertain access to electricity and frequent interruptions in its supply, and high rates of inequality and poverty are some of the key challenges facing South Africans.[42]
Sustainable and reliable access to electricity is persistently an issue in SA. In 2018, 84.7% of homes were linked to the country's main power supply, although black African-headed families had proportionally lower levels of access to electricity than white, Indian and coloured South Africans.[43] In recent years, SA has experienced constant load shedding.[43] Meanwhile, Eskom (the national electricity provider) charges have grown by 300% in the past decade.[44] For healthcare facilities and individuals who can afford to connect to the main grid, electricity delivery may also be inconsistent owing to insufficient capacity, equipment breakdowns and other difficulties.[43] This is pertinent to the use of NPWT, for which electricity is needed. Although a battery-operated NPWT exists, availability in SA is limited and this option is often not funded by private providers. In the SA context, NPWT therefore requires reliable access to electricity in order to use the negative-pressure source,[45,46] and unreliable access to electricity is a potential barrier to accessing treatment. Autologous WBC treatment does not require electricity to be effective,[9] which is a benefit for SA patients.
Transportation issues increase the likelihood of missed appointments and delayed care, leading to inadequate management of hard-to-heal wounds and resulting in negative health outcomes.[47] Although patients need to travel to receive both autologous WBC treatment and NPWT, the higher number of visits required for NPWT may increase the likelihood of missed appointments given transport challenges. Moreover, autologous WBC is a point-of-care treatment, and can be done at the patients home if necessary. Studies have shown that the high number of dressing changes necessitated by NPWT increases risk of infection.[48,49] Autologous WBC treatment requires one visit every 7 days, depending on the level of exudate secretion, compared with two to three times a week for NPWT. The less frequent visits required for autologous WBC treatment could decrease both the need for transportation and financial stress. However, further primary research is required in this area in the SA context.
Study limitations
The article by Blume et al.[28] was published in 2008, and the study population was resident in North America. However, after review and assessment of various published articles, this article was found to be the most relevant and recent article available to assess healing rates for NPWT. Furthermore, the sample size in the study by Naude et al.[22] was small; however, this was the only study conducted in SA that assessed healing rates for autologous WBC treatment.
Conclusion
Sustainable and cost-effective solutions to hard-to-heal wound care are essential. It is critical to ensure access to advanced treatments as soon as possible for South Africans with such wounds. An experienced and trained wound care specialist should choose which treatment is best for the patient, while considering cost implications and efficacy, as well as personal and individual considerations. In SA these include access to reliable transport and the cost thereof, and access to and reliability of the electricity supply. Although both NPWT and autologous WBC are safe and effective in treating hard-to-heal wounds, autologous WBC shows superiority in wound closure rates compared with NPWT Moreover, in terms of cost-effectiveness and several of the social considerations reviewed, the benefit of autologous WBC over NPWT is clear. Further primary research is needed, with clear endpoints when comparing different modalities in the treatment of hard-to-heal wounds, as well as to assess critical patient factors and with a larger sample size in the SA context.
Declaration. None.
Acknowledgements. None.
Author contributions. All authors contributed to the conceptualisation and writing of the article, and to analysis of its content.
Funding. None.
Conflicts of interest. None.
References
1. Aitken SJ, Choy OS, Monaro S. A qualitative study exploring patient concerns and values in chronic limb-threatening ischemia. J Surg Res 2019;243:289-300. https://doi.org/10.1016/j.jss.2019.05.055 [ Links ]
2. Boersema GC, Smart H, Giaquinto-Cilliers MG, et al. Management of nonhealable and maintenance wounds: A systematic integrative review and referral pathway. Adv Skin Wound Care 2021;34(1):11-22, https://doi.org/10.1097/01.ASW.0000722740.93179.9f [ Links ]
3. Woo K, Santos VD, Alam T. Optimising quality of life for people with non-healing wounds. Wounds Int 2018;9(3):6-14. [ Links ]
4. Sibbald RG, Elliott JA, Ayello EA, Somayaji R. Optimizing the moisture management tightrope with Wound Bed Preparation 2015. Adv Skin Wound Care 2015;28(10):466-476. https://doi.org/10.1097/01.ASW.0000470851.27030.98 [ Links ]
5. Woo KY Krasner DL, Kennedy B, Wardle D, Moir O. Palliative wound care management strategies for palliative patients and their circles of care. Adv Skin Wound Care 2015;28(3):130-140. https://doi,org/10.1097/01.ASW.0000461116.13218.43 [ Links ]
6. Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev 2019;146:209-239. https://doi.Org/10.1016/j.addr.2018.12.014 [ Links ]
7. Wang Y Armato U, Wu J. Targeting tunable physical properties of materials for chronic wound care. Front Bio engineering Biotechnol 2020;8:584. https://doi.org/10.3389/fbioe.2020.00584 [ Links ]
8. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci 2016;17(12):2085. https://doi.org/10.3390/ijmsl7122085 [ Links ]
9. Snyder RJ, Ead K. A comparative analysis of the cost effectiveness of five advanced skin substitutes in the treatment of foot ulcers in patients with diabetes. Ann Rev Res 2020;6(1):555678. https://doi.org/10.19080/ARR.2020.06.555678 [ Links ]
10. Olsson M, Jarbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen 2019;27(1):114-125. https://doi.org/10.1111/wrr.12683 [ Links ]
11. Gupta S, Sagar S, Maheshwari G, Kisaka T, Tripathi S. Chronic wounds: Magnitude, socioeconomic burden and consequences. Wounds Asia 2021;4:8-14. [ Links ]
12. Salome GM, de Almeida SA,de Jesus Pereira MT, et al. The impact of venous leg ulcers on body image andself-esteem. Adv Skin Wound Care 2016;29(7) :316-321. https://doi.org/10.1097/01.ASW.0000484243.32091.0c [ Links ]
13. Upton D, Upton P. Psychology of Wounds and Wound Care in Clinical Practice. Switzerland: Springer International Publishing, 2015. [ Links ]
14. Platsidaki E, Kouris A, Christodoulou C Psychosocial aspects in patients with chronic leg ulcers. Wounds 2017;29(10):306-310. https://doi.org/10.25270/wnds/2017.10.306310 [ Links ]
15. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4(9):560-582. https://doi.org/doi:10.1089/wound.2015.0635 [ Links ]
16. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17(6):763-771. https://doi.org/10.1111/j.1524-475X.2009.00543.x [ Links ]
17. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020;10(12):e045253. https://doi.org/10.1136/bmjopen-2020-045253 [ Links ]
18. Sen CK. Human wounds and its burden: An updated compendium of estimates. Adv Wound Care 2019;8(2) :39-48. https://doi.org/10.1089/wound.2019.0946 [ Links ]
19. Jarbrink K, Ni G, Sonnergren H, et al. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst Rev 2017;6:15. https://doi.org/10.1186/sl3643-016-0400-8 [ Links ]
20. Shankaran V, Brooks M, Mostow E. Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol Ther2013;26(3):215-221. https://doi.org/10.llll/dth.12050 [ Links ]
21. Snyder RJ, Kasper MA, Patel K, et al. Safety and efficacy of an autologous blood clot product in the management of Texas 1A or 2 A neuropathic diabetic foot ulcers: A prospective, multicenter, open label pilot study. Wounds 2018;30(7):84-89. [ Links ]
22. Naude L, Idensohn P, Bruwer F, et al. An observational pilot study to collect safety and efficacy data on wound care using whole blood clot technology on hard-to-heal wounds. Wounds Int J 2021;12(2):42-53. [ Links ]
23. RedDress. Summary. 14 May 2018. https://www.fda.gov/media/113744/download (accessed 18 January 2022). [ Links ]
24. LimaRV, Coltro PS, Farina JA. Negative pressure therapy for the treatment of complex wounds. Rev Col Bras Cir 2017;44(l):81-93. https://doi.org/10.1590/0100-69912017001001 [ Links ]
25. Othman D. Negative pressure wound therapy literature review of efficacy, cost effectiveness, and impact on patients' quality of life in chronic wound management and its implementation in the United Kingdom. Plast Surg Int 2012;2012:374398. https://doi.org/10.1155/2012/374398 [ Links ]
26. Priyatham K, Rao YP, Satyanavamani G, Poornima D. Comparison of vacuum assisted closure vs conventional moist dressing in the management of chronic wounds. IOSR J Dent Med Sci 2016;15(2):35-49. https://doi.org/10.9790/0853-15273549 [ Links ]
27. Siddha LV, Shetty SK, Varghese T. Efficacy of modified vacuum assisted closure in wound healing. Int J Sci Study 2015;2(11):52-59. https://doi.org/10.17354/ijss/2015/52 [ Links ]
28. Armstrong DG, Lavery LA; Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet 2005;366(9498):1704-1710. https://doi.0rg/10.1016/S0140-6736(05)67695-7 [ Links ]
29. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomised controlled trial. Diabetes Care 2008;31(4):631-636. https://doi.org/10.2337/dc07-2196 [ Links ]
30. Seidel D, Storck M, Lawall H, et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: Results of the German DiaFu-RCT. BMJ Open 2020;10(3):e026345. https://doi.org/10.1136/bmjopen-2018-026345 [ Links ]
31. Alipour V, Rezapour A, Ebrahimi M, Arabloo J. Cost-utility analysis of negative pressure wound therapy compared with traditional wound care in the treatment of diabetic foot ulcers in Iran. Wounds 2021;33(2):50-56. [ Links ]
32. Al-Mallah A, Al-Sayed A, Bayoumi A. Negative pressure wound therapy versus conventional dressing in treatment of diabetic foot wound. Egypt J Hosp Med 2018;72(3):4054-4059. https://doi.org/10.21608/ejhm.2018.9115 [ Links ]
33. Sajid MT, Mustafa QuA, Shaheen N, Hussain SM, Shukr I, Ahmed M. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers. J Coll Physicians SurgPak 2015;25(11):789-793. [ Links ]
34. Upton D, Andrews A. Pain and trauma in negative pressure wound therapy: A review. Int Wound J 2015;12(1):100-105. https://doi.org/10.1111/iwj.12059 [ Links ]
35. Kim YH, Hwang KT, Kim JT, Kim SW. What is the ideal interval between dressing changes during negative pressure wound therapy for open traumatic fractures? J Wound Care 2015;24(11):536-542. https://doi.org/10.12968/jowc.2015.24.11.536 [ Links ]
36. Searle R, Milne J. Tools to compare the cost of NPWT with advanced wound care: An aid to clinical decision-making. Wounds UK 2010;6(1):106-109 [ Links ]
37. Vaidhya N, Panchal A, Anchalia MM. A new cost-effective method of NPWT in diabetic foot wound. Indian J Surg 2015;77(2):525-529. https://doi.org/10.1007/sl2262-013-0907-3 [ Links ]
38. Liu S, He CZ, Cai YT, et al. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: Systematic review and meta-analysis. Ther Clin Risk Manag 2017;13:533-544. https://doi.org/10.2147/TCRM.S131193 [ Links ]
39. McCall B. Huge burden of foot ulcers doubles diabetes costs in US. Medscape Medical News, 13 March 2014, https://www.medscape.com/viewarticle/821908 (accessed 23 January 2022). [ Links ]
40. Kushnir I, Kushnir A, Serena TE, Garfinkel D. Efficacy and safety of a novel autologous wound matrix in the management of complicated, chronic wounds: A pilot study. Wounds 2016;28(9):317-327. [ Links ]
41. Mohseni S, Aalaa M, Atlasi R, Mohajeri Tehrani MR, Sanjari M, Amini MR. The effectiveness of negative pressure wound therapy as a novel management of diabetic foot ulcers: An overview of systematic reviews, J Diabetes Metab Disord 2019;18(2):625-641. https://doi.org/10.1007/s40200-019-00447-6 [ Links ]
42. World Bank Group. Overcoming poverty and inequality in South Africa: An assessment of drivers, constraints and opportunities. 1 March 2018. https://documents.worldbankorg/en/publication/documents-reports/documentdetail/530481521735906534/overcoming-poverty-and-inequality-in-south-africa-an-assessment-of-drivers-constraints-and-opportunities (accessed 2 March 2022). [ Links ]
43. Chingwete A, Felton J, Logan C Prerequisite for progress: Accessible, reliable power still in short supply across Africa. Afro barometer, 5 December 2019. https://afrobarometer.org/sites/default/files/publications/Dispatches/ab_r7_dipstachno334_papll_reliable_electricity_still_out_of_reach_for_most_africans.pdf (accessed 2 March 2022). [ Links ]
44. Burton J, Caetano T, McCall B. Coal transitions in South Africa: Understanding the implications of a 2°C-compatible coal phase-out for South Africa. IDDRI & Climate Strategies, 2018. https://www.iddri.org/sites/default/files/PDF/Publications/Catalogue%20Iddri/Rapport/20180609_ReportCoal_SouthAfrica.pdf (accessed 2 March 2022). [ Links ]
45. Novak A, Khan WS, Palmer J. The evidence-based principles of negative pressure wound therapy in trauma & orthopedics. Open Orthop J 2014;8:168-177. https://doi.org/10.2174/1874325001408010168 [ Links ]
46. McLaren ZM, Ardington C, Leibbrandt M. Distance decay and persistent health care disparities in South Africa. BMC Health Serv Res 2014;14(1):1-9. https://doi.org/10.1186/sl2913-014-0541-l [ Links ]
47. Syed ST, Gerber BS, Sharp LK. Traveling towards disease: Transportation barriers to health care access J Comm Health 2013;38(5):976-993. https://doi.org/10.1007/sl0900-013-9681-l [ Links ]
48. Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatol 2017;6(1). https://doi.Org/10.5314/wjd.v6.il.1 [ Links ]
49. CitakM, Backhaus M, Meindl R, Muhr G, Fehmer T. Rare complication after VAC-therapy in the treatment of deep sore ulcers in a paraplegic patient. Arch Orthop Trauma Surg 2010;130(12) :1511-1514. https://doi.org/10.1007/s00402-010-1091-6 [ Links ]
 Correspondence:
Correspondence:
L Naude
liezl@eloquent.co.za
Accepted 2 June 2022














