Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.8 Pretoria Ago. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i8.16480
RESEARCH
https://doi.org/10.7196/samj.2022.v112i8.16480
The tragedy of smoking, alcohol, and multiple substance use during pregnancy
L T BrinkI; P E SpringerII; D G NelIII; M D PotterIV; H J OdendaalV
IMSc; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIFCPaed (SA), PhD; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIPhD; Department of Statistics and Actuarial Science, Stellenbosch University, Stellenbosch, South Africa
IVDip General and Paediatric Nursing Science; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VFRCOG, MD; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: Antenatal substance use is a significant public health concern in South Africa (SA). Information on smoking, drinking and drug use during pregnancy was collected prospectively for the Safe Passage Study of the PASS (Prenatal Alcohol in Sudden infant death syndrome and Stillbirth) Network
OBJECTIVES: Data from 4 926 pregnant women in a community near Tygerberg Academic Hospital, Cape Town, were examined to determine whether associations between different substance use groups and postnatal infant outcomes at birth and 1 year were significant
METHODS: Gestational age (GA) was determined by earliest ultrasound. Maternal data were collected at enrolment or first antenatal visit. Substance use data were obtained at up to four occasions. Birthweight data were derived from medical records, and birthweight z-scores (BWZs) were specifically calculated using INTERGROWTH-21st study data. Statistical analyses were done with Statistica version 13
RESULTS: Women who used more substances enrolled later, were younger, and had smaller mid-upper arm circumferences (MUACs), less education and lower monthly income than women who used no substances (control group). Infants born to women who used more substances had lower GA at delivery, birthweight and BWZ than infants from the control group. At 1 year, infants born to women who used more substances had a lower weight, shorter length and smaller head circumference. Education was positively associated with all infant outcomes at birth and 1 year. MUAC was positively associated with infant BWZ, and weight and length at 1 year. Income was negatively associated with BWZ, but positively associated with all 1 -year outcomes
CONCLUSION: Substance use during pregnancy affects infant outcomes at birth and 1 year of age. The addictive properties of substance use make cessation difficult, so prevention strategies should be implemented long before pregnancy. Higher maternal education, associated with better infant outcomes at birth and 1 year and acting as a countermeasure to substance use, is of paramount importance
Substance use during pregnancy is on the increase worldwide[1-4] and is a significant public health concern.[5,6] In South Africa (SA), use of multiple substances during pregnancy is common. In a survey of 5 232 pregnant women visiting midwife obstetric units in Cape Town, it was found that 36.9% used alcohol and drugs, 34.9% alcohol only, and 1.6% drugs only[7] Also in Cape Town, a substudy of the Safe Passage Study (SPS) of the PASS (Prenatal Alcohol in Sudden infant death syndrome and Stillbirth) Network, on the value of maternal serum alpha-fetoprotein measurements, found that 61% of pregnant women smoked, 55% drank alcohol, and 9% and 5% used marijuana and methamphetamine, respectively[8] Methamphetamine use in pregnancy is associated with poorer neonatal outcomes, especially decreased birthweight, head circumference and body length.[9,10] The effects of marijuana use during pregnancy are less clear, with reports ranging from no adverse effect with regard to the likelihood of prematurity or low birthweight (LBW)[11-13] to a reduction in birthweight, length and head circumference[3] and an increase in preterm births and growth restriction (GR).[14,15]
The association of marijuana use with poor perinatal outcome seems to be attributable to concomitant use of tobacco and other confounding factors.[12] Perinatal outcome is particularly susceptible to socioeconomic conditions affecting lifestyle choices and behaviour.[16] Low socioeconomic status and lower educational attainment increase the risk of smoking during pregnancy significantly[17,18] Smoking is not only associated with complications such as preterm birth, GR and stillbirth,[17,19,20] but has long-term maternal implications such as lung cancer, cardiovascular and chronic respiratory disease, oral diseases and strokes, and long-term infant implications such as respiratory problems (e.g. childhood asthma), infections, obesity, cleft lip/palate, and neurodevelopmental and behavioural problems.[21-24]
Interestingly, only the effect of cocaine on birthweight remained significant after adjusting for confounding variables.[5] It is important to note that very few pregnant women use methamphetamine or marijuana on their own; most of them also use nicotine or alcohol, or both. In a study of 12 069 pregnant women, it was found that 45% of marijuana users also smoked.[13] The same applied to users of methamphetamine, of whom 78.6%, 42.9% and 39.3% used tobacco, alcohol, and marijuana, respectively.[2]
Of all three health-compromising behaviours, smoking, alcohol consumption and recreational drug use, cigarette smoking has been most studied and strongly implicated in reduced fetal growth.[25] Our previous finding that significantly more pregnant smokers than pregnant non-smokers engaged in heavy alcohol consumption[26] is supported by Okah et al.[27] They found that pregnant smokers were seven times more likely than non-smokers to use alcohol and/or drugs, and that the rate of heavy smoking and moderate/ heavy drinking increased with the number of health-compromising behaviours. Infants antenatally exposed to both alcohol and cigarettes had a substantially higher risk of sudden infant death syndrome compared with those who were unexposed, or exposed to alcohol or cigarettes alone. [28]
As the information on smoking, drinking and drug use for the SPS was collected prospectively, this database was ideal to examine the interactions of substance use during pregnancy on infant outcome.[29]
Methods
The SPS was designed to investigate the role of prenatal alcohol exposure in the outcome of 12 000 pregnancies in SA (Cape Town) and the USA (Northern Plains). Women recruited included those with low- and high-risk pregnancies, with a wide range of exposures to alcohol, nicotine, marijuana and methamphetamine.[29] The present study was limited to the SA arm of the SPS, where participants were recruited at a community health centre close to Tygerberg Academic Hospital (TAH), Cape Town. Participants were enrolled between August 2007 and January 2015 and infants were followed up until the end of August 2016. Gestational age (GA) was determined by earliest ultrasound before the second antenatal visit. Depending on the GA at enrolment, women had up to three further antenatal visits at TAH, at 20 - 24, 28 - 32 and 34 - 38 weeks. The revised Timeline Followback method was used at up to four occasions to obtain detailed information on drinking, cigarette smoking, and the use of marijuana, amphetamines and other substances during pregnancy[30] Anaemia was based on laboratory results of a haemoglobin value <11 g/dL during pregnancy and obtained from medical chart abstraction (MCA). Demographic and anthropometric information was obtained at enrolment or the first antenatal visit. Maternal weight was measured twice, using a regularly calibrated high-quality scale. For the mid-upper arm circumference (MUAC), the midpoint of the upper arm was first determined and then the circumference measured twice. If any two measurements differed by >1 kg (weight) or 2 mm (MUAC), a third measurement was taken and the mean of the closest two measurements used.
A pregnancy loss or fetal death before 20 weeks, according to the US definition for the SPS, was defined as a miscarriage, whereas a non-live birth at >20 weeks was regarded as a stillbirth.[31-33] Terminations of pregnancies after 20 weeks were done for medical reasons. Death of a liveborn infant before the age of 1 year was defined as an infant death. A social worker, employed for the SPS, was available to all women for counselling if necessary or requested.
Newborns were weighed immediately after birth and the information was entered in the maternal chart, from where it was obtained by MCA after delivery. The GA at delivery, obtained from the electronic data capturing (EDC) system, together with fetal sex was used to determine birthweight z-scores (BWZs) and centiles specifically for us upon request, from the international standards of the INTERGROWTH-21st study (available for GAs from 168 to 299 days, excluding twins).[34]
The infants were seen at 1 year of age and the assessment date was adjusted for prematurity, e.g. an infant born 10 weeks (70 days) early had a required 1 -year age of birth date + 365 + 70 days to birth date + 365 + 70 + 30 days at 1-year assessment. At the beginning of our study, infants born at term were required to have an age of 365 - 30 to 365 + 30 days at their 1-year examination, but this was soon changed to between birth date + 365 days and birth date + 365 + 30 days. Infants were weighed (1YW), and their length (1YL) and head circumference (1YHC) were measured by trained research workers according to a specific protocol. For weighing the infants, a Charder digital baby scale was used (Charder Electronic Co. Ltd, Taiwan). The child, dressed in a clean, dry diaper, with a vest during winter, was weighed to the nearest 0.1 kg. The process was repeated, and if the measurements differed by >0.2 kg, a third measurement was taken. A Seca 416 infantometer (Seca Deutchland, Germany) was used to measure the length to the nearest millimetre. The full procedure was repeated for a second measurement and if it differed by >2 mm, a third measurement was taken. A flexible tape measure was used to measure the head circumference to the nearest millimetre while the child was sitting on the mother's lap or lying down. The tape measure was placed over the occipital protuberance at the back of the head and around to just over the supraorbital ridge and the forehead in front. The procedure was repeated, and done a third time if the first two measurements differed by >2 mm. All the measurements were entered on a specific case report form, and later on the EDC system.
To examine the effects of various combinations of exposure to nicotine, alcohol, marijuana (Mar) and methamphetamine (Met), 11 different combinations were used, namely no exposure (Control), NoDrugsDrink, NoDrugsSmoke, NoDrugsDrinkSmoke, MarSmoke, MarDrink, MarDrinkSmoke, MetDrink, MetSmoke, MetDrinkSmoke, and All (used all four substances). Since only 12 and 2 participants used only marijuana or only methamphetamine, respectively, separate groups for these drugs were not developed and they were excluded from the cohort. Outcome variables studied were BWZ, 1YW, 1YL and 1YHC. Since we, and others, have shown that MUAC, maternal education and household income play important roles in newborn and 1-year outcomes, these were used as confounders.[35,36]
Statistical analyses were performed using the Statistica data analysis software system, version 13 (TIBCO Software Inc., USA). Descriptive statistics were used to describe continuous variables, which were compared between groups with analysis of variance (ANOVA). Bonferroni or least significant difference multiple comparisons identified significant differences between the means in the ANOVA. Non-parametric tests such as the Mann-Whitney U-test or the Kruskal-Wallis test compared differences between groups where responses were not normally distributed. Two-way ANOVAs were used to compare the influence of two factors on continuous response variables. The maximum likelihood x2 test determined significance in categorical data and was used to compare the substance use groups with the Control group. Spearman correlations measured correlations between ordinal/ continuous response variables. A p-value <0.05 indicated statistical significance. The three prespecified confounding variables were used in multiple regression analyses with 11 groups of smoking, drinking, marijuana and methamphetamine combinations for each of the four outcome variables to determine their association and the underlying effect of substance use.
Ethics approval for the study was obtained from the Health Research Ethics Committee of Stellenbosch University (ref. nos N06/10/210 and S19/07/119), as well as from the Western Cape Department of Health. Participants were able to withdraw at any time during the study.
Results
The full cohort consisted of 4 926 pregnant women, of whom 877 (17.8%) used no drugs, cigarettes or alcohol (Control), 825 (16.7%) used no drugs but drank (NoDrugsDrink), 862 (17.5%) used no drugs but smoked (NoDrugsSmoke), 1 801 (36.6%) used cigarettes and alcohol (NoDrugsDrinkSmoke), 64 (1.3%) used marijuana and cigarettes (MarSmoke), 27 (0.5%) used methamphetamine and cigarettes (MetSmoke), 20 (0.4%) used marijuana and alcohol (MarDrink), 11 (0.2%) used methamphetamine and alcohol (MetDrink), 274 (5.6%) used marijuana, alcohol and cigarettes (MarDrinkSmoke), 88 (1.8%) used methamphetamine, alcohol and cigarettes (MetDrinkSmoke), and 77 (1.6%) used all four substances (All). This equated to 65% of women who smoked, 63% of women who drank, 9% of women who used marijuana and 4% of women who used methamphetamine. Excluded from the cohort were twin pregnancies, withdrawals, participants lost to follow-up, women who used marijuana or methamphetamine alone or had missing substance use data, and multiple enrolments. Only the first enrolment of a participant was included in this cohort. Preterm birth (<37 weeks) and very preterm birth (<32 weeks) occurred in 598 (12.1%) and 85 (1.7%) women, respectively. Of the total cohort (4 926 women), 65 (1.3%) were HIV positive, 1 979 (40.2%) were anaemic, 8 (0.2%) had a miscarriage, 7 (0.1%) had a termination of pregnancy, 657 (13.3%) had LBW infants who weighed <2 500 g, 840 (17.1%) had small-for-gestational-age (SGA) infants who fell below the 10th birthweight centile, 44 (0.9%) had a stillbirth, and 45 (0.9%) had an infant death.
Information on the biometric measurements and socioeconomic conditions is provided in Table 1.
Table 2 summarises the maternal biometric measurements and socioeconomic conditions that were compared for the different substance use groups. Women in the Control group enrolled the earliest for antenatal care, had the largest MUAC and BMI, and also earned the highest mean income per month. Women in the MetSmoke group enrolled the latest, had the highest gravidity without being the oldest women, had the smallest mean MUAC, had the lowest average monthly income, and had the joint lowest education together with the MarSmoke and All groups. Women in the MarDrink group had the joint lowest gravidity and the highest education. Women in the MetDrink group were the oldest and had the joint highest gravidity. Women in the MarDrinkSmoke group were the youngest, had the joint lowest gravidity, had the lowest BMI, and were significantly the most anaemic.
Infant outcomes at birth and 1 year were compared in the different substance use groups and are summarised in Table 3. Infants from the Control group were heaviest at birth, had the largest BWZ, and were joint heaviest at 1 year. Infants from the NoDrugsSmoke group were significantly more premature, with more LBW and GR (SGA), and had more deaths compared with the Control group. Infants from the NoDrugsDrink group had the highest GA at birth and were joint heaviest at 1 year, whereas infants from the MarDrink group had the largest mean length and head circumference at 1 year. Infants from the MetDrink group had the lowest mean GA (<37 weeks) and more were premature: they had the lowest birthweight, and more were stillborn. Those alive at 1 year also had the lowest mean weight, lowest mean length and lowest mean head circumference, despite their adjusted age at 1 year. The MetSmoke group had the highest significant rate of infant deaths. Infants from the MarDrinkSmoke group had the lowest BWZ, and compared with the Control group had the highest proportion who had LBW and were SGA.
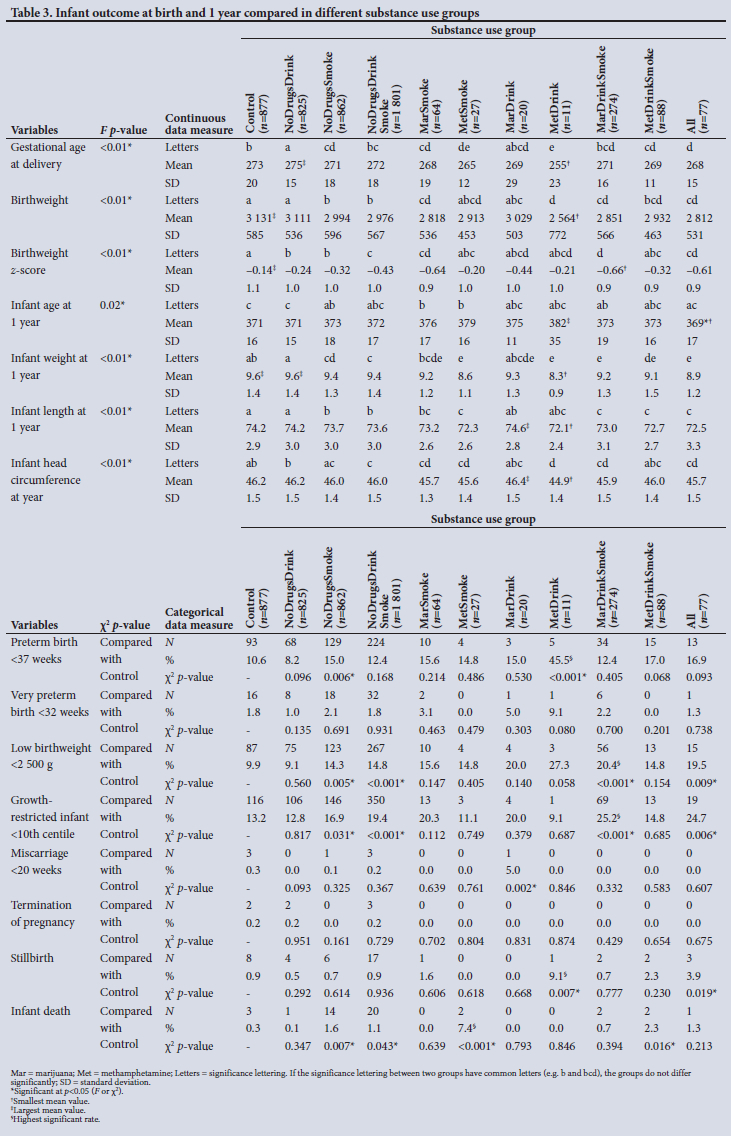
The maternal measures of GA at enrolment, age, MUAC and education as found in 11 substance use groups are presented in Figs 1, 2, 3 and 4, respectively. The birth outcomes of GA at delivery birthweight and BWZ in the different substance use groups are shown in Figs 5, 6 and 7, respectively. The 1-year visit outcomes of 1YW, 1YL and 1YHC in the different substance use groups are shown in Figs 8, 9 and 10, respectively
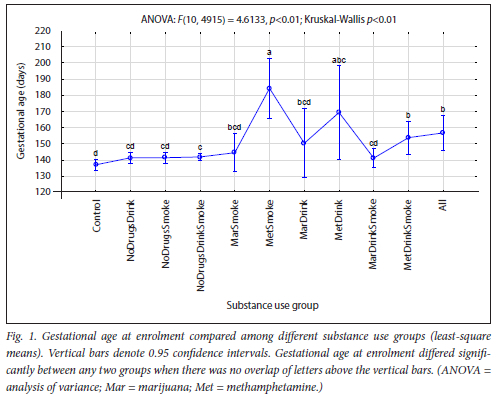
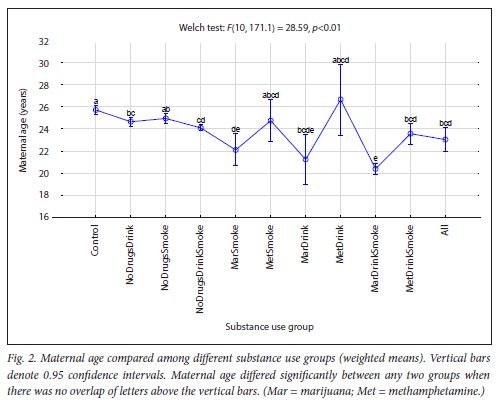
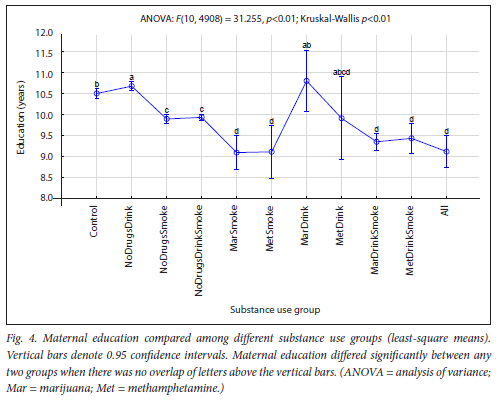
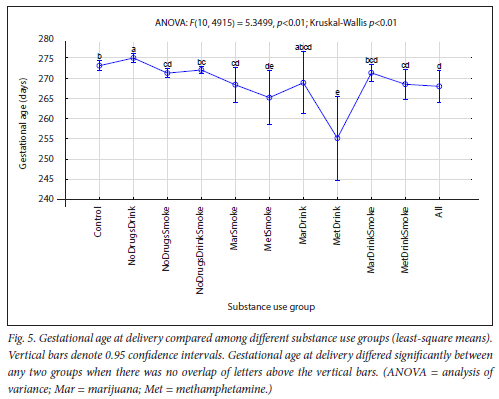
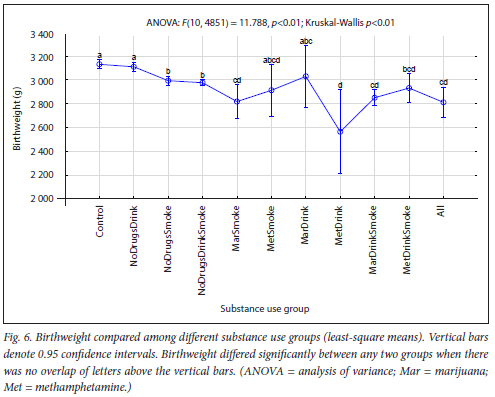
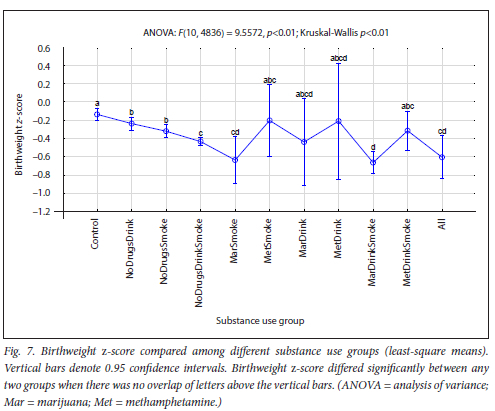
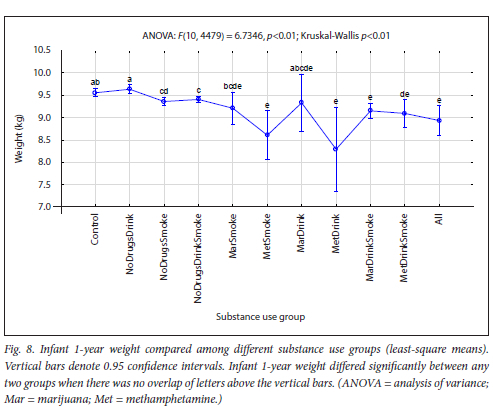
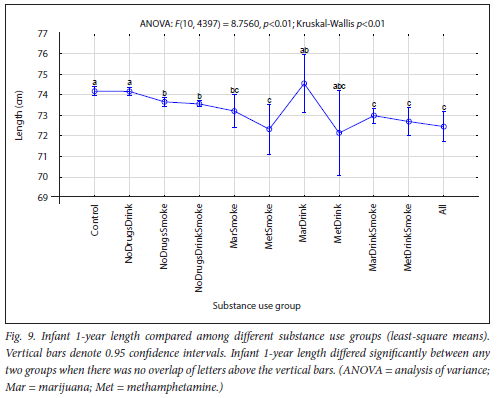
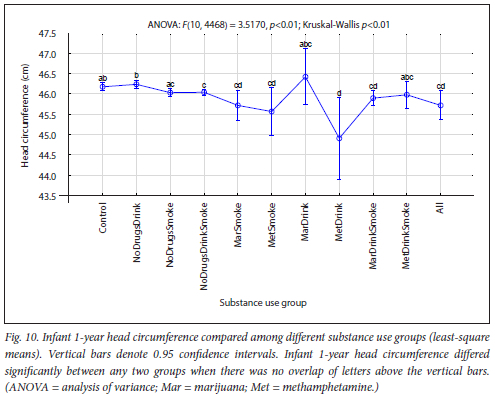
Table 4 summarises the multiple regression results for BWZ. There was a positive association between BWZ and MUAC for all the groups that did not use drugs. The strongest associations were in the Control and the NoDrugsDrink groups, which also had the largest MUACs. BWZ was positively associated with education in only two groups, NoDrugsDrink and MarDrink, and these two groups also had the highest education. BWZ was negatively associated with income in the MetDrink group only. In this group, a higher income was associated with a lower BWZ, whereas a lower income was associated with a higher BWZ.
Table 5 summarises the multiple regression results for 1YW There was a positive association between infant weight at 1 year and MUAC for all the groups that did not use drugs, apart from the All group. Mothers in the All group had 4th-lowest MUAC, that was associated with the 3rd-lowest weight at 1 year. There was also a positive association between 1-year weight of the infant and education of the mother for the Control, NoDrugsDrink NoDrugsSmoke, NoDrugsDrinkSmoke, MarDrinkSmoke and MetDrinkSmoke groups. There was a positive association between 1-year weight of the infant and income of the mother in the NoDrugsDrinkSmoke group. These mothers earned the 5th-highest income and had infants with the 3rd-largest weights at 1 year.
Table 6 summarises the multiple regression results for 1YL. A positive association between infant length at 1 year and MUAC was only found for the Control and NoDrugsSmoke groups. The Control group had the largest MUACs, which was associated with the tallest infants at 1 year, whereas the NoDrugsSmoke group had significantly smaller MUACs and significantly shorter infants at 1 year when compared with the Control group. Infant length at 1 year was also positively associated with education of mothers in the Control, NoDrugsDrink, NoDrugsSmoke, NoDrugsDrinkSmoke and MetDrinkSmoke groups. Education was highest in the Control and NoDrugsDrink groups, with the tallest infants at 1 year, and lowest in smoking plus drug use groups, and these infants were also significantly shorter at 1 year, as seen in the MetDrinkSmoke group. There was a positive association between 1YL of the infant and income of the mother for the NoDrugsSmoke and NoDrugsDrinkSmoke groups. Those who had a higher income in these groups had taller infants at 1 year.
Table 7 summarises the multiple regression results for 1YHC Infant head circumference at 1 year was not associated with MUAC, but was positively associated with maternal education for the Control, NoDrugsDrink, NoDrugsDrinkSmoke, MarDrinkSmoke and MetDrinkSmoke groups. Higher education was associated with larger head circumferences and lower education was associated with smaller head circumferences in these groups. In the NoDrugsDrinkSmoke group, 1YHC was positively associated with income. Those who had a higher income in this group also had infants with larger 1YHC.
Discussion
Maternal measures and trends
We found a significant trend in the GA at enrolment, when women booked for antenatal care, from the earliest GA in women who took no substances to a later GA in those who used all substances, but the MetSmoke and MetDrink groups enrolled even later (Fig. 1).
The finding of McCalla et al.[36] that, although recreational drug users had a wide range of social problems that compromised fetal growth and development and were in greater need of prenatal care, they were less likely to make use of antenatal care services, supports our finding.
There was also a trend in maternal age (Fig. 2), with the oldest women in the Control group to the youngest in the All group, except for the MarSmoke, MarDrink and MarDrinkSmoke groups. Women who used marijuana were the youngest. Our finding that marijuana users are young is in agreement with other researchers.[3,13,14]
The trend in MUAC (Fig. 3), from no substance users to users of all substances, was significantly smaller MUACs, but MUACs were even smaller in the MarSmoke, MetSmoke and MarDrinkSmoke groups. Our finding that women who smoked, whether combined with drugs and/or alcohol or not, had significantly smaller MUACs, has been confirmed by two previous studies.[26,35] The reduced MUAC, associated with cigarette smoking and indicating poorer nutritional status, was associated with an increased risk of spontaneous preterm birth as well as a lower infant BWZ.[26,35]
The trend in education (Fig. 4) and income (Table 2) from Control to All was lower education and lower income with more substances used. Women who smoked, in any combination, all had significantly lower education when compared with the Control group or drinkers only. Numerous studies that have reported on the association of cigarette smoking with a lower level of education[37-41] and income[39-43] support our finding. Compared with the women in the Control group, women in the NoDrugsDrink group had a higher education, and women who drank combined with marijuana or methamphetamine, but did not smoke, did not differ significantly. Women in the NoDrugsDrink, MarDrink and Control groups had the highest mean education, ranging from 10.5 to 10.8 years. This finding is validated by research by Patrick et al.[40] who reported that young adults with the highest family education and income were most prone to alcohol and marijuana use, and by Rees,[44] who found little evidence that drinking affected educational attainment.
Birth outcomes and trends
Gestation at delivery declined as the number of substances increased, although this did not apply to alcohol use alone. Compared with the Control group, GA at delivery was significantly lower for methamphetamine users and for smoking on its own or in combination with marijuana, while it was significantly higher for the NoDrugsDrink group (Fig. 5), with the highest mean GA of 39 weeks and 2 days. There was no significant difference between the Control and NoDrugsDrinkSmoke, MarDrink or MarDrinkSmoke groups. Our previous study also found that alcohol use alone was associated with a higher GA, while alcohol seemed to counteract the negative association of smoking with GA,[26] and lends support to our findings. The highest significant difference in GA was found when we compared the NoDrugsDrink group (highest GA) with the MetDrink group (lowest GA). This suggests a combined effect of methamphetamine and alcohol on GA. Not only did the MetDrink group have the most preterm births, but it also had the highest significant rate of stillbirths, despite being such a small group. Our results endorse the findings of other researchers that methamphetamine was associated with a lower GA atbirth[9,45-47] and with preterm birth.[46-48] However, according to England et al.[49] little is known about the co-use of other substances by women who drink during pregnancy. It appears that the combined effect of methamphetamine and alcohol on GA has not been reported previously. It is interesting that Sowell et al.[50] found that brain morphology was affected in children with prenatal methamphetamine and alcohol exposure above and beyond the effects of alcohol exposure alone, suggesting a synergistic effect between methamphetamine and alcohol.
The trend in birthweight from Control to All was lower birthweight with more substances used (Fig. 6). Okah et al.[27] reported that women with alcohol and/ or drug use during pregnancy did not appear to be at greater risk of giving birth to a term LBW infant than women who reported abstinence. However, the addition of smoking to either behaviour produces placental vasoconstriction that will decrease oxygen delivery to the fetus, limit fetal growth,[51] and increase the risk of LBW by 2- to 4-fold. Gibson et al.[52] found that infants born to smokers had lower birthweights and were more prone to GR. These reports support our findings of significantly more infants with LBW in the smoking groups (NoDrugsSmoke, NoDrugsDrinkSmoke, MarDrinkSmoke and All) and of the non-smoking groups (all but one) being the only groups with a mean birthweight >3 000 g (Table 3). The MetDrink group, being the exception, had the lowest mean birthweight and also the lowest mean GA at delivery (<37 weeks), with 45.5% of infants being preterm. Many researchers have found that methamphetamine was associated with lower birthweight, [10,45,53,54]and Black et al.[55] found antenatal drug use to increase the risk of LBW infants above that related to cigarette smoking. Odendaal et al.[56] and Jackson et al.[57] reported that the combined use of cigarettes and alcohol during pregnancy had a synergistic effect for LBW and GR, which also concurs with our findings.
The trend in BWZ from Control to All was lower BWZs with more substances used. The lowest BWZs were associated with marijuana and smoking, but not methamphetamine (Fig. 7). Significant GR was detected in the infants from the smoking groups (NoDrugsSmoke, NoDrugsDrinkSmoke, MarDrinkSmoke, and All), with >25% of the MarDrinkSmoke group being affected. El Marroun et al[58] reported that marijuana use during pregnancy resulted in more pronounced GR than tobacco use, while Sturrock et al.[59] also found that cigarette smoking was associated with a lower BWZ, but that women who both smoked and used marijuana during pregnancy had infants with a lower BWZ than those who used cigarettes alone. Spinillo et al.[60] reported on fetal GR among women who smoked throughout pregnancy, while Hayatbakhsh et al.,[61] after controlling for smoking, alcohol consumption and other drugs, showed that marijuana use in pregnancy was associated with SGA infants with lower BWZs. The abovementioned researchers all validate our findings.
One-year outcomes and trends
The trend in infant weight from Control to All was lower infant weight at 1 year with more substances used (Fig. 8). The lowest weights were in the methamphetamine-using groups, especially the MetDrink group, which had the lowest mean weight, with the most preterm births and infant ages adjusted for prematurity, and the MetSmoke group. In previous studies, weight and growth were reported as significantly decreased in methamphetamine-exposed children at ages 1-4 years,[54,62,63]which endorses our results.
The trend in infant length from Control to All was shorter infant length at 1 year with more substances used (Fig. 9). Smoking only, or smoking combined with drugs and/or alcohol, was associated with significantly shorter infants at 1 year. Many studies have shown a long-term negative effect of maternal smoking during pregnancy on height of infants, from birth to adolescence,[64,70] which supports our finding. Zabaneh et al,[71] Smith et al.[63] and Eriksson et al.[62] reported decreased height velocity throughout the first 3 years of life in methamphetamine-exposed children, corroborating our findings that infants from the MetDrink and MetSmoke groups, although adjusted for prematurity, had the shortest and second-shortest mean length at 1 year, respectively (Table 3).
The trend in infant head circumference from Control to All was a smaller 1YHC with more substances used. The smallest head circumferences were in the MetDrink group, despite adjustment for prematurity (Fig. 10). Other researchers have found that infants prenatally exposed to methamphetamine tended to show a significantly smaller head circumference at birth or 1 year,[54,62,72,73] supporting our findings.
Effects of combined drug use, smoking and drinking on maternal measures, birth and 1-year outcome
Many significant differences were found when the MarDrinkSmoke and MetDrinkSmoke groups, who used three substances, were compared with the Control group. Women using three substances (methamphetamine or marijuana with smoking and drinking) were younger, had a smaller MUAC, lower education and smaller income, and had infants with lower birthweight, 1 -year weight and 1 -year height than those from the Control group. These results are supported by the findings of other researchers.[2,13] Although polysubstance use in pregnancy is common,[74] there is little information available, and the full range of substance combinations and their health impacts remain incompletely understood.[75] Alcohol, tobacco and drug co-use during pregnancy is particularly problematic and compounds the adverse effects on fetal growth.[55,75,76]
Women in the methamphetamine three-substance (MetDrinkSmoke) group enrolled much later and had a lower GA at birth than Controls. They were also older than marijuana users but younger than abstainers. Smith et al[2] found that infants exposed to methamphetamine or tobacco during pregnancy were 3.5 times or 2 times more likely, respectively, to be SGA compared with unexposed infants, suggesting more GR if the infant was exposed to methamphetamine and smoking. GR together with our finding of lower GA in the MetDrinkSmoke group (17% preterm births, which was second highest after the 45.5% in the MetDrink group) supports the association of methamphetamine with preterm birth.
Women in the marijuana three-substance (MarDrinkSmoke) group were much younger (also younger than methamphetamine users), had lower gravidity, were significantly more anaemic, had infants with a lower BWZ and smaller head circumference, and had more LBW and SGA infants when compared with the
Control group. Interestingly, Chabarria et al[13] and Grzeskowiak et al.[77] reported decreased head circumference at birth to be associated with maternal marijuana use combined with smoking, or independent of tobacco use, respectively. This may help explain the association found between MarDrinkSmoke and smaller head circumference of infants at 1 year in our study. Although we agree with others that marijuana use in pregnancy is harmful to the fetus in that it was associated with low infant birthweight[3,13,77] and SGA infants,[14,78,79] our findings support those of Conner et al.[12] and Forray et al.[74] who reported that the association between maternal marijuana use and adverse outcomes appears to be attributable to comorbid substance use. Our findings are consistent with many reports of marijuana users being younger,[75] of lower parity, better educated, and more likely to use alcohol, cigarettes and hard drugs.[3,3,14] However, we found no direct association between marijuana use and spontaneous preterm birth, as others have reported.[13,14]
Confounders
Our finding that a larger MUAC, indicative of better nutritional status, was associated with a higher BWZ was supported by Smith et al.,[2] who found that lower maternal weight gain during pregnancy was more likely to result in an SGA infant. A larger MUAC was also associated with a taller, heavier infant at 1 year.
Higher education was positively associated with outcomes at birth (BWZ) and all outcomes at 1 year, resulting in a larger infant who weighed more, was taller and had a larger head circumference. Numerous researchers have reported a strong inverse relationship between education and cigarette smoking[37-41,80] and drug use.[81,82] By decreasing substance use, academic outcomes may improve, and therefore also birth and 1-year outcomes.
Higher income was associated with a lower BWZ, perhaps suggesting more methamphetamine and alcohol use while pregnant, but was also associated with a larger infant at 1 year who weighed more, was taller and had a larger head circumference.
Study strengths and limitations
The SPS was a unique, large study performed in population groups with similar socioeconomic circumstances and known to have a high incidence of antenatal substance use. A wealth of maternal, fetal and infant data were collected prospectively over a 9-year period. Substance use exposure data were collected on up to four occasions throughout pregnancy, and infant assessments were done at up to three time points throughout the first year of life. All measurements were taken twice, and we used validated recognised instruments and adjusted 1-year infant age for prematurity.
Limitations include that despite this being a large study with a high incidence of substance use, the small numbers in certain substance use groups limit the strength of the findings. Substance use was self-reported and may therefore be under-reported. Although we have detailed smoking and drinking exposure continuous data, drug information was not quantified, limiting us to nominal (yes or no) data for the various substances used.
Conclusion
The tragedy of substance use during pregnancy not only affects maternal and fetal health during pregnancy, but also infant growth and wellbeing at 1 year of age. Given that these substances are modifiable risk factors,[28] and that detailed information on the preventable adverse effects of smoking and drinking during pregnancy was not effective in the population studied,[83] it is clearly a major public health problem. The co-use of methamphetamine and alcohol (smallest group) seemed to have a confounding negative association with infant birth and 1-year outcomes, but reasons for this remain unknown. The addictive properties of substance use make cessation difficult, so prevention strategies should rather be addressed. As the prevalence of tobacco use among 13 - 15-year-old females in SA was 20% in 2002,[21] prevention strategies should be implemented long before pregnancy in order to limit the uptake of addictive substance use among young women. Higher maternal education, associated with better infant outcomes at birth and 1 year and acting as a countermeasure to substance use, is of paramount importance.
Declaration. None.
Acknowledgements. We thank the personnel of the SA arm of the SPS for their outstanding work, which included the recruitment of 7 060 pregnant women and the collection and capturing of valuable information at up to seven assessment time points per participant.
Author contributions. Concept and design: LTB, HJO; acquisition, statistical analysis or interpretation of data: LTB, PES, DGN, MDP, HJO; drafting of the manuscript: LTB, HJO; editing, revising or proofreading of the manuscript: LTB, PES, DGN, MDP, HJO.
Funding. The study was funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders (ref. nos U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991 and U01 AA016501). The funding body had no role in conducting the research or writing the article.
Conflicts of interest. None.
References
1. Kuczkowski KM. The effects of drug abuse on pregnancy Curr Opin Obstet Gynecol 2007;19(6):578-585. https://doi.org/10.1097/GCO.0b013e3282flbfl7 [ Links ]
2. Smith LM, LaGasse LL, Derauf C, et al. The infant development, environment, and lifestyle study. Effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics 2006;118(3):1149-1156. https://doi.org/10.1542/peds.2005-2564 [ Links ]
3. Fergusson DM, Horwood LJ, Northstone K. Maternal use of cannabis and pregnancy outcome. Int J Obstet Gynaecol 2002;109(1):21-27. https://doi.org/10.1111/j.l471-0528.2002.01020.x [ Links ]
4. Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol 2009;113(6):1285 1291.https://doi.org/10.1097/AOG.0b013e3181a5ec6f [ Links ]
5. Schempf AH, Strobino DM. Illicit drug use and adverse birth outcomes. Is it drugs or context? J Urban Health 2008;85(6):858-873. https://doi.org/10.1007/sll524-008-9315-6 [ Links ]
6. Godleski SA, Shisler S, Eiden RD, Huestis MA. Co-use of tobacco and marijuana during pregnancy. Pathways to externalizing behavior problems in early childhood. Neurotoxicol Teratol 2018;69:39-48. https://doi.org/10.1016/j.ntt.2018.07.003 [ Links ]
7. Petersen Williams P, Jordaan E, Mathews C, Lombard C, Parry CDH. Alcohol and other drug use during pregnancy among women attending midwife obstetric units in the Cape Metropole, South Africa. Adv Prev Med 2014;2014:871427. https://doiorg/10.1155/2014/871427 [ Links ]
8. Odendaal H, Geerts L, Nel D, Brink LT, Hitchcock E, Groenewald CA. Effects of alcohol, cigarettes, methamphetamine and marijuana exposure during pregnancy on maternal serum alpha-fetoprotein levels at 20-24 weeks' gestation. J Pediatr Neonatal Care 2018;8(1):73-82. https://doi.org/10.15406/jpnc.2018.08.00314 [ Links ]
9. Kalaitzopoulos DR, Chatzistergiou K, Amylidi AL, Kokkkinidis G, Dimitrios G. Effect of methamphetamine hydrochloride on pregnancy outcome. A systematic review and meta-analysis. J Addict Med 2018;12(3):220-226. https://doi.org/10.1097/ADM.0000000000000391 [ Links ]
10. Gabrhehk R, Skurtveit S, Nechanska B, Handal M, Mahic M, Mravcik V. Prenatal methamphetamine exposure and adverse neonatal outcomes. A nationwide cohort study. Eur Addict Res 2021;27(2):97- 106. https://doi.org/10.1159/000509048 [ Links ]
11. Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births'. Etiologic fraction attributable to prenatal drug exposure. J Perinatal 2005;25(10):631-637. https://doi.org/10.1038/sj.jp.7211378 [ Links ]
12. Conner SN, Bedell V, Lipsey K, et al. Maternal marijuana use and adverse neonatal outcomes. A systematic review and meta-analysis. Obstet Gynecol 2016;128(4):713-723. https://doi.org/10.1097/AOG.0000000000001649 [ Links ]
13. Chabarria KC, Racusin DA, Antony KM, Suter MA, Mastrobattista JM, Aagaard KM. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol 2016;215(4):506.el-506.e7. https://doi.org/10.1016/j.ajog.2016.05.044 [ Links ]
14. Luke S, Hutcheon J, Kendall T. Cannabis use in pregnancy in British Columbia and selected birth outcomes. J Obstet Gynaecol Can 2019;41(9):1311-1317. https://doi.org/10.1016/j.jogc.2018.11.014 [ Links ]
15. Metz TD, Borgelt LM. Marijuana use in pregnancy and while breastfeeding. Obstet Gynecol 2018;132(5):1198-1210. https://doi.org/10.1097/AOG.0000000000002878 [ Links ]
16. Joseph KS, Listen RM, Dodds L, Dahlgren L, Allen AC. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. Can Med Assoc J 2007;177(6):583-590. https://doi.org/10.1503/cmaj.061198 [ Links ]
17. Mund M, Louwen F, Klingelhoefer D, Gerber A. Smoking and pregnancy - a review on the first major environmental risk factor of the unborn. Int J Environ Res Public Health 2013;10(12):6485-6499. https://doi.org/10.3390/ijerphl0126485 [ Links ]
18. Chamberlain C, O'Mara-Eves A, Porter J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2017, Issue 2. Art. No.. CD001055. https://doi.org/10.1002/14651858.CD001055.pub5 [ Links ]
19. Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev 2007;83(11):713-720. https://doi.org/10.1016/j.earlhumdev.2007.08.002 [ Links ]
20. Baba S, Wikström AK, Stephansson O, Cnattingius S. Influence of smoking and snuff cessation on risk of preterm birth. Eur I Epidemiol 2012;27(4):297-304. https://doi.org/10.1007/sl0654-012-9676-8 [ Links ]
21. World Health Organization. WHO report on the global tobacco epidemic, 2008. The MPOWER package. 2008. https://apps.who.int/iris/handle/10665/43818 (accessed 11 July 2022). [ Links ]
22. Wehby GL, Prater K, McCarthy AM, Castilla EE, Murray JC. The impact of maternal smoking during pregnancy on early child neuro development. J Hum Cap 2011;5(2):207-254. https://doi.org/10.1086/660885 [ Links ]
23. Banderah G, Martelli A, Landi M, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation. A descriptive review. J Transl Med 2015;13(1):1-7. https://doi.org/10.1186/sl2967-015-0690-y [ Links ]
24. Shuffrey LC, Myers MM, Isler JR, et al. Association between prenatal exposure to alcohol and tobacco and neonatal brain activity. Results from the Safe Passage Study. JAMA Netw Open 2020;3(5):e204714. https://doi.org/10.1001/jamanetworkopen.2020.4714 [ Links ]
25. Abraham M, Alramadhan S, Iniguez C, et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017;12(2):e0170946. https://doi.org/10.1371/journal.pone.0170946 [ Links ]
26. Brink LT, Nel DG, Hall DR, Odendaal HJ. The intricate interactions between maternal smoking and drinking during pregnancy and birthweight Z-scores of preterm births. J Womens Health Care Manag 2021;2(2):10.47275/2692-0948-121.https://doi.org/10.47275/2692-0948-121 [ Links ]
27. Okah FA, Cai J, Hoff GL. Term-gestation low birth weight and health-compromising behaviors during pregnancy. Obstet Gynecol 2005;105(3):543-550. https://doi.org/10.1097/01.AOG.0000148267.23099.b7 [ Links ]
28. Elliott AJ, Kinney HC, Haynes RL, et al. Concurrent prenatal drinking and smoking increases riskfor SIDS. Safe Passage Study report. EClinMed 2020;19:100247. https://doi.org/10.1016/j.eclinm.2019.100247 [ Links ]
29. Dukes KA, Burd L, Elliott AJ, et al. The Safe Passage Studyr Design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol 2014;28(5):455-465. https://doi.org/10.1111/ppe.l2136 [ Links ]
30. Dukes K, Tripp T, Petersen J, et al. A modified Timeline Followback assessment to capture alcohol exposure in pregnant women. Application in the Safe Passage Study. Alcohol 2017;62:17-27. https://doi.org/10.1016/j.alcohoL2017.02.174 [ Links ]
31. Odendaal HJ, Elliott A, Kinney HC, et al., for the Prenatal Alcohol and SIDS and Stillbirth (PASS) Network. Consent for autopsy research for unexpected death in early life. Obstet Gynecol 2011;117(1):167-171. https://doi.org/10.1097/AOG.0b013e318200cbl7 [ Links ]
32. Odendaal H, Wright C, Brink L, Schubert P, Geldenhuys E, Groenewald C. Association of late second trimester miscarriages with placental histology and autopsy findings. Eur J Obstet Gynecol Reprod Biol 2019;243:32-35. https://doi.org/10.1016/j.ejogrb.2019.10.024 [ Links ]
33. Odendaal H, Dukes KA, Elliott AJ, et al. Association of prenatal exposure to maternal drinking and smoking with the risk of stillbirth. JAMA Netw Open 2021;4(8):e2121726. https://doi.org/10.1001/jamanetworkopen.2021.21726 [ Links ]
34. Villar J, Papageorghiou AT, Pang R, et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st project. The fetal growth longitudinal study and newborn cross-sectional study. Lancet Diabetes Endocrinol 2014;2(10):781-792. https://doi.org/10.1016/S2213-8587(14)70121-4 [ Links ]
35. Brink LT, Nel DG, Hall DR, Odendaal HJ. Association of socioeconomic status and clinical and demographic conditions with the prevalence of preterm birth. Int J Gynaecol Obstet 2020;149(3):359-369. https://doi.org/10.1002/ijgo.l3143 [ Links ]
36. McCalla S, Minkoff HL, Feldman J, Saiwin M, Valencia G, Glass L. The biologic and social consequences of perinatal cocaine use in an inner-city population. Results of an anonymous cross-sectional study. Am J Obstet Gynecol 1991;164(2):625-630. https://doi.org/10.1016/S0002-9378(11)80036-0 [ Links ]
37. Gilman SE, Martin LT, Abrams DB, et al. Educational attainment and cigarette smoking: A causal association? Int J Epidemiol 2008;37(3):615-624. https://doi.org/10.1093/ije/dym250 [ Links ]
38. Dhavan R, Melissa SH, Perry CL, Arora M, Reddy S. Is tobacco use associated with academic failure among government school students in urban India? J Sch Health 2010;80(ll):552-560. https://doi.org/10.1111/j.l746-1561.2010.00541.x [ Links ]
39. Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts. The influence of gender and education. Am J Public Health 1996;86(2):231-236.https://doLorg/10.2105/AJPH.86.2.231 [ Links ]
40. Patrick ME, Wightman P, Schoeni RF, Schulenberg JE. Socioeconomic status and substance use among young adults. A comparison across constructs and drugs. J Stud Alcohol Drugs 2012;73(5):772-782. https://doi.org/10.15288/jsad.2012.73.772 [ Links ]
41. Zhu BP, Giovino GA, Mowery PD, Eriksen MP. The relationship between cigarette smoking and education revisited. Implications for categorizing persons' educational status. Am J Public Health 1996;86(11):1582-1589. https://doi.org/10.2105/AJPH.86.11.1582 [ Links ]
42. Farmer S, Hanratty B. The relationship between subjective wellbeing, low income and substance use among schoolchildren in the north west of England. A cross-sectional study. J Public Health (Oxf) 2012;34(4):512-522. https://doi.org/10.1093/pubmed/fds022 [ Links ]
43. Addiction Center. Addiction and low-income Americans. 2022. https://www.addictioncenter.com/addiction/low-income-americans/ (accessed 15 June 2022). [ Links ]
44. Rees DI. Does substance use affect academic performance? IZA World Labor, April 2019, 1 - 10. https://doi.org/10.15185/izawol.66.v2 [ Links ]
45. Wright TE, Schuetter R, Tellei J, Sauvage L. Methamphetamines and pregnancy outcomes. J Addict Med 2015;9(2).111-117. https://doi.org/10.1097/ADM.0000000000000101 [ Links ]
46. Premchit S, Orungrote N, Prommas S, Smanchat B, Bhamarapravatana K, Suwannarurk K. Maternal and neonatal complications of methamphetamine use during pregnancy. Obstet Gynecol Int 2021;2021:8814168. https://doi.org/10.1155/2021/8814168 [ Links ]
47. Phupong V, Darojn D. Amphetamine abuse in pregnancy. The impact on obstetric outcome. Arch Gynecol Obstet 2007;276(2):167-170. https://doi.org/10.1007/s00404-007-0320-x [ Links ]
48. Gorman M, Orme K, Nguyen N, Kent J, Caughey A. 63h Outcomes in pregnancies complicated by methamphetamine use. A retrospective cohort study. Am J Obstet Gynecol 2014;210(1):S309. https://doi.org/10.1016/j.ajog.2013.10.664 [ Links ]
49. England LJ, Bennett C, Denny CH, et al. Alcohol use and co-use of other substances among pregnant females aged 12 - 44 years - United States, 2015 - 2018. MMWR Morb Mortal Wkly Rep 2020;69(31):1009-1014. https://doi.org/10.15585/mmwr.mm6931al [ Links ]
50. Sowell ER, Leow AD, Bookheimer SY, et al. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci 2010;30(11):3876-3885. https://doi.org/10.1523/JNEUROSCI.4967-09.2010 [ Links ]
51. Carter RC, Wainwright H, Molteno CD, et al. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res 2016;40(4):753-764. https://doi.org/10.1111/acer.13022 [ Links ]
52. Gibson G, Baghurst P, Colley D. Maternal alcohol, tobacco and cannabis consumption and the outcome of pregnancy. Aust N Z J Obstet Gynaecol 1983;23(1):15-19. https://doi.org/10.1111/J.1479-828x.l983.tb00151.x [ Links ]
53. Smith LM, Yonekura ML, Wallace T, Berman L, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr 2003;24(1):17-23. https://doi.org/10.1097/00004703-200302000-00006 [ Links ]
54. Wouldes TA, LaGasse LL, Huestis MA, DellaGrotta S, Dansereau LM, Laster BM. Prenatal methamphetamine exposure and neurodevelopmental outcomes in children from 1 to 3 years. Neurotoxicol Terato J 2014;42:77-84. https://doi.org/10.1016/j.ntt.2014.02.004 [ Links ]
55. Black M, Bhattacharya S, Fairley T, Campbell DM, Shetty A. Outcomes of pregnancy in women using illegal drugs and in women who smoke cigarettes. Acta Obstet Gynecol Scand 2013;92(1):47-52. https://doi.org/10.1111/j.l600-0412.2012.01519.x [ Links ]
56. Odendaai HJ, Steyn DW, Elliott A, Burd L. Combined effects of cigarette smoking and alcohol consumption on perinatal outcome. Gynecol Obstet Invest 2009;67(1):1-8. https://doi.org/10.1159/000150597 [ Links ]
57. Jackson DJ, Batiste E, Rendall-Mkosi K. Effect of smoking and alcohol use during pregnancy on the occurrence of low birthweight in a farming region in South Africa. Paediatr Perinat Epidemiol 2007;21(5):432-440.https://doi.org/10.1111/j.l365-3016.2007.00847.x [ Links ]
58. El Marroun H, Tiemeier H, Steegers EAP, Verhuist FC, van den Brink W, Huizink AC. Intrauterine cannabis exposure affects fetal growth trajectories. The Generation R Study. J Am Acad Child Adolesc Psychiatry 2009;48(12):1173-1181. https://doi.org/10.1097/CHI.0b013e3181bfa8ee [ Links ]
59. Sturrock S, Williams E, Ambulkar H, Dassios T, Greenough A. Maternal smoking and cannabis use during pregnancy and infant outcomes. J Perinat Med 2020;48(2):168-172. https://doi.org/10.1515/jpm-2019-0422 [ Links ]
60. Spinillo A, CapuzzoE, Piazzi G, Baltaro F, Iasci A, Nicola S. Effect measures for behavioral factors adversely affecting fetal growth. Am J Perinatol 1996;13(2):119-123. https://doi.org/10.1055/s-2007-994305 [ Links ]
61. Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res 2012;71(2):215-219. https://doi.org/10.1038/pr.2011.25 [ Links ]
62. Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Cross-sectional growth of children whose mothers abused amphetamines during pregnancy. Acta Paediatr 1994;83(6):612-617. https://doi.org/10.1111/j.l651-2227.1994.tbl3091.x [ Links ]
63. Smith LM, Diaz S, LaGasse LL, et al. Developmental and behavioral consequences of prenatal methamphetamine exposure. A review of the Infant Development, Environment and Lifestyle (IDEAL) Study. Neurotoxicol Teratol 2015;51:35-44. https://doi.org/10.1016/j.ntt2015.07.006 [ Links ]
64. Muraro AP, G on calves-Silva RMV, Moreira NF, et al. Effect of tobacco smoke exposure during pregnancy and preschool age on growth from birth to adolescence. A cohort study. BMC Pediatr 2014;14(1):1-9. https://doi.org/10.1186/1471-2431-14-99 [ Links ]
65. Karvonen M, Saari A, Sund R. Maternal smoking during pregnancy and offspring head growth in comparison to height and weight growth up to 6 years of age. A longitudinal study. Clin Epidemiol 2021;13(September):959-970.https://doi.org/10.2147/CLEP.S327766 [ Links ]
66. Matijasevich A, Brion MJ, Menezes AM, Barros AJD, Santos IS, Barros FC. Maternal smoking during pregnancy and offspring growth in childhood. 1993 and 2004 Pelotas cohort studies. Arch Dis Child 2011;96(6):519-525.https://doi.org/10.1136/adc.2010.191098 [ Links ]
67. Howe LD, Matijasevich A, Tilling K, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity. Comparing maternal and paternal associations. Int J Epidemiol 2012;41(3):722-732. https://doi.org/10.1093/ije/dys025 [ Links ]
68. Lcary S, Smith GD, Ness A. Smoking during pregnancy and components of stature in offspring. Am J Hum Biol 2006;18(4):602-512.https://doi.org/10.1002/ajhb.20518 [ Links ]
69. Koshy G, Delpisheh A, Brabin BJ. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health 2011;21(3):286-291. https://doi.org/10.1093/eurpub/ckql73 [ Links ]
70. Gigante DP, Horta BL, Lima RC, Barros FC, Victora CG. Early life factors are determinants of female height at age 19 years in a population-based birth cohort (Pelotas, Brazil). J Nutr 2006;136(2):473-478.https://doi.org/10.1093/jn/136.2.473 [ Links ]
71. Zabaneh R, Smith LM, Lagasse LL, et al. The effects of prenatal methamphetamine exposure on childhood growth patterns from birth to 3 years of age. Am J Perinatol 2012;29(3):203-210. https://doi.org/10.1055/s-0031-1285094 [ Links ]
72. LaGasse LL, Wouldes T, Newman E, et al. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol 2011;33(1):166-175. https://doi.org/10.1016/j.ntt.2010.06.009 [ Links ]
73. Zhang Y, Gong F, Liu P, Wang H. Effects of prenatal methamphetamine exposure on birth outcomes; brain structure, and neurodevelopmentai outcomes. Dev Neurosci 2021;43(5):271-280. https://doi.org/10.1159/000517753 [ Links ]
74. ForrayA. Substance use during pregnancy [version 1,referees. 2 approved]. F1000Research,2016,5(F100C Faculty Rev):887. https://doi.org/10.12688/F1000research.7645.1 [ Links ]
75. Qato DM, Zhang C, Gandhi AB, Simoni-Wastila L, Coleman-Cowger VH. Co-use of alcohol, tobacco, and licit and illicit controlled substances among pregnant and non-pregnant women in the United States. Findings from 2006 to 2014 National Survey on Drug Use and Health (NSDUH) data. Drug Alcohol Depend 2020;206:107729. https://doi.org/10.1016/j.drugalcdep.2019.107729 [ Links ]
76. Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children. Alcohol Res Health 2000;4(4)242-249. [ Links ]
77. Grzeskowiak LE, Grieger JA, Andraweera P, et al. The deleterious effects of cannabis during pregnancy on neonatal outcomes. Med J Aust 2020;212(11):519-524. https://doi.org/10.5694/mja2.50624 [ Links ]
78. Janisse JJ, Bailey BA, Ager J, Sokol RJ. Alcohol, tobacco, cocaine, and marijuana use. Relative contributions to preterm deli very and fetal growth restriction. Subst Abus 2014;35(1):60-67. https://doi.org/10.1080/08897077.2013.804483 [ Links ]
79. Hatch EE, Bracken MB. Effect of marijuana use in pregnancy on fetal growth. Am J Epidemiol 1986;124(6):986-993. https://doi.org/10.1093/oxfordjournals.aje.a114488 [ Links ]
80. Wagenknecht L, Perkins L, Cutter G, et al. Cigarette smoking behavior is strongly related tc educational status. The CARDIA study. Prev Med 1990;19(2):158-169. https://doi.org/10.1016/0091-7435(90)90017-e [ Links ]
81. Fothergill KE, Ensminger ME, Green KM, Crum RM, Robertson J, Juon H-S. The impact of early school behavior and educational achievement on adult drug use disorders. A prospective study Drug Alcohol Depend 2008;92(1-3):191-199. https://doi.org/10.1016/j.drugalcdep.2007.08.001 [ Links ]
82. King KM, Meehan BT, Trim RS, Chassin L. Substance use and academic outcomes. Synthesizing findings and future directions. Addiction 2006;101(12):1688-1689. https://doi.org/10.1111/j.1360-0443.2006.01695.x [ Links ]
83. Odendaai HJ, Brink LT, Nel DG, et al. Smoking and drinking habits of women in subsequent pregnancies after specific advice about the dangers of these exposures during pregnancy. S Afr Med J 2020;110(11):1100-1104. https://doi.org/10.7196/SAMJ.2020.vll0ill.14667 [ Links ]
 Correspondence:
Correspondence:
H J Odendaal
hjo@sun.ac.za
Accepted 8 April 2022














