Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 n.10 Pretoria Oct. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i10.16427
RESEARCH
Training, guideline access and knowledge of antiretroviral interactions: Is the South African private sector being left behind?
B S ChisholmI; A M SwartII; M BlockmanIII
IBPharm, Dip HIV TB Man; National HIV and TB Healthcare Worker Hotline, Medicines Information Centre, Division of Clinical Pharmacology, Faculty of Health Sciences., Department of Medicine, University of Cape Town, South Africa
IIBSc (Pharm); National HIV and TB Healthcare Worker Hotline, Medicines Information Centre, Division of Clinical Pharmacology, Faculty of Health Sciences., Department of Medicine, University of Cape Town, South Africa
IIIMB ChB, MMed (Clin Pharmacol); Division of Clinical Pharmacology, Faculty of Health Sciences, Department of Medicine, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: South Africa (SA) has the largest antiretroviral therapy programme in the world. While the majority of the country accesses healthcare in the public sector, 15.2% access private healthcare. In 2019, dolutegravir was introduced as first-line treatment for HIV. Dolutegravir has clinically significant interactions with numerous commonly used medicines, e.g. rifampicin and cation-containing medicines such as calcium and iron. They require dosage adjustments, detailed in public and private HIV guidelines
OBJECTIVES: To describe SA healthcare workers' guideline access, training and knowledge of dolutegravir's interactions, focusing on differences between the public and private sectors
METHODS: A cross-sectional, descriptive study was done using an online survey of healthcare workers in the field of HIV in SA, conducted by the National HIV and TB Healthcare Worker Hotline. Convenience sampling was used, with electronic dissemination to users of the hotline and by relevant HIV-focused organisations. Simple descriptive statistics and statistical analyses were used
RESULTS: A total of 1 939 surveys were analysed, with 22% from the private sector. Training on the dolutegravir guidelines was received by significantly fewer healthcare workers in the private sector v. the public sector: 42.4% (95% confidence interval (CI) 37 - 48) v. 67.5% (95% CI I 65 - 70), respectively. Significantly fewer healthcare workers in the private sector had access to the guidelines (63.8%; 95% CI 59 - 69 v. 78.8%; 95% CI 77 - 81). When asked if they were aware that dolutegravir has interactions, just over half (56.9%) of healthcare workers in the private sector responded 'yes', 24.6% responded 'no' and 18.5% did not answer. Of those who were aware that dolutegravir has interactions, 48.9% knew that dolutegravir interacts with calcium, 44.6% with iron and 82.0% with rifampicin. Private sector knowledge of dosing changes was lower for all interacting drugs, with the difference only significant for calcium and iron. Private sector healthcare workers reported significantly lower levels of counselling on dolutegravir use in all appropriate situations
CONCLUSION: Private sector healthcare worker access to HIV training and guidelines requires attention. In a high-burden HIV setting such as SA, it is vital that healthcare workers across all professions, in both the public and private sector, know how to adjust antiretroviral dosing due to clinically significant interactions. Without these adjustments, there is a risk of treatment failure, increased mother-to-child transmission and morbidity and mortality
With an estimated 8.2 million people living with HIV in South Africa (SA) in 2021, and an estimated adult (15 - 49 years old) population prevalence of 19.5%, SA has the largest antiretroviral therapy (ART) programme in the world.[1] In terms of the Joint United Nations Programme on HIV and AIDS (UNAIDS) 90-90-90 targets, 92% of South Africans knew their status, 72% were on ART and 66% were virally suppressed in 2020.[2]
The majority of South Africans receive healthcare in the public sector. However, 9 million people (15.2%) were covered by a medical aid scheme (private sector) in 2020.[3] According to the Council for Medical Schemes 2020/21 annual report, 47.7/1 000 members were registered on an HIV management programme.[4] Govender et al'.s[5] interview-based study of patients receiving care at private and public facilities in SA found that 48% usually visited public facilities, 32% private and 20% both. With the high HIV prevalence in SA, it is vital that healthcare workers (HCWs) in both the public and private sector have up-to-date ART knowledge.
In line with World Health Organization recommendations,[6] SA adopted dolutegravir-based ART as the preferred first line at the end of 2019.P[7,8] Dolutegravir is preferred over efavirenz-based ART because of its safety, efficacy, high barrier to resistance and smaller propensity for drug-drug interactions (DDIs).[9,10] While these characteristics make dolutegravir preferable, the drug does have some clinically important interactions with other commonly used drugs, including cation-containing drugs such as calcium and iron, metformin, rifampicin and some anti-epileptic drugs (AEDs).[11-17] These require changes in dosing and/or dosing regimen, detailed in both public and private sector guidelines (Table 1). Non-adherence to the recommended adjustments may cause HIV-1 resistance, treatment failure and increased HIV transmission.
While information on DDIs, and the steps to take to mitigate them, is freely available, data on HCW knowledge of ART DDIs are sparse, especially in the SA setting and among all HCWs involved in HIV care. Two small international studies of HCWs' knowledge of ART DDIs showed low levels of knowledge: a UK-based hospital study showed that only 36% of clinically relevant interactions were identified by physicians,[18] and a US-based survey using 10 case-based ART questions, including 4 on DDIs, showed that a mean of 33% of residents, 37% of attending physicians and 93% of infectious disease specialists correctly answered the questions.[19] One study in an African setting found that 43% of patients on ART included in a prospective observational study at a Tanzanian chronic disease clinic had one or more clinically relevant DDIs that were not recognised or incorrectly managed by the treating physician.[20]
An online survey to describe SA HCWs' knowledge of dolutegravir's interactions, guideline access and training, and the variables associated with gaps in knowledge was conducted. The full study has been reported on previously,[21] and this article focuses on gaps in the private sector
Methods
Study design
A cross-sectional, descriptive study using an online survey of HCWs in the field of HIV in SA (appendix: https://www.samedical.org/file/1878) ran for 8 weeks in August and September 2020. Full details of the survey design have been previously published.[21]
The study was conducted by the National HIV and TB Healthcare Worker Hotline, based at the Medicines Information Centre, in the Division of Clinical Pharmacology at the University of Cape Town. The hotline has been running since 2008 and is toll-free. Specially trained pharmacists answer around 500 HIV- and tuberculosis (TB)-related clinical queries a month from HCWs across SA, mainly telephonically but also via email and WhatsApp.
Study setting and participants
SA HCWs (i.e. doctors, nurses, pharmacists, community HCWs or other HCWs) were invited to participate, targeting those working in the field of HIV, with dissemination via email, SMS and social media to users of the hotline and by relevant HIV-focused organisations (convenience sampling). The survey was in English, and therefore required participants to be able to read English, and was designed to exclude respondents who did not fit within the inclusion criteria, i.e. those who stated they were from outside of SA or not involved in HIV care.
Statistical analysis
Simple descriptive statistics (frequencies, median, interquartile range (IQR)), were calculated in Excel (Microsoft, USA), and statistical analyses were performed using Stata software (StataCorp., USA). Branching logic was used in the survey - for example, only those who responded that they were aware that dolutegravir has interactions saw the question on which drugs interact. The denominator used was the total number of responses to each question. Blanks (where a respondent did not answer that question) were included, where appropriate, in the descriptive statistics, and excluded in the inferential analyses. A full description of the analyses has been published in the primary article.[21]
Ethical considerations
Research ethics approval was obtained from the University of Cape Town's Human Research Ethics Committee (ref. no. 357/2020). The survey was conducted anonymously, and an opt-in draw for a prize hamper was offered at the end. Personal details entered for the draw were collected and stored independently from survey answers.
Results
Due to the online nature of the survey, response rates could not be calculated, but 1 950 analysable surveys were submitted. Of these, 11 did not specify their sector, so were excluded from the analysis, leaving 1 939 surveys analysed: 427 respondents were from the private sector (22%). Demographics are detailed in Table 2, and provincial distribution in Fig. 1.


Training on the dolutegravir guidelines had been received by significantly fewer HCWs in the field of HIV in the private sector: 42.4% (95% confidence interval (CI) 37 - 48) compared with 67.5% (95% CI 65 - 70) in the public sector. Most training in both sectors included training on dolutegravir. Training was desired across the board, regardless of whether any had been received before (Table 3).

When asked about the preferred format of training, HCWs in the private sector were significantly more likely than their public sector colleagues to prefer computer-based online training (61.0; 95% CI 55 - 67 v. 44.9%; 95% CI 42 - 48), and less likely to prefer cellphone-based online training (31.5%; 95% CI 26 - 37 v. 40.8; 95% CI 38 - 44) (Table 3).
Less than two-thirds of HCWs in the private sector had access to HIV guidelines, significantly fewer than those in the public sector (63.8%; 95% CI 59 - 69 v. 78.8%; 95% CI 77 - 81). Private sector HCWs were more likely than those in the public sector to access online ART guidelines (79.2%; 95% CI 74 - 84 v. 58.5%; 95% CI 56 - 62) and less likely to have access to hard copy ART guidelines (34.3%; 95% CI 28 - 41 v. 61.2%; 95% CI 58 - 64). Less than half of HCWs in both the private and public sectors reported having access to prevention of mother-to-child transmission guidelines (both online and hard copy). Access to apps was similarly low in both sectors, at -15% (Table 4).

When asked if they were aware that dolutegravir has interactions, just over half (56.9%) of HCWs in the private sector responded 'yes', 24.6% responded no' and 18.5% did not answer. Looking by profession, just under half of private sector nurses (49.0%) answered 'yes', 30.6% 'no' and 20.4% left this question blank, compared with 72.5%, 11.2% and 16.4% of those practising in the public sector, respectively. Two-thirds of doctors and three-quarters of pharmacists answered 'yes', compared with >80% of their public sector colleagues (Table 5).
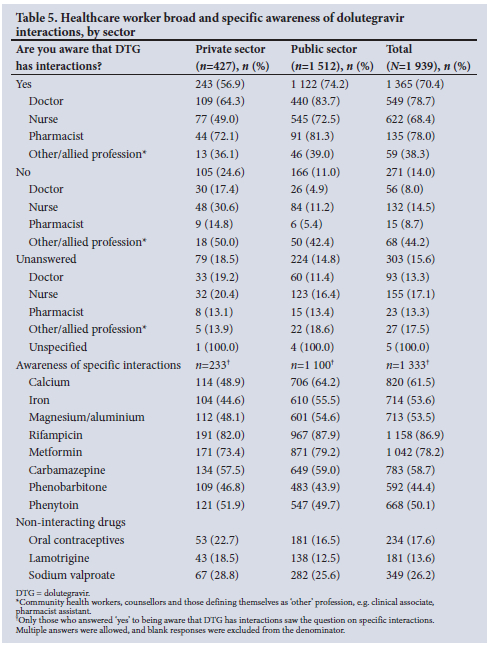
Of the private sector HCWs who were aware that dolutegravir has interactions, less than half knew that dolutegravir interacts with calcium (48.9%), iron (44.6%) and magnesium/aluminium (48.1%). Over 80% knew there is an interaction with rifampicin, and 73.4% knew it interacts with metformin (Table 5).
When asked about interactions with AEDs, 57.5% were aware of the interaction with carbamazepine, 46.8% with phenobarbitone and 51.9% with phenytoin. With AEDs that do not interact with dolutegravir, 18.5% thought that there are interactions with lamotrigine and 28.8% with sodium valproate (Table 5).
Only the respondents who marked that they were aware of each interaction saw the question on dosing/dosage adjustments needed when using those drugs with dolutegravir. Looking at knowledge of the required dosing in combination with dolutegravir (correct dosing adjustment in boxes on Fig. 2), private sector knowledge was lower for all interacting drugs, but the difference was only significant for calcium (48.9%; 95% CI 42 - 56 v. 61.3 - 67) and iron (44.6%; 95% CI 38.1-51.3 v. 55.5%; 95% CI 52.5 - 58.4).

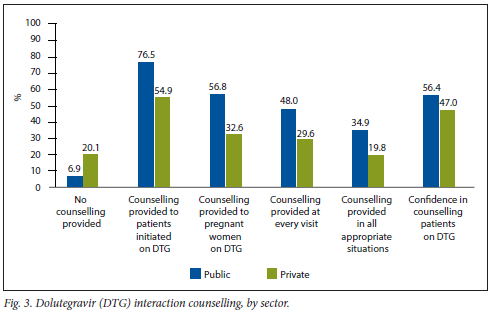
When asked about which patients were counselled on dolutegravir interactions, private sector HCWs reported significantly lower levels of counselling in all appropriate situations than public sector HCWs (19.8%: 95% CI 15 - 25 v. 34.9%; 95% CI 32 - 38), which was reflected across the board, and in their confidence in counselling (47.0%; 95% CI 43 - 50 v. 56.4%; 95% CI 55 - 58) (Fig. 3).
Discussion
To the authors' knowledge, this is the first study done in SA to quantify HCWs in the field of HIV's guideline access, training on updated guidelines and knowledge of what is in those guidelines. Our study provides sector-level insight on this. There are significant gaps in SA HCW awareness that dolutegravir has interactions, which drugs interact with it and the changes in administration needed to mitigate the effects of the interaction.
The gaps in knowledge are understandable, especially in the private sector, considering that less than half of the respondents in this sector reported having received training on dolutegravir (42.4%), and under two-thirds (63.8%) reported having easy access to HIV guidelines. This was significantly lower than those in the public sector. High computer proficiency and good internet access would be expected in the private sector, and HIV guidelines are available electronically, which begs the question: why is private sector guideline access poor? The root cause(s) of poor access are beyond the scope of this study, and importantly, require further research. A study conducted at public sector urban and rural facilities in the Western Cape Province reinforced that training, mentorship and clinical experience were associated with HIV knowledge and confidence in professional nurses.[23]
Less than half of private sector-based HCWs reported that they felt confident to counsel patients on how to take dolutegravir with interacting medicines, with only 19.8% reporting counselling their patients on dolutegravir in all appropriate situations. This highlights the tact that gaps in knowledge and a lack of guideline access and training ultimately affect patient care. While not implicitly comparable, a standardised patient study of private sector SA general practitioners showed that they did not provide optimal management of HIV in the context of TB and did not perform as well as their public sector colleagues in previous studies.[24] Our results add to this knowledge: only 64.3% of private sector doctors in our study responded that they were aware that dolutegravir has interactions. With the interaction between dolutegravir and rifampicin requiring a doubling of the dolutegravir dose, a lack of awareness will contribute to poor management and outcomes of HIV/TB-co-intected patients.
Our study s main strength was the good response - which was due to the online nature of the study, the large database of contacts collected through the hotline over 5 years and long-standing associations with HIV-based organisations. The proportion of public and private respondents also reflect the SA healthcare landscape.
Limitations of the study include the risk of both self-selection and non-response bias. Secondly, there is the potential for positive skewing because those invited are'interactive HCWs who have called the hotline or are members of relevant organisations and followers of relevant Facebook pages. Finally due to the branching nature of the survey, those who said 'no' to the question asking if they were aware of dolutegravir's interactions did not see further questions on which drugs interact, and those who did not mark interacting drugs did not see the questions on dosing - meaning that the proportions reported may overestimate knowledge.
Conclusion
Of concern is that under half of private sector-based HCWs in this study, across all professions, had received training on dolutegravir, only two-thirds had access to HIV guidelines and just over half were aware that dolutegravir has interactions. In a high-burden HIV setting such as SA, it is vital that all HCWs know how to use, and dose-adjust, dolutegravir owing to clinically significant interactions. Without these dose adjustments, there is a risk of treatment failure, increased mother-to-child transmission, and morbidity and mortality. Private sector HCW access to HIV training and guidelines and in particular, their awareness that dolutegravir has clinically significant interactions, require attention.
Declaration. None.
Acknowledgements. Chloe van Biljon and Noelle van Biljon conducted the statistical analysis of the study. We kindly acknowledge the many organisations and associations who assisted with dissemination of the survey to their members and social media audience.
Author contributions. BC, AS and MB conceived the research project. BC designed the survey with input from AS and MB. The survey was tested, piloted, disseminated and conducted by BC. Data were extracted by BC, cleaned by BC, in consultation with AS and MB, and analysed by BC and statisticians. The first draft of the manuscript was written by BC, and revised by B C, AS and MB. All authors have read and approved the final manuscript.
Funding. This research was made possible under funding provided by the Global Fund to Fight AIDS, Tuberculosis and Malaria through the SA National Department of Health (NDoH) and the NDoH Pharmacovigilance Centre for Public Health Programmes.
Conflicts of interest. None.
References
1. Statistics South Africa. Mid-year population estimates. 2021. Pretoria. StatsSA, 2021. http://www.statssa.gov.za/publications/P0302/P03022021.pdf (accessed 30 December 2021). [ Links ]
2. Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2021. Geneva. UNAIDS, 2021. https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (accessed 4 January 2022). [ Links ]
3. Statistics South Africa. General household survey. 2020. Pretoria. StatsSA, 2021. http://www.statssa.gov.za/publications/P0318/P03182020.pdf (accessed 30 December 2021). [ Links ]
4. Council for Medical Schemes. Council for Medical Schemes Annual Report 2020/21. Annexure E. Utilisation of healthcare services. Pretoria. CMS, 2021. https://www.mediealschemes.co.za/cms-annual-report-2020-21/(accessed 18 January 2022). [ Links ]
5. Govender K, Girdwood S, Letswaio D, Long L, Meyer-Rath G, Miot J. Primary healthcare seeking behaviour of low-income patients across the public and private health sectors in South Africa. BMC Public Health 2021;21;1649. https://doi.org/10.1186/sl2889-021-11678-9 [ Links ]
6. World Health Organization. WHO recommends dolutegravir as preferred HIV treatment option in all populations. Mexico City. WHO, 2019. https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations (accessed 13 September 2021). [ Links ]
7. National Department of Health, South Africa. National Consolidated Guidelines for the Management of HIV in Adults, Adolescents, Children and Infants and Prevention of Mother-to-Child Transmission. Pretoria. NDoH, 2020. https://www.knowledgehub.org.za/system/files/elibdownloads12020-07/National%20Consolidated%20Guidelines%2030062020%20signed%20PRINT%20v7.pdf (accessed 11 October 2021). [ Links ]
8. Nel J, Dlamini S, Meintjies G, et al. Southern African HIV clinicians society guidelines for antiretroviral therapy in adults. 2020 update. South Afr J HIV Med 2020;21(l):alll5. https://doi.org/10.4102/sajhivmed.v21il.1115 [ Links ]
9. Kanters S, Vitoria M, Zoratti M, et al. Comparative efficacy, tolerabiiity and safety of dolutegravir and ef avirenz 400 mg among antiretroviral therapies for first-line HIV treatment. A systematic literature review and network meta-analysis. E Clin Med 2020;28:100573. https://doi.org/10.1016/j.eclinm.2020.100573 [ Links ]
10. Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL, Delgado R. Genetic barrier to resistance for dolutegravir. AIDS Rev 2015;17( l):56-64. [ Links ]
11. Patel P, Song I, Borland J, et al. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 coadministered with acid-reducing agents and multivitamins in healthy volunteers. J Antimicrob Chemother 2011;66(7):1567-1572.https://doi.org/10.1093/jac/dkrl39 [ Links ]
12. Song I, Borland J, Arya N, Wynne B, Piscitelli S. Pharmacokinetics of dolutegravir when administered with mineral supplements in healthy adult subjects. J Clin Pharmacol 2015;55(5):490-496. https://doi.org/10.1002/jcph.439 [ Links ]
13. Song I, Zong J, Borland J, et aL The effect of dolutegravir on the pharmacokinetics of metformin in healthy subjects. J Acquir Immun Defic Syndr 2016;72(4):400-407. https://doi.org/10.1097/qai.0000000000000983 [ Links ]
14. WangX,Cerrone M, Ferretti F, et aL Pharmacokinetics of dolutegravir 100 mg once daily with rifampicin. Int J Antimicrob Agents 2019,54(2).202-206. https://doi.org/10.1016/j.ijantimicag.2019.04.009 [ Links ]
15. Dooley KE, Kaplan R, Mwelase N, et aL Dolutegravir-based antiretroviral therapy for patients coinfected with tuberculosis and human immunodeficiency virus. A muiticenter, noncomparative, open-labeL randomised trial. Clin Infect Dis 202;70(4):549-556. https://doi.org/10.1093/cid/ciz256 [ Links ]
16. Song I, Welier S, Patel J, et aL Effect of carbamazepine on dolutegravir pharmacokinetics and dosing recommendation. Eur J Clin Pharmacol 2016;72(6):665-670. https://doi.org/10.1007/s00228-016-2020-6 [ Links ]
17. Tivicay (summary of product characteristics). Amersfoort. ViiV Healthcare, 2018. https://www.ema.europa.eu/en/documents/product-information/tivicay-epar-product-information_en.pdf (accessed 31 December 2021). [ Links ]
18. Evans-Jones JG, Cottle LE, Back DJ, et al. Recognition of risk for clinically significant drug interactions among HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2010;50(10):1419-1421. https://doi.org/10.1086/652149 [ Links ]
19. Arshad S, Rothberg M, Rastegar DA, Spooner LM, Skiest D. Survey of physician knowledge regarding antiretroviral medications in hospitalised HIV-infected patients. J Int AIDS Soc 2009,12.1. https://doiorg/10.1186/1758-2652-12-1 [ Links ]
20. Kuemmerle A, Sikalengo G, Vanobberghen F, et al. Recognition and management of clinically significant drug-drug interactions between antiretrovirals and co-medications in a cohort of people living with HIV in rural Tanzania. A prospective questionnaire-based study. J Antimicrob Chemother 2021;76(10):2681-2689. https://doi.org/10.1093/jac/dkab254 [ Links ]
21. Chisholm B, Swart A, Blockman M. South African healthcare workers' knowledge of dolutegravirs drug-drug interactions in the first year of its rollout A cross-sectional online survey. J Int AIDS Soc 2022;25(3):e25885.https://doi.org/10.1002/jia2.25885 [ Links ]
22. Statistics South Africa. Mid-year population estimates. 2020. Pretoria. StatsSA, 2021. http://www.statssa.gov.za/publications/P0302/P03022020.pdf (accessed 7 June 2021). [ Links ]
23. Solomons DI, van der Merwe AS, Esterhuizen TM, Crowley T. Factors influencing the confidence and knowledge of nurses prescribing antiretroviral treatment in a rural and urban district in the Western Cape province. South Afr J HIV Med 2019;20(1):323. https://doi.org/10.4102/sajhivmed.v20il.923 [ Links ]
24. Boffa J, Moyo S, Chikovore J, et ai. Quality of care for tuberculosis and HIV in the private health sector. A cross-sectional, standardised patient study in South Africa. BMJ Glob Health 2021;6:e005250. https://doi.org/10.1136/bmjgh-2021-005250 [ Links ]
 Correspondence:
Correspondence:
B S Chisholm
briony.chisholm@uct.ac.za
Accepted 7 June 2022














