Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 n.7 Pretoria Jul. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i7.16478
RESEARCH
Phase I of the DiaVACCS screening trial: Study design, methods, population demographics and baseline results
G DreyerI; L C SnymanII; F H van der MerweIII; K L RichterIV; G J DreyerV; C VisserVI; M H BothaVII
IMMed (O&G), PhD; Department of Obstetrics and Gynaecology and Gynaecological Oncology Unit, Faculty of Health Sciences, University of Pretoria, South Africa
IIMMed (O&G), PhD; Department of Obstetrics and Gynaecology and Gynaecological Oncology Unit, Faculty of Health Sciences, University of Pretoria, South Africa
IIIMMed (O&G), FCOG: Department of Obstetrics and Gynaecology and Unit for Gynaecological Oncology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IVFC Path (SA) Viro, MMed Path (Med Virol); Department of Medical Virology, Faculty of Health Sciences, University of Pretoria, South Africa
VBCom (Statistics and Actuarial Science), BCom Hons (Actuarial Science): Department of Statistics and Actuarial Science, Faculty of Commerce, Stellenbosch University, Cape Town, South Africa
VIBSc Hons, MSc; Department of Obstetrics and Gynaecology and Gynaecological Oncology Unit, Faculty of Health Sciences, University of Pretoria, South Africa
VIIMMed (O&G), PhD; Department of Obstetrics and Gynaecology and Unit for Gynaecological Oncology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: Human papillomavirus (HPV)-based primary screening guidelines are based on screening test performance and prevalence data generated in high-resource areas with low HIV infection rates. There is an urgent need for local data on infection and disease prevalence, as well as screening test performance, among both HIV-positive and HIV-negative South African (SA) women, in order to inform updated screening guidelines
OBJECTIVES: This study describes the baseline characteristics of participants in the cross-sectional phase of the multicentric DIAgnosis in Vaccine And Cervical Cancer Screen (DiaVACCS) screening trial. The objective was to determine the prevalence of positive screening and pre-invasive disease using different tests and strategies in the SA HIV-positive and HIV-negative population
METHODS: A total of 1 104 women aged 25 - 65 years and eligible for screening were included, 465 HIV positive and 639 HIV negative. Visual inspection and molecular and cytological screening tests were done on self-sampled and healthcare worker-collected specimens. All participants who screened positive and 49.1% of those who screened negative were invited for colposcopy and biopsy, and those qualifying for treatment were recalled for large loop excision of the transformation zone as part of the trial. The worst histology result for each participant was used, and for untested women, multiple imputation was used to estimate verification bias-adjusted histology values
RESULTS: Visual inspection was positive in 50.4% of HIV-positive v. 20.9% of HIV-negative women, cytology (atypical squamous cells of undetermined significance) in 39.9% v. 17.0%, and high-risk HPV DNA in 41.2% v. 19.6%. Overall, high-grade squamous intraepithelial lesion-positive cytology peaked in the age group 30 - 39 years at 16.7%. After adjustment for verification bias, histological diagnosis of cervical intraepithelial neoplasia (CIN)2+ was suspected in 44.7% v. 23.5% and CIN3+ in 23.3% v. 10.2% of HIV-positive and negative women, respectively. Invasive cancer was diagnosed in 15 women (1.95% of histological studies performed), and verification bias adjustment suggested 20 cases (1.8% of the study population
CONCLUSION: The baseline findings from the DiaVACCS trial confirm a high prevalence of HPV-related cervical pathology in the SA HIV-negative screening population, showing a clear need to reach these women with a screening programme. Among HIV-positive women, prevalence values were almost doubled. The prevalence of existing invasive cervical cancer was 1 - 2% of all women. Further analysis of the performance of single and multiple screening tests between the two subgroups will contribute to the choice of the most effective strategies to identify women at risk of developing invasive cancer
South Africa (SA) suffers a double epidemic of cervical cancer and HIV infection, two diseases competing for health resources and aggravating each other. Around 12 000 new cervical cancer cases are diagnosed in SA per year, with an estimated age-standardised incidence rate of 35.3 per 100 000 women.[1] It has been estimated that 23.9% of women between the ages of 15 and 49 years were HIV positive in 2021 [2] and that more than half of patients with cervical cancer in southern Africa are women living with HIV (WLWH).[3]
Infection with HIV and poor immunity influence acquisition and the clinical course of human papillomavirus (HPV)-related disease.[4] Several studies have demonstrated a significantly higher prevalence of cervical cancer, precancerous lesions and cervical HPV infection in HIV-infected women compared with HIV-uninfected women, and this effect may be increased by late initiation of antiretroviral therapy
(ART).[5] In SA the average CD4 count at initiation of ART has been reported as 350 cells/(µL, and in 2012 only 34.4% of those eligible for ART were receiving it.[6] Compared with HIV-uninfected women, HIV-infected women more often have multiple types[7] and have an altered HPV genotype distribution,[8,9] persistence or reactivation of infections are more likely,[7,10] and the average age at diagnosis is younger. Recently, however, an analysis from the SA National Cancer Registry showed an incidence rate as high as 242 cases per 100 000 person years for HIV-positive women aged >60 years.[11]
The Vaccine And Cervical Cancer Screen (VACCS) consortium is a multidisciplinary group of SA researchers focusing on clinical research aimed at implementable strategies that can improve cervical cancer control in our unique context.[12,13] This diagnostic trial, named VACCS4 or DIAgnosis in Vaccine And Cervical Cancer Screen (DiaVACCS), is a screening trial among HIV-positive and HIV-negative women in Gauteng and Western Cape provinces, the countrys two economic epicentres. Phase I, of which the baseline data are reported here, is a cross-sectional screening study with the objectives of determining the positivity rates of different screening tests and the prevalence of pre-invasive disease, and measuring the performance of several screening and triage strategies against histological end-points.
This article describes the study design and methodology demographics and HIV-related findings, and reports prevalences of abnormal cytology, high-risk HPV (hrHPV) and histologically confirmed cervical disease in the HIV subgroups. The performance of each primary screening test and of different triage strategies will be discussed in detail in further articles, and phase II will involve longitudinal follow-up of treated and untreated participants for >5 years. The protocol was approved by the faculty of health sciences research ethics committees of the University of Pretoria (ref no. 196/2014) and Stellenbosch University (reciprocal approval 2015), registered as a clinical trial (ClinicalTrials.gov identification number NCT02956031), and conducted according to the principles of the Declaration of Helsinki.
Methods
Design and setting
From December 2016 to March 2020, 1 104 women were recruited to phase I at three study sites. Site A, at Tshwane District Hospital, recruited mostly WLWH (n=423) who receive ART from this public hospital; site B, at Kalafong Provincial Tertiary Hospital, included a mixed population (n=260); and site T, at Tygerberg Academic Hospital, recruited a mixed group of 421 participants. All sites were equipped to screen, test and treat participants and had cold-storage facilities. Clinical research staff received training in naked-eye visual inspection methods, colposcopy and the treatment of pre-invasive lesions. Specialist cover was available at all times, visual inspection test results were randomly reviewed, and staff were retrained when needed.
Study population
The baseline design, recruitment and test algorithm for phase I with eligibility and exclusion criteria is shown in Fig. 1. The study aim was to enlist 1 000 women of a typical screening population, 500 women each with and without HIV infection, to allow for subgroup analysis. Sample size calculation was based on previously reported high prevalences of HPV infection and related disease. [14] By the end of recruitment, 456 women known to be or newly tested as HIV positive were enrolled. Investigators invited women from the public, outpatient clinics and ART clinics, who were eligible for screening according to national guidelines, to participate.[15] We obtained written informed consent for screening and appropriate further tests, and consent for treatment if needed. Participants did not receive any remuneration or reimbursement, but all tests and treatments were cost free.
HIV status did not influence enrolment, testing or treatment, but for subgroup analysis the status was assigned according to the participants' known or disclosed status. A rapid test was available but optional, and women who declined were assigned to the HIV-negative group according to national screening policy. Information on duration of ART use and CD4 values were collected for HIV-positive women.
Sample collection and visual inspection
The investigators used a picture to demonstrate self-sampling with the digene HC2 DNA Collection Device (Qiagen, USA) or the Evalyn Brush (Rovers Medical Devices, Netherlands). After sampling, the brush tip was broken off or removed with disposable tweezers and placed in digene Specimen Transport Medium (STM) (Qiagen, USA). The healthcare worker (HCW) performed a vaginal speculum examination to locate and visualise the cervix and collected a specimen from the transformation zone for cytological and molecular analysis, which was placed in SurePath (BD, USA).
The cervix was drenched with 3-5% acetic acid, then inspected with the naked eye for visible lesions and staining (visual inspection with acetic acid (VIA)). Final sampling for molecular analysis using a cervical broom placed in SurePath, ThinPrep Pap Test PreservCyt Solution (Hologic, USA) and/or Qiagen digene STM followed. Lugol's iodine was then applied to the visible cervix with a cotton swab and the area again inspected with a light source to evaluate the presence and pattern of abnormal iodine staining (visual inspection with Lugol's iodine (VILI)). Participants were classified for both VIA and VILI as positive, negative or uncertain, with lesions affecting two quadrants or more with abnormal staining considered positive, no lesions as negative, and small lesions or other findings as uncertain. All non-negative findings were considered positive during baseline data analysis.
Colposcopy and biopsy
Women with any abnormality reported within 6 months of recruitment on visual inspection, an HPV test or cytology were invited for colposcopy and one targeted or two blind cervical biopsies at 6° and 12°. In addition, a sample of screen-negative women was selected for colposcopy and biopsy to comprise a negative control group. Colposcopy and biopsy were planned for a second visit if the visual inspection was not positive in the initial phase of the study. Following poor attendance for this second visit, the research protocol was amended to perform colposcopy and biopsy at the initial visit for all consenting participants. Standard colposcopy examination techniques were used, and characteristics of any lesion were recorded and used to draw a diagram, estimate a clinical impression and calculate the Reid's Colposcopic Index score.[16]
LLETZ procedure
Large loop excision of the transformation zone (LLETZ) was offered as part of the trial to all women who required this treatment based on national guidelines. Information included aftercare, and written consent was obtained. The cervix was anaesthetised with 2% m/v lignocaine with adrenaline (1:80 000) using a dentistry syringe and needle. Using an appropriate electrocautery loop to enable total excision of any iodine-negative lesion in one or more fragments, and a cut-coagulation blend on the electrocautery machine, the lesion was resected from side to side or top to bottom and the specimen placed in formalin. Haemostasis was achieved with ball cautery, compound tincture of benzoin or silver nitrate.
Transport, storage and laboratory testing
All specimens were marked with a unique study number and identifier, and stored in a temperature-controlled fridge until cold-transported in batches to the designated laboratory. The initial round of tests used to describe the baseline characteristics included cytology, a cobas HPV test (Roche Diagnostics, Switzerland) and histology. Unused specimens were stored according to individual manufacturers' instructions until additional screening and triage tests could be performed, including advanced cytology, further HPV DNA and RNA tests, and methylation marker analysis. All molecular tests were performed by trained staff in accredited facilities, and reported according to the manufacturers' instructions.
We initially performed conventional cytology at sites A and B, but later in the study all sites migrated to liquid-based cervical cytology. Cytology results were reported according to the Bethesda system and classified as negative, uncertain or positive for epithelial abnormality using different cut-offs. The cobas HPV test was performed on 4 mL of the Thinprep sample. HPV DNA results were recorded as positive or negative for any of the hrHPV types in the test, HPV16 or HPV18, or invalid. All cervical biopsy samples were processed in total, and at least two sections of all LLETZ samples were examined microscopically by conventional routine. For each participant the 'worst' histological result between the biopsies or LLETZ was considered the final histological result. A single pathologist per case, blinded to other test results, performed the histological examination and reported the findings using the standard classification of cervical intraepithelial neoplasia (CIN).
Data collection and analyses
Excel 2016 (Microsoft Corp., USA) was the primary software for data recording, cleaning and analysis. Prevalence values were determined across different age groups for all screening and diagnostic tests in the total group and for the two HIV subgroups. Selective recall for colposcopy and histological verification of final diagnosis caused some verification bias, and multiple imputation (using the statistical programming language R; R Foundation for Statistical Computing, Austria) was employed to adjust for this bias.
Missing histology values were simulated based on age, HIV status, ARV use and screening results and replaced with multiple possible values rather than with a single value. The program ran several iterations of the imputation process from which the ultimate imputed values were gathered. The process of verification bias adjustment (VBA) retains the randomness one could expect from a sample balanced by maintaining relative consistency with the existing data structure. By accounting for uncertainty, the method allows calculation of standard errors and 95% confidence intervals.[17] The crude data as well as the adjusted histology results are shown, but the VBA values will be used as the best estimate of true disease prevalence and to calculate the performance of screening strategies.
Results
Population demographics
The age range was 25 - 65 years (mean 41.26, median 40) and was similar for the two HIV groups. The age distribution for the two groups differed (Fig. 2). Between ages 35 and 45 years, the study population comprised more WLWH than HIV-negative women, while all other groups had more HIV-negative women.
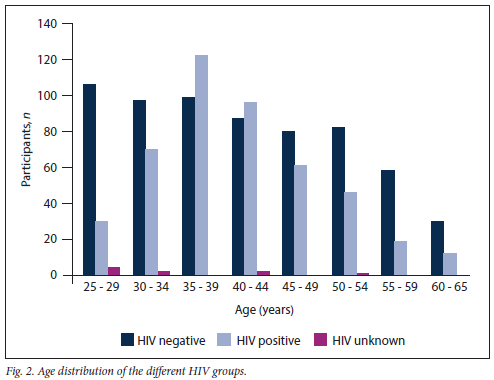
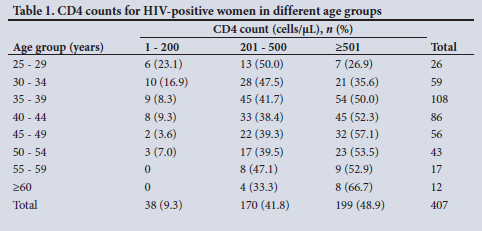
The study included 456 women known to be HIV positive (41.3%), and the latest CD4 count was available for 407 (89.3%) of these, with 51.1% having CD4 counts below the normal value of 500 cells/µL (Table 1). The median (interquartile range) CD4 count was 500 (367.5) cells/µL and the average (range) 519.6 (11 - 1 401) cells/µL; there was a trend to lower CD4 values in younger women. Data on ART use were available for all WLWH; 23 were not using ART, and 59 reported using it for <1 year and 374 for > 1 year.
Screening test results
The overall test positivity for the total group and for the two HIV groups is shown for visual inspection at a cut-off of uncertainty for cytology using two different cut-offs, and for HPV screening using the Roche cobas HPV DNA test performed on HCW-collected specimens (Table 2). Overall, visual inspection tests were positive most often, followed by high-risk HPV without genotyping (Any hrHPV) and then cytology using a cut-off value of atypical squamous cells of undetermined significance (ASCUS). Visual inspection, cytology and hrHPV DNA were positive in 50.4%, 39.9% and 41.2%, respectively, of WLWH v. 20.9%, 17.0% and 19.6% of HIV-negative women.
Visual inspection without magnification identified 339 women (30.7%) for further investigation in the total screening population when acetic acid was used, and 365 (33.1%) when Lugols iodine was used. WLWH had significantly more positive visual inspection tests than HIV-negative women, and in this cohort of WLWH, >50% tested positive (p<0.0001). With regard to age distribution, women aged 35-39 years had the highest probability (>40% for both VIA and VILI) of a positive visual inspection test. Results for the different age and HIV groups are shown in Table 3.
Cytology results showed the highest prevalence of positive cytology among HIV-positive women (37.1% at cut-off ASCUS) (p<0.0001) and overall in the age group 35 - 39 years (31.7% at cut-off ASCUS). The severity of abnormal cytology findings was also much higher among HIV-positive women: high-grade squamous intraepithelial lesion-positive (HSIL+) cytology was the most common abnormality among HIV-positive women (20.6%), while ASCUS was the most common abnormal result among HIV-negative women (6.8%) (Table 4).
HPV DNA results for the two HIV subpopulations are shown in Table 5, with two levels of cut-off for positivity. HIV-positive women were more likely to have positive findings for all hrHPV types than HIV-negative women (p<0.0001), and the prevalence was highest and constant in the youngest three age groups.
Histological diagnosis
We recalled all screen-positive women (n=605) for colposcopy and biopsy or LLETZ, and another 245 of 499 women from the screen-negative study population, but not everyone attended. Histology results were available for 768 women (69.6% of the study population): 42.7% of screen-negative and 91.7% of screen-positive women, and 77.2% of WLWH and 64.2% of HIV-negative women. The histology test results per age and HIV group are shown in Table 6. The unadjusted histological diagnosis is shown as a percentage of the total group in the table and text and also in the table as a percentage only of those with histology. In addition, the histology results as adjusted for the known verification bias (VBA) are shown per age and HIV group, representing the best estimate of the true disease prevalence on tissue diagnosis.
HIV-positive women had more abnormal biopsy results than HIV-negative women, especially in more severe disease categories. CIN3+ was diagnosed on histology in 92 WLWH (20.2%) and 51 HIV-negative women (7.9%), while VBA data suggest a prevalence as high as 23.2% v. 10.2%, respectively. The 143 confirmed CIN3+ lesions also included 15 (1.4%) confirmed cases of invasive cancer (VBA results: 172 cases including 22 cancers).
The results use two cut-off points (CIN2 and CIN3) to allow comparison with published data. CIN2, CIN3 and invasive cancer were all most prevalent between 35 and 39 years. The youngest woman with an invasive neoplasm was 35 years old and the oldest was 63 years old.
Discussion
The age distribution of HIV-negative women represents that of the local cervical screening population, while the age distribution of WLWH in this study mimics that of adult women receiving ART Because of differences between the two subgroups, bias is introduced that may complicate interpreting the age distribution of test results. This study comprised a larger percentage of WLWH than the adult female HIV prevalence (41.3% v. the estimated 23.9%),[2] which was by trial design and was aimed to create two subpopulations to inform possible different screening policies for HIV-positive and HIV-negative women.[15,18]
Positivity rates for all screening and diagnostic tests were unusually high among both subgroups, reflecting the largely unscreened and untreated status of the population and the local HPV epidemiology. Positivity for all abnormalities was much higher than reported from population-based studies performed in the rest of the world.[19,20] In addition, WLWH had double or more the prevalence of positivity on all tests than HIV-negative women. This could be due to current and previous impaired immunity, low CD4 nadir, incomplete reconstitution of cellular immunity, possible direct virus interaction, epithelial disease, and other factors. These findings support the need for a local screening policy, differentiating between the HIV groups. This study was not powered to explore differences according to CD4 count and ARV duration.
VILI positivity rates were -10% higher than when using acetic acid only. Naked-eye screening was positive in almost half of HIV-positive women, in agreement with recently published findings by Kelly et al.[21] (41.5 - 46.2%) from WLWH in Johannesburg. The performance of VIA, VILI and colposcopy as screening and triage tests to predict cytology and histology endpoints will be explored further among HIV-positive and negative groups.
Cytology data show a poorly understood increase at all thresholds when compared with historical data from a similar screening study performed previously in a population unselected for HIV.[22] Abnormalities were then seen in 17.3% (now 24.6%), HSIL+ in 9.6% (now 12.6%) and suspicion of malignancy in 0.5% (now 9.1%).[22] Possible explanations include a lower HIV prevalence in the earlier study, time since HIV infection, time exposed to HPV, and a change in HPV epidemiology over time. This increase has occurred despite a doubling in ART initiation and access between 2008 and 2012.[6] Regarding recent local data, cytology results corresponded to those reported for WLWH from Johannesburg,[21] but were worse than among women recruited from Cape Town.[23]
More severe abnormalities dominated, and HSIL+ occurred three times more than low-grade squamous intraepithelial lesions in both subgroups, while in WLWH, atypical squamous cells were replaced by more severe lesions. In this small trial, the total number of women participating purely in a screening trial who had HSIL cytology (n=126/l 104) was similar to or exceeded the numbers used to calculate screening test performance in several single large international screening studies such as the ATHENA (n=146/46 887), [24] ARTISTIC (n=105/6 124)[25] and Onclarity (n=104/33 634)[26] trials, confirming our sample size calculation. Many screening/diagnostic studies artificially enrich the sample by including clinical samples from patients referred for cervical precancer and cancer, including Horizon (n= 106/5 068, of whom 4 793 were from the screening population)[27] and a large Chinese study by Li et al.[28] (100 CIN3 and 84 invasive cancers on histology/1 122 women comprising 911 screen participants).
The prevalence of detected hrHPV infection varies widely according to population characteristics such as age group, previous screening status, HIV and treatment status, and sensitivity of the sampling method and assay used. Data shown here were tested on a high-throughput laboratory-based, dedicated screening platform that is used globally and includes 14 high-risk HPV types. HPV positivity rates using one of the accepted screening assays are of clinical importance to the National Department of Health, which in its screening policy has committed to the phased implementation of HPV-based screening to be performed by the National Health Laboratory Service.
Both overall and HPV 16/18 positivity were lower in the present study than previously reported for the historical comparison group described above[22] tested with the Roche Linear Array research platform (37 types including low risk) (28.5% and 11.5% v. 54.3% and 19.5%, respectively). The plateau-shaped HPV age distribution curve (flattened up to the age of 39 years) persisted in our cohort, but is explained by the skewed age distribution of HIV-positive women. When corrected for HIV status, hrHPV positivity is highest in the youngest group and drops steadily among both HIV-positive and negative women (data not shown). When considering current data from southern Africa, our data show a lower hrHPV prevalence than that among sexually experienced high-school girls in Eastern Cape Province, SA (54.5%),[29] but a similar prevalence to other samples of adult women from the same province (28.5% overall, 40.6% v. 21.4% in WLWH and HIV negative[30]) and to HIV-positive and negative women from Botswana.[31,32]
Even in the HIV-negative subgroup, the prevalence of hrHPV, HPV16 and HPV18 (23.5%, 6.0% and 1.9%, respectively) is higher than figures reported in screening populations in the developed world in countries including Denmark (hrHPV 16.2%),[33] the USA (14.7%, 2.7% and 0.8%[26] and 12.6%, 2.8% and 1.0%,[24] respectively) and the UK (hrHPV 15.6%)[25] when using screen-adapted HPV tests. This difference is more pronounced among WLWH (48.5%, 11.4% and 7.7% in the present study).
Positivity rates for the various screening tests did not differ sufficiently within subgroups to allow a choice of screening test. For all tests, referral of all women with positive results for either treatment or colposcopic examination will result in overwhelming of the country's service capacity. The resulting over-referral confirms the urgent need for a practical and simple referral and treatment algorithm, which must include a solution for the further management of screen-positive but triage-negative women. Further analysis will be performed to describe sensitivity, specificity and numbers referred for treatment of different strategies. The optimal strategy will allow treatment for the majority of women at highest risk for future invasive cancer with the lowest referral numbers.
Histological findings confirm the considerable burden of disease in both subgroups. Crude positivity rates based on histologically confirmed disease are inherently inaccurate. They would simulate an accurate population prevalence only if either all women without biopsy data have no disease (express data as a percentage of total subpopulation), or those with valid histological results were chosen randomly (express as percentage of biopsied women). There were, however, a surprising number of positive biopsies in screen-negative women, both HIV negative and positive, sampling was not random, and some screen-positive women did not have a histology result.
An important strength of this trial is the calculation of VBA histology data for all disease categories. As expected, the estimated prevalence values fall between the two crude values described above. Crude histological values are included as baseline data and allow direct comparison with trials with similar study design. This study population had up to 20 times higher histologically proven disease prevalence than other large screening trials, showing the need to recalculate the performance of different screening strategies for this setting and for the two groups separately.[24,26,34]
CIN2+ and CIN3+ on histology were significantly more common among WLWH than among HIV-negative women (odds ratios 1.91 and 2.28, respectively), and disease severity in WLWH was also significantly worse. In this cohort, >20% of WLWH and 10% of HIV-negative women had significant precancerous lesions (CIN3+) and needed treatment. The estimated prevalence of existing invasive cervical cancer among women similar to those in this trial is 1 - 2%, with implications for cancer treatment readiness when improving screen coverage.
Other strengths of this trial include the availability of histology for the majority of women in the trial and for >90% of screen-positive women, and the high prevalence of screen positivity and of histologically confirmed disease in both subgroups. For ethical reasons, we offered treatment as part of the trial, which also improved the quality of histology, reduced the number of patients lost to follow-up, and will enable the longitudinal follow-up phase of the trial. The multicentric nature of the study, staff training, audit and retraining, and the inclusion of all WHO screening options are also strengths. The results of this study can be extrapolated to populations of women in sub-Saharan Africa that are similar to ours in most respects, but not to highly resourced, screened and treated cohorts. The relatively small sample size is a limitation, as it may not allow accurate subanalyses.
Conclusion
Phase 1 of the DiaVACCS trial enrolled 1 104 SA women using the public healthcare sector, in subgroups of 465 HIV-positive and 648 HIV-negative women. The demographics and the prevalence of HPV infection, positive screening test results, and biopsy-confirmed pre-invasive and invasive disease in both subgroups confirm the suitability of this cohort to calculate the performance of various screening and triage strategies using tests mentioned in this article and others that have not been included. By study design and owing to differences in demographics and epidemiology shown in these baseline results, HIV-positive and HIV-negative groups need to be considered separately in any further analysis.
Declaration. None.
Acknowledgements. The authors thank Bertha Grond and the HPV Cervical Cancer Fund for the management of all financial and legal matters. We gratefully acknowledge assistance in the clinical management of screen-positive women from clinical staff at the gynaecological oncology units at the universities of Stellenbosch (SU) and Pretoria (UP).
Author contributions. GD was the project leader and designed the study with KLR, with input from LCS, FHvdM, MHB and CV, and had access to all data. GD drafted the manuscript and is the guarantor. CV was responsible for database management. GJD did all data analyses. LCS. FHvdM, GD and MHB were finally responsible for managing recruitment, sample collection and storage, visual inspection, colposcopy and LLETZ treatment at the different study sites. All authors critically reviewed the manuscript and approved the final version.
Funding. We gratefully acknowledge the financial support of Roche, manufacturer of the cobas HPV DNA test, towards participant recruitment, sample and data collection and performance of the baseline tests described in this article. Financial support was also received from the 1st For Women Foundation and the SU and UP gynaecological oncology funds.
Conflicts of interest. Roche had insight but not editorial rights in the study design and the final manuscript. No employee of Roche was on the project team or took part in the writing of the manuscript. All authors have attended meetings with funders and with manufacturers of other molecular tests for cervical screening. None of the authors received remuneration for their work on the project, payment, bonuses or stocks from the funders.
References
1. Bruni L, Albero G, Serrano B, et al.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in South Africa. Summary report 22 October 2021 https://hpvcentre.net/statistics/reports/ZAF.pdf?t=1632401549610 (accessed 2 February 2022). [ Links ]
2. Statistics South Africa. Mid-year population estimates 2021. Statistical release P0302. http://www.statssa.gov.za/publications/P0302/P03022021.pdf (accessed 2 February 2022). [ Links ]
3. Stelzle D, Tanaka LF, Lee KK, et al Estimates of the global burden of cervical cancer associated with HIV Lancet Glob Health 2021;9(2):e161-e169. https://doi.org/10.1016/S2214-109X(20)30459-9 [ Links ]
4. Dreyer G. Clinical implications of the interaction between HPV and HIV infections. Best Pract Res Clin Obstet Gynaecol 2018;47:95-106. https://doi.org/10.1016/j.bpobgyn.2017.08.011 [ Links ]
5. Looker KJ, Ronn MM, Brock PM, et al. Evidence of synergistic relationships between HIV and human papillomavirus (HPV). Systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc 2018;21(6):e25110. https://doi.org/10.1002/jia2.25110 [ Links ]
6. Zuma K, Shisana O, Rehle TM et al. New insights into HIV epidemic in South Africa. Key findings from the National HIV Prevalence, Incidence and Behaviour Survey 2012. Afr J AIDS Res 2016;15(1):67-75. https://doi.org/10.2989/16085906.2016.1153491 [ Links ]
7. Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018;32(6):795-808. https://doi.org/10.1097/QAD.0000000000001765 [ Links ]
8. Bogale AL, Belay NB, Medhin G, et al. Molecular epidemiology of human papillomavirus among HIV infected women in developing countries. Systematic review and meta-analysis. Virol J 2020;17:179. https://doi.org/10.1186/s12985-020-01448-1 [ Links ]
9. Van Aardt MC, Dreyer G, Pienaar HE et al. Unique human papillomavirus-type distribution in South African women with invasive cervical cancer and the effect of human immunodeficiency virus infection Int J Gynecol Cancer 2015;25(5):919-925. https://doi.org/10.1097/IGC0000000000000422 [ Links ]
10. Lekoane KMB, Kuupiel D, Mashamba-Thompson TP, Ginindza TG. The interplay of HIV and human papillomavirus-related cancers in sub-Saharan Africa. Scoping review Syst Rev 2020;9(1):88. https://doi.org/10.1186/s13643-020-01354-1 [ Links ]
11. Dhokotera T, Bohlius J, Spoerri A, et al The burden of cancers associated with HIV in the South African public health sector 2004 - 2014. A record linkage study Infect Agents Cancer 2019;14:12. https://doi.org/10.1186/s13027-019-0228-7 [ Links ]
12. Dreyer G, Botha MH, Snyman LC, et al. Combining cervical cancer screening for mothers with schoolgirl vaccination during human papillomavirus (HPV) vaccine implementation in South Africa. Results from the VACCS1 and VACCS2 trials. Int J Gynecol Cancer 2022;32(5):592-598. https://doi.org/10.1136/ijgc-2021-003079 [ Links ]
13. Botha MH, van der Merwe FH, Snyman LC, Dreyer G. The vaccine and cervical cancer screen (VACCS) project. Acceptance of human papillomavirus vaccination in a school-based programme in two provinces of South Africa. S Afr Med J 2015;105(1):40-43. https://doi.org/10.7196/SAMJ.8419 [ Links ]
14. Van Aardt MC, Dreyer G, Richter KL, Becker P. Human papilloma virus-type distribution in South African women without cytological abnormalities. A peri-urban study South Afr J Gynaecol Oncol 2013;5(2):S21-S27. https://doi.org/10.1080/20742835.2013.11441218 [ Links ]
15. National Department of Health, South Africa. Cervical cancer prevention and control policy 2017. https://www.health.gov.za/wp-content/uploads/2021/07/cervical-cancer-policy.pdf (accessed 18 January 2022). [ Links ]
16. Wentzensen N, Walker J, Smith K, et al. A prospective study of risk-based colposcopy demonstrates improved detection of cervical precancers. Am J Obstet Gynecol 2018;218(6):604.e1-604.e8. https://doi.org/10.1016/j.ajog.2018.02.009 [ Links ]
17. Katitas A. University of Virginia Library Research Data Services + Sciences. Getting started with multiple imputation in R. University of Virginia, 2019. https://data.library.virginia.edu/getting-started-with-multiple-imputation-in-r/ (accessed 3 February 2022). [ Links ]
18. Botha MH, Dreyer G. Guidelines for cervical cancer screening in South Africa. South Afr J Gynaecol Oncol 2017;9(1).8-12. https://journars.co.za/doi/pdf/10.10520/EJC-9584c6bc7 (accessed 1 June 2022). [ Links ]
19. Staler MH, Wright TC Jr, Parvu V, Yanson K, Cooper CK, Andrews J. Stratified risk of high-grade cervical disease using Onclarity HPV extended genotyping in women, >25 years of age, with NILM cytology Gynecol Oncol 2019;153(1):26-33. https://doi.org/10.1016/j.ygyno.2018.12.024 [ Links ]
20. Iftner T, Becker S, Neis KJ, et al Head-to-head comparison of the RNA-based Aptima human papillomavirus (HPV) assay and the DNA-Based Hybrid Capture 2 HPV test in a routine screening population of women aged 30 to 60 years in Germany. J Clin Microbiol 2015;53(8):2509-2516. https://doi.org/10.1128/JCM.01013-15 [ Links ]
21. Kelly HA, Chikandiwa A, Sawadogo B, et al. Diagnostic accuracy of cervical cancer screening and screening-triage strategies among women living with HIV-1 in Burkina Faso and South Africa. A cohort study. PLoS Med 2021:18(3):e1003528. https://doi.org/10.1371/journal.pmed.l003528 [ Links ]
22. Richter K Becker P, Horton A, Dreyer G. Age-specific prevalence of cervical human papillomavirus infection and cytological abnormalities in women in Gauteng Province, South Africa. S Afr Med J 2013;103(5):313-317. https://doi.org/10.7196/SAMJ.6514 [ Links ]
23. Kuhn L, Wang C, Tsai WY, Wright TC, Denny L. Efficacy of human papillomavirus-based screen-and-treatfor cervical cancer prevention among HIV-infected women. AIDS 2010;24(16):2553-2561. https://doi.org/10.1097/QAD.0b013e32833el63e [ Links ]
24. Wright TC Jr, Staler MH, Behrens CM, Apple R Derion T, Wright TL. The ATHENA human papillomavirus study. Design, methods, and baseline results. Am J Obstet Gynecol 2012;206(1):46.e1-46.e11. https://doi.org/10.1016/j.ajog.2011.07.024 [ Links ]
25. Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC). A randomised controlled trial. Lancet Oncol 2009; 10(7):672-682. https://doi.org/10.1016/S1470-2045(09)70156-1 [ Links ]
26. Staler MH, Wright TC Jr, Parvu V, et al. The Onclarity Human Papillomavirus Trial Design, methods, and baseline results. Gynecol Oncol 2018;149(3):498-505. https://doi.org/10.1016/j.ygyno.2018.04.007 [ Links ]
27. Bonde J, Rebolj M, Ejegod DM, Preisler S, Lynge E, Rygaard C. HPV prevalence and genotype distribution in a population-based split-sample study of well-screened women using CLART HPV2 human papillomavirus genotype microarray system. BMC Infect Dis 2014;1:413. https://doi.org/10.1186/1471-2334-14-413 [ Links ]
28. Li T, Wu Z, Jiang M, et al. Clinical performance of Onclarity HPV assay and Cobas HPV test in detection of cervical precancer and cancer in Chinese women. Gynecol Oncol 2020;157(1):202-208. https://doi.org/10.1016/j.ygyno.2020.01.011 [ Links ]
29. Mbulawa ZZA, Somdyala NI, Mabunda SA, Williamson AL. High human papillomavirus prevalence among females attending high school in the Eastern Cape Province of South Africa. PLoS ONE 2021;16(6):e0253074.https://doi.org/10.1371/journal.pone.0253074 [ Links ]
30. Taku O, Businge CB, Mdaka ML, et al. Human papillomavirus prevalence and risk factors among HIV-negative and HIV-positive women residing in rural Eastern Cape, South Africa. Int J Infect Dis 2020;95:176-182. https://doi.org/10.1016/j.ijid.2020.02.051 [ Links ]
31. Castle PE, Varalio JE, Bertram MM, Ratshaa B, Kitheka M, Rammipi K. High-risk human papillomavirus prevalence in self-collected cervicovaginal specimens from human immunodeficiency virus (HIV)-negative women and women living with HLV living in Botswana. PLoS ONE 2020;15(2):e0229086. https://doi.org/10.1371/journal.pone.0229086 [ Links ]
32. Luckett R, Mogowa N, Li HJ, et al. Performance of two-stage cervical cancer screening with primary high-risk human papillomavirus testing in women living with human immunodeficiency virus. Obstet Gynecol 2019;134(4):840-849. https://doi.org/10.1097/AOG.0000000000003496 [ Links ]
33. Preisler S, Rebolj M, Untermann A, et al. Prevalence of human papillomavirus in 5,072 consecutive cervical SurePath samples evaluated with the Roche cobas HPV real-time PCR assay. PLoS ONE 2013;8(3):59765. https://doi.org/10.1371/journal.pone.0059765 [ Links ]
34. Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomised controlled trial of human papillomavirus (HPV) testing for cervical cancer screening. Trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010;10:111. https://doi.org/10.1186/1471-2407-10-111 [ Links ]
 Correspondence:
Correspondence:
C Visser
visser.cathy@gmail.com
Accepted 6 April 2022














