Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 n.2b Pretoria Feb. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i2b.15559
RESEARCH
Vascular Society of southern Africa (VASSA) 2020 clinical practice guidelines on the management of peripheral arterial disease
N G NaidooI; M G VellerII; T V MulaudziIII; B PillayIV; P H MistryV; D A Le RouxVI; Writing committee on behalf of the Vascular Society of Southern Africa
IMB ChB, FCS; Department of Surgery, Faculty of Health Sciences, University of Cape Town, South Africa
IIMB BCh, FCS, MMed (Surg); Department of Surgery, Faculty of Health Sciences Faculty, University of the Witwatersrand, Johannesburg, South Africa
IIIMB ChB, MMed (Surg), FCS, Cert Vasc Surg; Department of Surgery, Steve Biko Academic Hospital, Pretoria, South Africa
IVBSc, MB ChB, FCS, Cert Vasc Surg, PhD; Department of Vascular Surgery, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
VMB ChB, MMed (Surg), FCS, Cert Vasc Surg; Sunninghill Hospital, Johannesburg, South Africa
VIMB ChB, FCS, Cert Vasc Surg; Sunninghill Hospital, Johannesburg, South Africa
Introduction
The concept of best medical, interventional or surgical vascular practice pertaining to peripheral arterial disease (PAD) is best informed by the level of available clinical evidence, local expertise and practices, availability of resources and affordability. While it is generally accepted that the scientific basis for any practice guideline or clinical recommendation is level A evidence supported by multiple large prospective randomised controlled trials (RCTs) and meta-analyses of RCTs (Table 1), such evidence is surprisingly rare in a condition as common as PAD.

In an effort to develop practice guidelines for the management of patients with PAD in South Africa (SA), a meeting of SA vascular surgeons and allied disciplines was convened in November 2019 in Cape Town. In attempting to compile these guidelines, contributing authors at this consensus meeting were requested to review existing international practice guidelines for PAD developed by various vascular societies and consensus groups, to supplement these guidelines with an updated literature review of the latest publications and recommendations, and to consider local expertise and resources when providing recommendations adapted for local conditions.
These are official guidelines of the Vascular Society of Southern Africa (VASSA). They are intended to guide vascular surgical practice and inform other interested parties. As mentioned in previous practice guidelines developed by VASSA, 'It is essential to note that these guidelines are not intended to be absolute dictates, but should provide a framework within which the reasonable physician can and should practice. Undoubtedly, future technological, pharmaceutical and other therapeutic developments and progress in the understanding of the diseases will become available. These guidelines will therefore have to be revised on a regular basis and it is envisaged that similar meetings will be held on a regular basis for this purpose.'
Current clinical practice needs to be undertaken in a setting of evidence-based medicine, with an emphasis on patient safety. The extent of evidence and its varying levels of quality makes integration of such evidence into practice challenging. In addition, the evidence obtained in controlled studies rarely conforms to the other capricious factors found in the real-world clinical setting. Factors including patient expectations, funding and market forces also have an impact on what is considered the standard of care. Clearly, no guideline can integrate all of this. This can only be done by applying judgment based on many medical literature sources which include guidelines. Finding this balance is the quintessential hallmark of competent clinical practice.
Furthermore, while guidelines have become an integral component of clinical practice, guidelines are just that - a guideline and not a rule. Therefore:
• The expectation is that all clinical decisions and actions require a thorough evaluation of the available information regarding the specific case and circumstance at that time, often also having to consider factors for which good evidence does not exist.
• Adherence to guidelines does not suggest a successful outcome nor are they a guarantee that harm will not occur.
• Guidelines do not set legal standards for clinical care but can provide the court with a benchmark by which to evaluate and judge conduct.
Many clinical practice guidelines attempting to help guide clinicians through the complexity described above have been developed by a wide range of organisations. These guidelines have raised concerns about the value of clinical discretion in the face of such directives, uncertainty as to the validity and authority of these guidelines, and questions being asked about the role of such guidelines in defining the quality of clinical practice. Therefore, it is important that guidelines are of a high quality.
In a 2012 survey of 130 guidelines selected at random from the US National Guideline Clearinghouse, less than half of the guidelines met 50% of the Institutes of Medicine standards for such guidelines, the most significant being that conflicts of interest were either not listed or, more importantly, it was not considered at all.[1]
Empirical evidence suggests that guidelines improve patient outcomes; however, adherence to guidelines is variable. Guidelines must therefore be actively disseminated, and implementation strategies must be devised.[2]
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system popularised by the GRADE working group is often used in published American and European practice guidelines, with a few modifications.[3-7] A proposal was made to adopt the modified GRADE system used by the global vascular guidelines on chronic limb-threatening ischaemia (CLTI).[8] After carefully considering the strength of recommendations needed in an economy such as ours, the following modification of the GRADE system was utilised in drawing up these clinical practice guidelines (Tables 1 and 2).
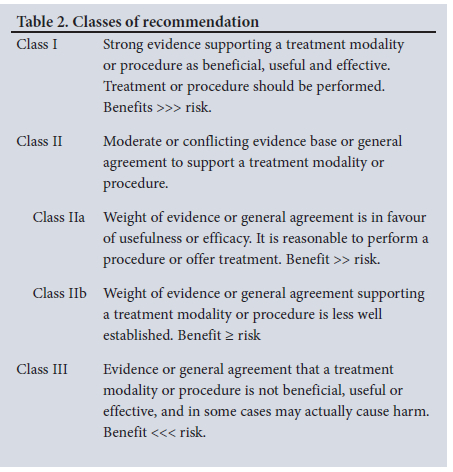
Good clinical practice recommendations
Such ungraded recommendations are supported by a wealth of indirect evidence but no direct evidence. The benefit of pursuing the recommended action(s) is considered to outweigh any plausible harm. The intention of these good practice recommendations is to draw attention and remind providers of known and noncontroversial principles of general medical and surgical care.
Epidemiology and aetiopathology
PAD is defined as an established occlusive disease involving the circulation of the extremities. PAD is one component of cardiovascular disease affecting mainly the lower limbs. More than 90% of the pathology in PAD is due to atherosclerosis. Indeed, in Western literature, atherosclerotic PAD is synonymous with PAD. A resting ankle brachial index (ABI) <0.9 is caused by a haemodynamically significant arterial stenosis and is universally accepted as the haemodynamic definition of PAD.
The community prevalence of atherosclerotic PAD averages 10% by the age 65 years in most studies.[9-11] The prevalence is age-related - it is low in patients between the ages of 50 and 59 years (2.5 - 5%) and increases with advancing age. The prevalence is >20% for patients older than 70 years. A worrying trend is the increasing prevalence of PAD in sub-Saharan Africa (SSA), especially southern SSA. Previously, the only available data from a general African population were reported by the Southern African Stroke Prevention Initiative (SASPI) study.[12] In this study, PAD was reported in 25% of patients >60 years. PAD prevalence of 15% and 32.4% have been reported in Bangui and Brazzaville, respectively.[13]-The higher prevalence in Brazzaville has been attributed to urbanisation and the adoption of a more Western lifestyle. Smoking correlated with a higher prevalence of PAD in southern SSA compared with other regions in Africa.[14] A review of PAD in SSA reported that the prevalence of PAD may be equal to or higher than that in developed countries, exceeding 50% in some high-risk populations.[15] The global prevalence of PAD has increased by 24% in the span of 10 years (2000 - 2010) from 164 million to 202 million.[16] The number of individuals living with PAD is increasing, as a result of total population increase, global ageing, increased incidence of diabetes mellitus, and smoking in developing countries.'16- This study also reported that the prevalence of PAD was higher in women than men in developing countries, which is the opposite in developed countries. The increase in PAD burden observed in women and in younger people is worrisome.
Patients with PAD can have asymptomatic, symptomatic or complicated disease. The ratio of asymptomatic to symptomatic PAD is independent of age, and is usually in the range of 3:1 - 4:1, respectively. It is important to define the population at risk for PAD based on the following predictive factors:
• Age <50 years with diabetes mellitus and one additional risk factor (e.g. smoking, dyslipidaemia, and hypertension);
• Age 50 - 69 years with history of smoking and diabetes;
• Age >70 years;
• Leg symptoms with exertional symptoms (suggestive of claudication) or rest pain (ischaemic foot pain);
• Abnormal lower-extremity pulse examination;
• Known atherosclerotic coronary, renal and carotid disease.
The prevalence of claudication is also age-related and ranges from 3% in patients >40 years to >6% in patients >60 years. In general, the prevalence of PAD is in the range of 3 - 10%, increasing to 15 - 20% in people >70 years. Approximately 10 - 50% of claudicants do not consult their doctor. More than 50% of patients with PAD have no symptoms or have atypical claudication. The PARTNERS study'17-reported that PAD afflicted 29% of all patients >70 years, aged 50 - 69 years with >10-year history of smoking, and aged 50 - 69 with a history of diabetes. More than 70% of treating physicians in this study were unaware of established PAD in their patients. It is estimated that <20% of family practitioners examine the feet of patients at risk for PAD, especially diabetic patients. Among Danish males aged 65 - 74 years, the prevalence of PAD was 10%, of whom only one third had symptoms of intermittent claudication.[18]
The prevalence of CLTI is more difficult to determine. In general, for every 100 claudicants, one patient will present with CLTI.
The dominant pathology in PAD is atherosclerosis which affects multiple vascular beds. The risk factors for atherosclerotic PAD are comprehensively addressed in the Transatlantic Intersociety Consensus (TASC) II document.[19] Potent risk factors for atherosclerosis include smoking, diabetes mellitus, advancing age, hypertension and hypercholesterolaemia. Other risk factors include black ethnicity, obesity, sedentary lifestyles, hyperfibrinogenaemia, hyperhomocysteinaemia, elevated C-reactive protein (CRP) and chronic kidney disease. Young PAD patients (<55 years old) may present with accelerated or precocious atherosclerotic PAD. These are generally high-volume smokers with or without other risk factors for atherosclerosis. However, they may have other non-atherosclerotic pathologies that may require an extensive diagnostic appraisal by way of an expanded blood work, imaging and histological specimens to confirm.
The poly-vascular implications related to PAD are comprehensively addressed in the TASC II document.'19- Approximately 40 - 60% of patients with PAD have associated coronary artery disease (CAD) or cerebrovascular disease (CVD). The REACH registry[20] provides compelling data on 1-year outcomes (death, myocardial infarction (MI) or stroke) in outpatients at risk (i.e. patients with CAD, CVD, PAD or patients with at least 3 risk factors for atherosclerosis).
Patients with established PAD have a 1-year death, MI or stroke rate approaching 5.35%. Patients with CAD, CVD and PAD have a 1-year death, MI and stroke rate approaching 26.2%. PAD is a potent surrogate marker for cardiovascular death, MI or stroke. Currently, PAD is regarded as a CAD risk equivalent. An ABI <0.9 is an independent predictor of mortality.
Pattern and distribution of atherosclerotic PAD
Based on the pattern and distribution of the occlusive disease that define the pulse status, PAD can be categorised into either suprainguinal disease (aorto-iliac disease), or infrainguinal disease, which may be further sub-classified as femoropopliteal disease and tibio-peroneal disease, also known as infrapopliteal or below-the-knee (BTK) disease. The anatomic profile of occlusive disease varies according to risk factors (e.g. tibio-peroneal disease is a common profile in diabetic patients and end-stage renal failure patients). Categorisation of lesion characteristics and extent of PAD has been previously attempted by the TASC, and more recently by the Global Vascular Guidelines (GVG) on CLTI. These need to be factored into decision-making regarding evidence-based revascularisation strategies. Vascular runoff is known to impact on outcomes of revascularisation but appear difficult to quantify. Methods used have been the Society of Vascular Surgeons (SVS) runoff score, the Bollinger score in the Bypass v. Angioplasty in Severe Ischaemia of the Leg (BASIL) trial, etc. However, none appear to be user-friendly. More recently, angiosome-targeted revascularisation has been encouraged to improve clinical outcomes.
Clinical spectrum of PAD
Individuals with PAD present in clinical practice in one of the following ways:
• Asymptomatic
• Symptomatic
• Intermittent claudication (ischaemic claudication)
• Erectile dysfunction
• Complicated
• Acute lower-limb ischaemia
• Chronic limb threatening ischaemia (CLTI)
• Isolated ischaemic rest pain
• With tissue loss (ischaemic ulcer or gangrene).
Intermittent claudication
Patients with intermittent claudication (IC) classically present with exertional calf symptoms (lameness, stiffness, giving way, cramping, etc.) which are relieved by standing still for 3 - 5 minutes. Patients with aorto-iliac disease may present with associated thigh and buttock claudication. Patients with the Leriche syndrome classically present with a triad of buttock claudication, erectile dysfunction, and absent femoral pulses. Differential diagnoses include spinal claudication (generally associated with spinal stenosis), venous claudication (classically in patients with severe venous outflow obstruction), and other causes of leg pain (osteo-arthropathy, fibromyalgia, etc.)
The clinical severity of PAD can be categorised using the Fontaine or Rutherford grading systems (Table 3).[19] Current GVG on CLTI encourage the use of the wound, ischaemia and foot infection (WIfI) staging system.[8]
Ischaemic rest pain
Severe ischaemic neuropathic pain is experienced when lying in a recumbent position, classically at night, involving the toes and the forefoot. The pain is relieved by limb dependency.
Tissue loss
This may present as ischaemic necrosis (focal skin necrosis or gangrene of the digits and/or forefoot) or ischaemic ulceration. A foot ulcer is considered to be due to PAD unless proven otherwise. Palpation of foot pulses is essential. Doppler pressures and ABI should be evaluated in the absence of foot pulses. Due to the calcification of arteries in diabetic patients, the ankle pressures may be falsely elevated. In diabetic patients with tissue loss, an ABI >0.6 is not reliable. These patients may be further assessed with toe pressure measurements or transcutaneous oxygen tension measurements of the foot, when available.
Acute lower-limb ischaemia
These patients present with an acute circulation disorder involving the lower extremities. The duration of symptoms is <2 weeks. Patients may present with any of the following clinical features: pain; pulselessness; paraesthesia; pallor; poikilothermia or paralysis. A comprehensive history, clinical appraisal and Doppler interrogation is mandatory at baseline patient evaluation.
Chronic limb-threatening ischaemia
The term critical limb ischaemia (CLI) is no longer recommended by the recent GVG. The recommended terminology is chronic limb-threatening ischaemia (CLTI). The basic definition of CLTI includes the following:
• Established PAD (absent foot pulses; 1.4< ABI <0.9)
• Ischaemic rest pain >2 weeks and associated with one or more abnormal haemodynamic parameters:
• Ankle-brachial index (ABI) <0.4
• Absolute highest ankle pressure <50 mmHg
• Toe pressure <30 mmHg
• Transcutaneous partial pressure (TcPO2) <30 mmHg
• Flat or low amplitude pulse volume recording (PVR/waveform)
• Tissue loss
• Gangrene
• Non-healing ulcer >2 weeks.
PAD is a progressive disease as reported in the TASC and American College of Cardiology (ACC)/American Heart Association (AHA) practice guidelines on PAD.[19,2U2,32] PAD is progressive for both the symptomatic and asymptomatic disease profiles.
Fate of the leg in PAD[11, 23-26]
The prognosis for the limb is generally benign in asymptomatic or symptomatic PAD. Only a quarter of patients with IC will significantly deteriorate, and only a very small percentage will progress to CLTI requiring intervention. This is most frequent during the first year after diagnosis (7 - 9% compared with 2 - 3% per annum thereafter).
Major amputation is relatively rare in patients with IC (1.0 - 3.3% of patients in this group will over a 5-year period require a major amputation). A changing ABI is the best predictor of progression. Also, those with a low ankle systolic pressure (40 - 60 mmHg) are at risk of progression to severe ischaemia or limb loss (~8.5% per annum).
Most patients with CLTI receive some form of revascularisation. In the subgroup with non-reconstructible disease or where reconstruction has failed, 40% will lose their legs within 6 months, and up to 20% will die in the first year.[27]
Fate of the patient in PAD[19,21,22,32]
PAD is a potent surrogate marker and predictor of cardiovascular events. Patients with PAD have multiple risk factors for atherosclerosis and extensive atherosclerotic polyvascular disease, placing them at an increased risk for cardiovascular events. The increased risk of cardiovascular events in patients with PAD is related to the severity of the disease in the legs as defined by ABI. Atherosclerosis tends to affect all vascular territories. The annual overall major cardiovascular event rate (MI, stroke and vascular death) is ~5 - 7%.
Excluding CLTI, patients with PAD have a 2 - 3% annual incidence of non-fatal MI. The 5-, 10- and 15-year morbidity and mortality rates are 30%, 50% and 70%, respectively. CAD is the most common cause of death in PAD patients (40 - 60%).
The ABI is a good predictor of mortality. There is a linear relationship between ABI and fatal, non-fatal, and cardiovascular events. Each decrease in ABI of 0.1 is associated with a 10% increase in a relative risk of a major vascular event. The lower the ABI, the higher the 5-year risk of a cardiovascular event in diabetic patients.
Future directions
More studies on people living with PAD from African countries are desperately needed. We need more data regarding the epidemiology of PAD in these countries. We also desperately need data on the management of people living with PAD in these countries.
The concern is that limb-salvaging vascular treatments are not implemented in most African countries, and that major amputation is the standard of care for CLTI. Of even greater concern is the relative lack of implementation of evidence-based medical treatments in people living with PAD in most African countries. The economic impact of such health practice deficiencies is not defined currently. This needs to be better defined to drive a more comprehensive PAD programme aimed not only at patient identification, education and treatment, but also at upgrading desperately needed health resources in these African countries.
A more concerted effort should be made to identify patients with PAD earlier so that disease-altering, evidence-based medical therapies can be instituted to reduce the incidence of cardiovascular death, MI and stroke in SA. This may take the form of more aggressive and sustained PAD awareness campaigns, physician education, community workshops, etc.
Recommendation 1
PAD is an independent predictor of mortality and a potent surrogate marker of future cardiovascular and cerebrovascular events. Identification of patients at risk is recommended to improve outcomes in people living with PAD in Africa.
Diagnosis of PAD
Basic clinical and diagnostic appraisal
Atherosclerosis is the most common cause for occlusive disease in multiple vascular territories. PAD is associated with significant morbidity and mortality, much of which is related to cardiovascular and cerebrovascular complications. PAD is also associated with a greater risk of carotid stenosis (~19% of patients with PAD).[28] Upper-extremity arterial disease, especially proximal subclavian artery stenosis, is prevalent in 9% of patients with PAD. A blood pressure difference >15 mmHg in both arms is highly specific for subclavian artery stenosis. In patients with PAD, 27% have a >50% stenosis in one of the mesenteric vessels.[29,30] PAD is also recognised as a risk factor for abdominal aortic aneurysm, especially in symptomatic PAD.[31]
While atherosclerosis risk factor modification is aggressively promoted regardless of severity in patients with coronary and cerebrovascular disease, screening patients with PAD for asymptomatic coronary or extracranial cerebrovascular disease does not improve clinical outcome.[32]
History and examination
A personal and family history should be thoroughly investigated. A family history of CAD, abdominal aortic aneurysm, PAD, hypertension, dyslipidaemia, and diabetes mellitus should be sought. A patient history of other arterial disorders must be investigated.
Smoking history is very important as smoking is a potent risk factor for atherosclerosis and PAD. Lifestyle habits, dietary history and levels of physical activity must be assessed.
Claudication symptoms and other exertional non-joint related limb symptoms are assessed, as well as non-healing wounds, ischaemic rest pain and gangrene. Symptoms related to other vascular territories must also be evaluated.
Vascular examination must include palpitation of all pulses (carotids, upper limbs, abdominal aortic and lower limbs). Palpation of arteries (brachial, radial and femoral) may reveal heavily diseased calcified vessels. Auscultation for bruits (carotid, subclavian, abdominal, iliac and femoral) is advisable. The legs and feet must be inspected for dystrophic features, as well as signs suggestive of CLTI, such as resting pallor, bluish mottling or reactive hyperaemia of the foot ('sunset foot'). Elevation pallor and dependency rubor (a positive Buerger's test) are very suggestive of critical ischaemia.
Diagnostic appraisal
The ABI is the ratio between the best ankle pressure (numerator) compared with the best brachial pressure (denominator). The ABI has good validity as a first-line investigation in the diagnosis of PAD (sensitivity 64 - 84% and specificity 84 - 99%).[331
A resting ABI <0.90 is diagnostic of PAD. The ABI should be reported as abnormal (1.4< ABI <0.90), normal (1.00 - 1.40) or borderline (0.91 - 0.99). Values above 1.40 (often seen in diabetic patients because of medial calcinosis, and patients with advanced chronic kidney disease) suggest heavily calcified crural vessels. Plain X-rays of the legs will reveal extensive vascular calcification. Toe pressures and toe pressure to brachial index (TBI) can be utilised when the ABI is >1.40.
Exercise treadmill testing should be reserved for patients with exertional leg symptoms.
In patients with CLTI, the following tests are indicated:
• The absolute ankle pressures, and the ABI.
• Pulse volume recordings ('waveforms') using Duplex ultrasound (DUS) or photoplethysmography.
• Transcutaneous oxygen measurements (TcPO2).
• Toe pressures and toe brachial index (TBI).
Routine laboratory testing in patients with PAD should include:
• Fasting blood glucose. The HbA1c should be reserved for patients who are prediabetic, or diabetic patients on treatment
• A fasting lipid profile
• Serum creatinine levels
• Full blood count
• Urine for proteinuria
• Uric acid levels in patients suspected of having gout.
Recommendation 1
PAD is an independent predictor of mortality and a potent surrogate marker of future cardiovascular and cerebrovascular events. Identification of at-risk patients is recommended to improve outcomes in people living with PAD in Africa. (Good practice statement)
Recommendation 2
Patients at increased risk of PAD should undergo a comprehensive medical evaluation, and a review of symptoms (exertional leg symptoms, including claudication or other walking impairment, ischaemic rest pain, and non-healing wounds). (Good practice statement)
Recommendation 3
Blood pressures should be measured in both arms. (Good practice statement)
Recommendation 4
In patients with a history or examination suggestive of PAD, a resting ABI is recommended. (Good practice statement)
Recommendation 5
The resting ABI should be reported as normal (1.0 - 1.4), abnormal (<0.9), borderline (0.91 - 0.99) and non-compressible (>1.4). (Good practice statement)
Recommendation 6
Toe pressures and a TBI are recommended in patients with CLTI and non-compressible vessels. (Good practice statement)
Vascular imaging
Vascular imaging in patients with PAD is dictated by their clinical status, the clinically determined anatomical location of occlusive disease, their renal function and the availability of imaging modalities. Imaging modalities could be non-invasive or invasive. Non-invasive investigations commonly used are DUS, computed tomographic angiography (CTA), magnetic resonance imaging or contrast-enhanced angiography (MRI/CE-MRA). Invasive investigations include conventional digital subtraction angiography (DSA), CO2 angiography and perfusion angiography. Imaging is also influenced by availability of institutional resources and expertise. Vascular imaging in patients with PAD should be performed when there is an indication to treat or occasionally when patients present with unusual symptoms.
• Chronic limb-threatening ischaemia in patients who are candidates for revascularisation.
• Severe lifestyle-limiting, medically refractory claudication (the literature supports a lower threshold for treating aorto-iliac disease rather than femoropopliteal disease).
When indicated, patients with PAD should preferably have non-invasive vascular imaging to identify the location and extent of the occlusive disease prior to any form of vascular intervention.
Duplex ultrasound
Duplex ultrasound (DUS) is universally the first-line vascular imaging modality for PAD. The information provided by DUS identifies the anatomical location of the occlusive disease and maps the extent of occlusive disease. DUS can identify occlusions and estimate the degree of stenoses using velocity criteria such as peak systolic velocities (PSV) and PSV ratios. The non-invasive nature, low cost, and wide availability of DUS make it an attractive imaging tool.[8] However, DUS is operator-dependent, and findings correlate with the expertise and experience of the ultrasonographer.
DUS imaging of the aorto-iliac segment may be limited by overlying bowel gas and the deep-seated location of the pelvic vessels, especially in obese patients. Assessment in this region can be made indirectly by assessing common femoral artery (CFA) waveforms. A normal common femoral arterial waveform is triphasic. An iliac artery PSV >200 cm/s and a PSV ratio of more than 2 is indicative of an iliac artery stenosis of >50%.[34] This has a sensitivity and specificity of 90% and 95%, respectively.
DUS can also identify PAD patients with associated abdominal aortic aneurysms and iliac aneurysms. Extensive calcification, especially in the infra-popliteal segments in diabetic patients, can make imaging with DUS challenging.[8]
Two observational studies reported on the utility of DUS when compared with other modalities in treatment planning for PAD patients with CLTI.[34,35] One study[34] highlighted the treatment planning difficulties for fem-crural bypass, reporting that tibial calcification was the most common reason for incomplete examinations. The other study assessed the accuracy of diagnostic ultrasound in operation planning.[35] Thirty-six patients with CLI had DUS and DSA, and the accuracy for predicting operations was compared. Thirty of the actual operations were correctly predicted by DUS, and 32 were correctly predicted by DSA (95% CI 81 - 99; p=0.5).The study concluded that DUS can reliably predict infrainguinal reconstruction strategies.[35]
Contrast-enhanced Duplex ultrasound (CEUS) has been suggested as a tool to improve the diagnostic accuracy of DUS.[8] A random effect meta-analysis with meta-regression analysis was conducted to compare time to peak intensity using CEUS in PAD v. healthy individuals. Fourteen studies (322 PAD v. 314 normal) were analysed. Time to peak intensity was 18.55 seconds in normal individuals v. 33.40 seconds in PAD patients (p<0.00009). ABI, age and sex were not significantly associated with time to peak intensity. This study concluded that CEUS could be a good diagnostic tool for PAD based on time to peak intensity.[36] However, the cost and availability of contrast agents limits the use of CEUS in routine vascular clinical practice.
In patients with PAD, screening for asymptomatic carotid disease is controversial. However, in the Asymptomatic Carotid Stenosis and Risk of Stroke study (ACSRS), patients who did not have symptoms of cerebrovascular arterial occlusive disease, with 60 - 99% carotid artery stenosis and an overall plaque area of <40 mm, had a 1% annual risk of ipsilateral stroke, whereas those with plaque areas of 40 - 80 mm and those with plaque areas >80 mm had an annual ipsilateral stroke risk of 1.4% and 4.6%, respectively.[37] Furthermore, the presence of 3 or more micro-plaque ulcers on 3D DUS was associated with 6% annual stroke risk compared with 0.6% for patients with 0 - 2 micro-ulcers.[37,38]
Recommendation 7
Vascular imaging should only be considered when revascularisation is clearly indicated, feasible and appropriate. (Good practice statement)
Recommendation 8
DUS (Duplex arteriography) should be the first-line vascular imaging modality, when available. (Class I; Level C)
Recommendation 9
Infrainguinal bypass decisions can be made solely based on a good-quality DUS report. (Class IIb; Level C)
Recommendation 10
Additional vascular imaging may be requested when the DUS is equivocal, inadequate or suboptimal. (Class I; Level C)
Recommendation 11
Where the expertise is available, Duplex-based percutaneous transluminal revascularisation may be attempted in patients with renal impairment. (Class IIb; Level C)
Recommendation 12
A carotid DUS should only be requested in select patients with PAD based on an appropriate clinical indication, consistent with contemporary carotid guidelines. (Class I; Level C)
Computed tomography angiography[34,39,40]
Contrast-enhanced multi-detector computed tomography angiography (CTA) provides high-resolution images that can be viewed in multiple planes as 2D or 3D reformatted images. It has the advantage of shorter procedure times than both magnetic resonance angiography (MRA) and DSA, as well as lower radiation exposure compared with DSA. It is used by many as the initial diagnostic tool for aorto-iliac and femoropopliteal disease.
The disadvantages of this imaging modality include decreased sensitivity in the infra-popliteal segment due to the presence of calcification and the small vessel sizes, contrast-induced nephropathy, and exposure to ionising radiation.
Met et al.[39] reported a sensitivity of 96%, 97% and 95% and a specificity of 98%, 94% and 91% in the aorto-iliac, femoropopliteal and infra-popliteal segments, respectively.[39]
Recommendation 13
CTA is an extremely useful imaging modality for aorto-iliac disease. (Class I; Level C)
Recommendation 14
CTA may be considered for infrainguinal imaging when a vascular ultrasound service is unavailable. (Class IIb; Level C)
Magnetic resonance angiography[8]
Magnetic resonance angiography (MRA) is non-invasive, does not rely on ionising radiation and is not affected by arterial calcification. Furthermore, MRA allows for 3D reconstruction of images. Additionally, time-based sequences increase the sensitivity in the infra-popliteal segment.
The value of MRA is limited by a tendency to over-estimate stenoses, and the failure to identify calcifications. Long scanning times and patients who experience claustrophobia limit its applicability, as do patients with incompatible pacemakers, defibrillators, and some metal clips. Metal clips in the region of arteries can cause artifacts that mimic occlusions.
CE-MRA is an evolving tool, especially for infrapopliteal imaging, but is limited in a few patients at risk of developing gadolinium-induced progressive nephrogenic sclerosis, particularly if the glomerular filtration rate is <30 mL/min/1.73 m3.
Recommendation 15
MRA may be considered instead of CTA based on availability, institutional expertise, and the need to assess infrapopliteal runoff.
(Class IIb; Level C)
Digital subtraction angiography[8]
Digital subtraction angiogram (DSA) is considered the gold standard for lower-limb arterial imaging. Selective catheterisation enhances the image resolution, minimises the volume of contrast required and increases the sensitivity. The disadvantages include the exposure to ionising radiation, catheter access-related complications, and the inability to assess vessel wall pathology. DSA is currently reserved for those patients requiring endovascular interventions for PAD.
Recommendation 16
DSA should be reserved for endovascular procedures. (Class I; Level C)
Recommendation 17
DSA provides better imaging of the infrapopliteal and foot vessels than non-invasive modalities. A DSA should be performed before condemning a limb to a major amputation, where feasible and appropriate. (Class IIa; Level C)
Carbon dioxide angiography[8]
Carbon dioxide (CO2) angiography should be limited to patients with allergy to contrasting material and those with severe chronic kidney disease. Historically, CO2 angiography can cause severe discomfort, limiting its widespread use. Current systems are less painful and can be performed under local anaesthesia. Images fade down the leg and have been found to be less accurate compared with iodinated angiography.
Recommendation 18
CO2 angiography should be considered as an option for patients with severe chronic kidney disease. (Class IIa; Level C)
Screening for PAD
Approximately 75% of the 200 million people with PAD are asymptomatic, globally.[41] PAD remains largely undetected in routine clinical practice as only 10% of patients present with IC.[42] This has prompted the need for a screening programme. Asymptomatic disease in healthy individuals devoid of a risk profile is low compared with those with atherosclerotic risk factors (2% v. 6.6%). Between 2000 and 2010, the incidence of PAD increased from 13 - 28.7%.[16] PAD (asymptomatic and symptomatic) has an equivalent/similar mortality risk as MI and stroke,[43] and may be complicated by amputations.[44] The asymptomatic patients are more sedentary with a poor functional performance and quality of life (QoL) in comparison with patients with IC.[45] The PARTNERS population-based study demonstrated a PAD prevalence in one-third of patients with common risk factors for atherosclerosis.[17] The Rotterdam[46] study identified conventional risk factors most strongly associated with PAD (older age, smoking, diabetes mellitus, hypercholesterolaemia and hypertension). These risk factors can be used as a guide for targeted ABI screening in the at-risk population.[47] A review of the guidelines for screening in respect of PAD is characterised by divergent recommendations. The absence of a randomised study has resulted in critical questions in terms of objectives, appropriateness and optimal approach to screening.
The rationale for screening is to identify the at-risk patient, thereby facilitating the potential for intervention with the aim of preventing disease progression and cardiovascular complications.
Risk factors for developing PAD are:[32]
• Age >65 years.
• Age 50 - 64 years, with atherosclerotic risk factors (smoking, hypertension, diabetes mellitus, hyperlipidaemia, and family history of PAD).
• Age <50 years, with diabetes and one additional risk factor.
• Atherosclerotic disease in another vascular bed (coronary, carotid, subclavian, renal, mesenteric artery stenosis or AAA).
Screening procedures may comprise questionnaires (World Health Organization leg pain and Edinburgh Claudication questionnaires), history, physical examination and physiological testing.[32,33]
History taking for claudication has a low sensitivity (54%) and predictive value (9%). The presence of a femoral bruit, pulse abnormalities and ischaemic skin changes may be reflective of significant PAD with moderate to severe obstruction. While these signs may be specific to PAD, their sensitivity is low. Although physical examination is often performed, the benefit to harms ratio has not been completely evaluated.
Tools for screening comprise non-invasive and invasive diagnostic modalities. Non-invasive tools include the ABI, TBI, DUS and pulse oximetry. The resting ABI is the most commonly used screening tool in clinical practice.
The ABI is an inexpensive, safe, non-invasive test using a hand-held Doppler machine. An ABI <0.9 is adequate to confirm the diagnosis of established PAD. The yield of the ABI screening test depends on the prevalence of traditional risk factors, as positive results increase the 10-year cardiovascular estimates. The diagnostic value of ABI is limited in disease that causes arterial calcification and non-compressibility (elderly, diabetes, renal disease). However, scanty data are available for the asymptomatic screening population. A modified approach is suggested (lowest ankle systolic pressure divided by the highest brachial pressure) in the screening population yielding a higher positive yield. This approach requires further validation. The association between a low ABI and cardiovascular risk is well established.
The TBI is recommended in patients with a high ABI (>1.4), especially in patients with diabetes and calcified crural vessels. DUS visualises the artery with sound waves and measures the blood flow to ascertain blockage. This modality has a sensitivity of 80% and specificity of 100% for detecting lesions in the femoral and popliteal arteries, but is less reliable for stenotic lesions in the infra-popliteal vessels. The threshold value for the diagnosis of PAD is a TBI <0.7.
Pulse oximetry is used to measure arterial oxygen saturation. The probe is placed on the toe and index finger with the patient lying in the supine position. An abnormal pulse oximetry is defined as a oxygen saturation (SaO2) value <2% of the finger value or a decrease of >2% on limb elevation.
Asymzptomatic disease remains undiagnosed, with missed opportunities in clinical practice for secondary prevention. Clinical detection of asymptomatic at-risk population paves the way for early initiation of therapy.
Future directions
High-quality research is required to assist clinicians in determining the effectiveness of screening patients with asymptomatic PAD and its overall impact in reducing morbidity (cardiovascular and PAD complications), mortality, and improving the QoL. Trial designs should consider outcome measures, population variability and test reliability.
Recommendation 19
Patients at risk of PAD should undergo a complete examination to include groin and leg pulses, bruits, and examination of the feet. (Good practice statement)
Recommendation 20
Patients at risk for PAD, or with a history or physical examination suggestive of PAD, should have a resting ABI measurement taken. (Class I; Level B)
Recommendation 21
Treadmill testing is indicated in patients with exertional non-joint-related limb symptoms or borderline ABI (>0.90 and <1.40). (Class
IIa; Level B)
Medical management of PAD
Smoking cessation strategies
A correlation between PAD and smoking was reported first by Erb[48-] in 1911. Cigarette smoking is one of the most potent risk factors for PAD. Smoking increases the risk of PAD by several fold and is a more influential risk factor for PAD than CAD. Taking into account other risk factors such as hypercholesterolaemia and diabetes, ~75% of PAD is attributable to smoking.[49,50]
In the Framingham Study population, the risk of IC was double in smokers compared with non-smokers and the odds of developing IC was 1.4 per 10 cigarettes smoked daily.[49,51] The Edinburgh study reported that the odds ratio (OR) of IC, major asymptomatic PAD, and minor asymptomatic PAD in current smokers was 3.7, 5.6 and 2.4, respectively.[52] In addition, PAD is diagnosed a decade earlier in smokers than in non-smokers.[49,52] The severity of PAD tends to increase with the number of cigarettes smoked. For patients with IC, rapid improvement in incidence of severe symptoms has been reported with smoking cessation.'531
The progression of PAD from asymptomatic to claudication to ischaemic rest pain is strongly associated with cigarette smoking, with a linear relationship to the highest tertile of pack years of exposure (>48 years), yielding an OR of 1.6.[53,54] Amputation rates also correlate significantly with smoking history. For smokers with CLI, the amputation rate was 11 - 23% v. 0 - 22% in non-smokers.[55] For patients with bypass grafts, the incident of graft failure is 3-fold higher in smokers and can be reduced to that of non-smokers with smoking cessation instituted at the time of revascularisation.[56]
For patients with thromboangiitis obliterans (Buerger's disease), the presumed pathogenesis hinges on causative components in the tobacco product. Smoking cessation is therefore a cornerstone of treatement.[57]
The risk of PAD in smokers is dose-dependent, and is related to both the number of cigarettes smoked per day and the number of years smoked.[49,50,53-]
Pathophysiology of PAD in smokers
Multiple pathophysiological mechanisms may account for the prevalence of atherosclerosis in cigarette smokers. These include abnormal endothelial function, lipoprotein metabolism, coagulation, and platelet function.
Cigarette smoke contains more than 4 000 compounds, many of which are toxic. The compounds that have drawn the most attention are nicotine and carbon monoxide, although some studies have recently reported that components of cigarette smoke other than these two may be implicated in the development of atherosclerosis.'58-Smoking affects lipoproteins and cholesterol homeostasis.'59-Smoking reduces high-density lipoprotein cholesterol (HDL), and increases low-density lipoprotein (LDL), very low-density lipoprotein cholesterol (VLDL), and triglyceride (TG) levels. Smoking increases monocyte adhesion to endothelial cells, an initial process in atherogenesis. It also facilitates the oxidation of LDL molecules, which is central to atherosclerotic plaque development and progression.'60,61-
Smoking may contribute to a prothrombotic predisposition. Smoking increases levels of fibrinogen, factor VII, and other factors involved in the fibrin clotting cascade, and decreases the concentration of plasminogen.'62- Smoking activates platelets, increasing their reactivity and their ability to adhere to the vessel wall.[61]
As a stimulant, nicotine creates a hyper-adrenergic state, resulting in increased heart rate and myocardial contractility as well as vasoconstriction, all of which may increase myocardial oxygen demand.'63- Carbon monoxide has an affinity for haemoglobin that is ~200 times higher than that of oxygen, and thus, smoking increases the levels of carboxyhaemoglobin, leading to hypoxia. This effectively reduces the blood oxygen concentration and amount of oxygen delivery.[64,65]
Strategies used for smoking cessation include:
• Abruptly quitting without assistance ('going cold turkey').
• Gradually reducing the number of cigarettes smoked, then quitting.
• Behavioural counselling.
• Pharmacotherapy (novel antidepressants, partial nicotine receptor agonists, cysteine, and nicotine replacement therapy).
• Electronic cigarettes ('vaping').
• Nicotine vaccine.
• Complementary medicine (hypnotherapy and acupuncture) -currently no evidence supports these therapies.
Only 3 - 6% of quit attempts without assistance are successful in the long term.[66] Behavioural counselling and medications increase the rate of successfully quitting smoking, and a combination of behavioural counseling with medication such as bupropion is more effective than either intervention alone.[67] A meta-analysis conducted on 61 RCTS reported that ~20% of people who quit smoking with cessation medication and some behavioural assistance were still abstaining from cigarettes a year later compared with 12% who did not take medication.[68] A quarter of smokers who use medications can remain free from smoking for >6 months.
Nicotine replacement therapy
The Food and Drug Administration (FDA) agency in the USA has approved five medications to deliver nicotine in forms that do not involve the risk of smoking. These include nicotine patches, nicotine gum, nicotine lozenges, nicotine spray and nicotine inhalers. Nicotine replacement therapies (NRTs) increase the chance of smoking cessation by 50 - 60% compared with placebo, or no treatment^69-
Antidepressants (bupropion, bupropion SR, nortriptyline)
The antidepressant bupropion (Zyban) is considered a first-line medication for smoking cessation and has been shown in many studies to increase long-term success rates.
Nortriptyline (not registered in SA) is a moderately effective drug for smoking cessation and is generally considered second-line therapy for those who have failed NRT and bupropion.
Partial nicotine receptor agonists (varenicline)
Varenicline is a partial nicotine receptor agonist and is an effective smoking cessation therapeutic option. However, there are concerns about incidents of suicide and suicidal ideation with the use of varenicline. Two nicotine receptor partial receptor antagonists have been marketed: varenicline (Champix - registered in SA), and Citysine (Tabex - not registered in SA). By acting as a partial agonist, they stimulate dopamine release and reduce nicotine withdrawal symptoms. Varenicline has been shown to be the most effective drug for smoking cessation. Champix is a schedule 5 drug in SA and patients should be monitored regularly, with particular attention to changes in their emotional state, behavioural patterns and suicidal ideation. A 2016 Cochrane review[70] concluded that the most recent evidence does not indicate that there is a link between depression moods, agitation or suicidal thinking in smokers taking varenicline to decrease the urge to smoke.
Nicotine vaccines
The theory behind nicotine vaccines is that they induce antibodies that bind to nicotine, reducing its availability to central receptors. Nicotine vaccines are still in the developmental stages.
Electronic cigarettes (e-cigarettes)[71]
The e-cigarettes are battery-operated devices, similar in appearance to the conventional cigarettes that vaporise nicotine. There is very limited supporting evidence that e-cigarettes are effective aids to smoking cessation, although they may reduce the number of cigarettes smoked.
Recommendation 22
Patient counselling plus medication to treat nicotine addiction is more effective than either intervention alone. (Class I; Level A)
Recommendation 23
Varenicline (Champix) has been shown to be the most effective single drug for smoking cessation, but patients should be monitored regularly for mood disorders. (Class IIa; Level A)
Recommendation 24
Bupropion (Zyban) at a dose of 150 mg twice daily for 7 - 12 weeks is an effective drug as a treatment strategy for smoking cessation (dose range 75 - 300 mg twice daily). (Class I; Level A)
Recommendation 25
Smoking cessation strategies must be implemented successfully before any consideration for revascularisation in claudicants. (Class IIa; Level B)
Recommendation 26
Smoking cessation strategies must be implemented in all patients with established PAD. (Class I; Level A)
Recommendation 27
The effectiveness of NRTs as a 'stand alone' therapy is unproven, and hence cannot be recommended currently. (Good practice statement)
Lipid-lowering strategies Statin therapy
Patients with PAD often have simultaneous CAD and CVD, with high attendant morbidity and mortality related to these vascular territories. The mortality risk is also increased in patients with PAD without co-existing CAD, and in asymptomatic patients with PAD diagnosed through routine screening.[72] For all patients with PAD, aggressive risk factor modification in conjunction with early intensive optimal medical therapy is indicated, and should be diligently implemented.
The Heart Protection Study reported that treatment with simvastatin (40 mg daily) reduced the rate of major vascular events by 25%, independent of the baseline cholesterol, and also reduced the rate of peripheral vascular events by 16%, mainly because of a relative reduction of non-coronary revascularisations and amputations.[73] Simvastatin treatment reduced the rate of first major vascular events in patients with PAD even without pre-existing CAD, and also prevented the occurrence of subsequent events. Similar risk reduction was observed in patients with prior peripheral arterial revascularisations or amputations, and in patients with less severe PAD. The most consistent benefits on cardiovascular mortality and morbidity were shown by statins, particularly simvastatin, when used in patients with a high serum cholesterol (>3.5 mmol/L).
Current professional society guidelines recommend statin therapy for all individuals with PAD.[32,33] With respect to functional capacity, lipid-lowering therapy has also proven to be beneficial, with studies showing an improvement of walking performance and claudication.[74]
The benefits of statins are also explained by their non-lipid-lowering (pleiotropic) effects. Statins play an important role in stabilisation and regression of atherosclerotic plaques. Moderate-intensity atorvastatin (20 mg/day) reportedly showed a significant effect on CFA intima-medial thickness (IMT). This difference was noticeable within 4 weeks of treatment.[75] This effect was attributed to the anti-inflammatory properties of statins. Besides stabilisation and regression of atherosclerotic plaques, statins were shown to reduce inflammation (reflected in lower levels of HS-CRP, fibrinogen, serum neutrophils), which in patients with PAD correlates with better survival and event-free survival rates.[76]
The JUPITER trial examined the use of intensive statin therapy (rosuvastatin 20 mg daily v. placebo) in a primary prevention trial.[77] In total, there were 17 802 individuals who had low levels of LDL-C but an elevated vascular risk based on HS-CRP. Investigators demonstrated a 44% reduction in major vascular events, including a 54% reduction in MI, a 48% reduction in stroke, a 46% reduction in arterial revascularisation, a 43% reduction in deep venous thrombosis or pulmonary embolism, and a 20% reduction in mortality. The greatest absolute risk reduction was observed in those with the highest levels of HS-CRP.
Statins not only block the formation of cholesterol by inhibiting HMG-CoA reductase but also decrease the amount of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) that bind to the Rho-GDP receptor on the cell membrane, thus inhibiting protein kinase and other effectors. This decreases the proliferative, inflammatory and fibrotic effects in the vessel wall, and this ultimately influences cholesterol plaque development.
The JUPITER[78] trial assessed the impact of low levels of LDL-C (<1.3 mmol/L) on cardiovascular events and adverse effects, and found that the rates of adverse effects were similar in the placebo and rosuvastatin groups, except for muscle symptoms during a median follow-up period of 2 years. Although these symptoms were more frequent in the rosuvastatin group, they were not different in patients with LDL-C levels < or >1.3 mmol/L. Rates of neuropsychiatric disorders, renal dysfunction, haemorrhagic stroke and cancer were not significantly different between patients treated with statins who reached a LDL-C level <1.3 mmol/L and patients on placebo. Moreover, rosuvastatin reduced the rate of cardiovascular events by 44% compared with placebo, and by 65% in patients who attained an LDL-C <1.3 mmol/L. The all-cause mortality was also reduced by 20% in patients receiving rosuvastatin, and by 46% in patients who attained LDL-C <1.3 mmol/L, which clearly shows that the benefits of intensive statin treatment outweigh the possible adverse effects.
The ACC/AHA guidelines recommend a baseline alanine transaminase (ALT) before statin institution, and only to be repeated if there is clinical evidence of hepatotoxicity. The European Society of Cardiology (ESC) guidelines require ALT after 8 weeks of treatment, and annually if liver enzymes are not elevated 3 times the upper limit. Higher values prompt interruption of treatment and institution at a lower dose, until ALT returns to normal. With regards to myalgia, if severe with creatine kinase (CK) levels above 5 times normal, it should prompt cessation of therapy, with institution at a lower dose post CK level normalisation.
Bezafibrates, ezetimibe and monoclonal antibodies
Bezafibrates do not seem to have an effect on overall coronary or cerebrovascular events but do decrease non-fatal coronary events on the basis of decreasing triglyceride and LDL levels, and increasing HDL levels.[79]
The addition of ezetimibe does not seem to decrease the cardiovascular risk and prevent the progression of disease in PAD despite reducing LDL and elevating HDL levels, but the addition of niacin does seem to add to the beneficial cardiovascular effects of statins.[80]
Monoclonal antibodies that inhibit proprotein convertase subtilisin-kexin type 9 (PCSK9) appear to be a promising new class of drugs effective in lowering LDL-C. A recent meta-analysis that included 24 trials evaluated the effects of PCSK9 antibodies on patients who had not reached LDL-C goals with statin therapy or who were statin intolerant.[81] PCSK9 inhibition led to a 47% reduction in LDL-C, and the relative reduction was similar in patients receiving statin therapy and those that did not receive statin therapy, which makes PCSK9 inhibitor a good adjunctive treatment in patients with inadequate response to statins. Treatment with PCSK9 inhibitors showed a significant reduction in all-cause mortality, cardiovascular mortality and MI. Larger studies are however required to better characterise these drugs, and to assess their possible role in peripheral atherosclerotic disease.
Recommendation 28
Use moderate- or high-intensity statin therapy to reduce cardiovascular events and vascular mortality in all patients with PAD, especially patients with CLTI. (Class I; Level A)
Recommendation 29
Statin therapy should target LDL-C levels <2.5 mmol/L, optimally below 1.8 mmol/L in all patients with PAD.(Class I; Level A)
Recommendation 30
When the target LDL-C level cannot be reached, a reduction >50% should be attempted. (Class I; Level A)
Recommendation 31
For patients with PAD, and high triglycerides or low HDL-C, but normal LDL-C, fibric acid derivatives may be considered. (Class IIa; Level B)
Recommendation 32
Consideration should be given to using a statin that does not use the same elimination pathway as the antiretroviral drugs for HIV patients with PAD. (Good practice statement)
Recommendation 33
Patients with PAD, suspected of familial lipid syndromes, or who are medically refractory, should be referred to a lipidologist or lipid clinic.
(Good practice statement)
Antithrombotic therapy
Patients with PAD due to atherosclerosis have a high risk of cardiovascular death, MI, and stroke, and are six times more likely to die from cardiovascular disease within 10 years.[82] Antiplatelet therapy is one of the pharmacological interventions used to modify that risk and ensure better long-term outcomes for these at-risk individuals.
In patients with asymptomatic PAD, evidence for the use of antiplatelet therapy as primary prophylaxis is lacking. In the POPADAD trial, diabetic patients with an ABI of 0.99 or less, but with no symptoms, were randomised to receive aspirin or placebo and followed up for a median length of 6.7 years. There was no difference in the primary composite endpoint of cardiovascular death, MI, or stroke (18.2% in the aspirin v. 18.3% in the placebo group (hazard ration (HR) 0.98; 95% CI 0.76 - 1.26)).[83] In patients without diabetes mellitus, similar results were seen in the aspirin group for asymptomatic atherosclerosis trial. In 3 350 patients with no symptoms and an ABI <0.95, aspirin was compared with placebo, and the patients were followed up for a mean of 8.2 years. There was no difference in the number of patients who reached the primary composite endpoint of vascular death, MI, stroke, and revascularisation (10.8% v. 10.5%; HR 1.03; 95% CI 0.85 - 1.27).[84]
The Antithrombotic Trialists Collaboration published a meta-analysis in 2002 that evaluated the use of antiplatelet drugs in 135 000 patients at high risk for vascular events. There was a 22% proportional reduction in cardiovascular events in the patients treated with antiplatelet agents v. placebo (10.7% v. 13.2%; p<0.0001), and a 25% reduction when the acute stroke group was excluded.[85] In the subgroup of 9 214 patients with symptomatic PAD, the reduction in vascular death, MI and stroke was 23% (5.8% v. 7.1%; p=0004). However, nearly two-thirds of the 42 PAD trials evaluated antiplatelet agents other than aspirin. There was no difference in event rate with different aspirin dosage regimens (low-dose 75 - 150 mg, medium-dose 160 - 365 mg or high-dose 500 - 1 500 mg).[85] Berger et al.[86] performed a meta-analysis only looking at aspirin in the PAD population. A total of 5 269 patients were included, and aspirin did not significantly reduce the risk of vascular events (8.9% v. 11.05%; relative risk (RR) 0.88; 95% CI 0.76 - 1.04). Therefore, although antiplatelet therapy seems to be beneficial in symptomatic PAD, there is uncertainty on the best agent or combination.
In the CAPRIE[87] trial, the administration of clopidogrel was more effective than aspirin in reducing the risk of vascular death, MI and stroke in patients with symptomatic atherosclerosis, and this advantage was most pronounced in the subgroup with symptomatic PAD, with a RR reduction of 23% in cardiovascular events. When clopidogrel and aspirin were compared with aspirin alone in 15 603 patients with either multiple risk factors or symptomatic atherosclerosis in the CHARISMA[88] trial, there was no decrease in major adverse cardiovascular events (MACE) with dual antiplatelet treatment but an increase in moderate bleeding. Ticagrelor was also shown not to be more effective than clopidogrel alone to prevent MACE, or acute limb ischaemia in symptomatic PAD patients, despite having similar bleeding risks.[89] The addition of vorapaxar to the medical management of PAD in the TRA20 P-TIMI 50 trial did not reduce MACE, although it did significantly reduce the risk of acute limb ischaemia (ALI) and the need for revascularisation at the cost of increased bleeding.[90] In a meta-analysis of 49 RCTs that included 34 518 patients by Katsanos et al.,[91] antiplatelet agents were evaluated for the prevention of MACE in PAD. Aspirin, vorapaxar, picotamide and cilostazol were found to be ineffective. Ticagrelor plus aspirin (n=66), clopidogrel (n=80), ticlopidine (n=87) and clopidogrel plus aspirin (n=98) all reduced the risk of MACE. There was an increased bleeding risk with ticlopidine (n=25) and vorapaxar (n=130). Therefore, clopidogrel monotherapy had the most favourable benefit/harm ratio in the PAD population.[91] This sentiment was echoed by a review in the Journal of the American Heart Association in 2014 on the comparative effectiveness of antiplatelet agents in PAD. They concluded that aspirin has no benefit in asymptomatic PAD patients, clopidogrel monotherapy is more beneficial than aspirin in IC, and dual antiplatelet therapy is not significantly better than aspirin at reducing cardiovascular events in claudicants or patients with CLI.[92] This review included 11 studies with 15 500 PAD patients.[92] It does seem that there is some evidence to suggest that clopidogrel monotherapy is the antiplatelet agent most suitable for high-risk group of patients.
The Cardiovascular Outcomes for People using Anticoagulation Strategies (COMPASS) trial investigated the use of low dose rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg once daily), or rivaroxaban alone (5 mg twice daily), or aspirin alone (100 mg once daily + rivaroxaban placebo twice daily) in patients with CAD (CABG within 14 days) and PAD (defined as previous revascularisation, ABI <0.9, documented peripheral stenosis >50% or carotid stenosis >50%).[93] The study enrolled 27 395 patients in total. The PAD sub-study had 7 470 patients. The primary outcome events (cardiovascular death, stroke, and MI) were lower in the rivaroxaban plus aspirin group v. aspirin alone (4.1% v. 4.9%; p<0.001; number needed to treat (NNT)=125). There was a small but statistically significant decrease in major adverse limb events (MALE), major amputation and ALI when compared with aspirin alone (4.1% v. 5.4%; p<0.001; NNT=77). The rivaroxaban group was associated with an increase in clinically relevant bleeding (3.1% v. 1.9%; p<0.001; numbers needed to harm (NNH)=83). Although NNT and NNH are similar, the investigators had a pre-specified formula for net clinical benefit, and this was lower in the rivaroxaban group than in the aspirin group (4.7% v. 5.9%).
The results of patients with PAD in the COMPASS trial were published in a separate article in the Lancet.[94] The PAD sub-study had 6 048 patients, and 1 422 patients with CAD and an ABI <0.9, totalling 7 470. Of the 6 048 patients, 55% (n=4 129) had symptomatic PAD (undefined symptoms), 26% had previous carotid surgery or an asymptomatic stenosis of at least 50%. Moreover, ~26% of the PAD cohort had previous intervention for PAD, 46% had IC or an ABI of <0.9 or a substantial stenosi >50%. The ABI's however were normal (>0.9) in 49% of the patients, 0.7 - 0.9 in 39% of patients and <0.7 in 7% of patients, and the ABI was measured using a pulse not a Doppler machine in 74% of subjects.
The findings were reported as follows: 'The combination of rivaroxaban plus aspirin compared with aspirin alone reduced the composite endpoint of cardiovascular death, MI, or stroke (5% (n=126/2 492) v. 7% (n=174/2 504); HR 0.72; 95% CI 0.57 - 0.90; p=0.0047), and MALE including major amputation (1% (n=32) v. 2% (n=60); HR 0.54; 95% CI 0.35 - 0.82; ρ=0·0037). Rivaroxaban 5 mg twice a day compared with aspirin alone did not significantly reduce the composite endpoint (6% (n=149/2 474) v. 7% (n=174/2 504); HR 0.86; 95% CI 0.69 - 1.08; p=0.19), but reduced MALE including major amputation (2% (n=40) v. 2% (n=60); HR 0.67; 95% CI 0.45 - 1.00; p=0.05). The median duration of treatment was 21 months. The use of the rivaroxaban plus aspirin combination increased major bleeding compared with the aspirin alone group (3% (n=77/2 492) v. 2% (n=48/2 504); HR 1.61; 95% CI 1.12 - 2.31; p=0.0089), which was mainly gastrointestinal. Similarly, major bleeding occurred in 3% (n=79) of 2 474 patients with rivaroxaban 5 mg, and in 2% (n=48/2 504) in the aspirin alone group (HR 1.68; 95% CI 1.17 - 2.40; p=0.0043)'.
It is extremely important to note that PAD is not a homogenous vascular disorder. MACE are different for CLTI patients and claudicants, and for symptomatic v. asymptomatic carotid disease.[19] However, these were grouped and analysed together. The COMPASS study demonstrates a clear signal in patients with PAD; however, this trial has significant methodological flaws, especially with the definition of PAD. Their definitions of common vascular surgical terms are unconventional, for example:
• ALI was defined as limb-threatening ischaemia with evidence of acute arterial obstruction by radiological criteria or a new pulse deficit leading to an intervention (surgery, thrombolysis, peripheral angioplasty, or amputation) within 30 days of symptoms onset. This does not follow the criteria of ALI defined by the Society for Vascular Surgery.
• Chronic limb ischaemia was defined as severe limb ischaemia leading to a vascular intervention. This encompasses IC and CLTI as a single entity including rest pain, ulceration, or gangrene as one entity. Interventions for IC are often subjective and were not defined.
• ABIs were measured by palpation of a pulse and not a Doppler probe. This is unconventional and the lack of reliability of pulse palpation is well documented. Also, no account is made for patients with ABIs >1.4, indicative of calcified vessels. This is a high-risk group.
• Patients requiring dual antiplatelets were excluded; however, patients post peripheral angioplasty were included. Dual antiplatelets have become the standard of care post peripheral angioplasty.
• Asymptomatic carotid stenosis >50% was included as symptomatic PAD.
There is certainly a signal, albeit not overwhelming when considering the bleeding risk as well as the cost. The greatest reduction is in the composite endpoint of non-fatal MI, stroke, and cardiovascular death (p=0.014). However, patients with PAD were grouped together and they have contrasting baseline risks. It is not clear who will benefit within this cohort. Patient selection will be important and perhaps those at the highest risk for cardiovascular outcomes will benefit the most, but those recommendations cannot be made currently.
The addition of a vitamin K antagonist to a antiplatelet agent in PAD patients without an indication for oral anticoagulation does not offer any benefit with regards to reducing major cardiovascular events, and also increases the risk of life-threatening bleeding.[95] In a patient with an indication for oral anticoagulation and vascular disease, the addition of a antiplatelet agent to the vitamin K antagonist also does not reduce the risk of vascular events, but does increase the risk of bleeding, and is therefore not recommended.[96]
Future directions
The role of combined low-dose direct oral anticoagulants with single antiplatelet therapy needs to be better defined. A dedicated study that is sufficiently powered to investigate the subgroups of patients with PAD (e.g. post bypass, post angioplasty, CLTI, IC) is required. The recent global vascular guidelines on CLTI suggested a class 2/level B recommendation in favour of rivaroxaban.'8-
Recommendation 34
Single antiplatelet therapy is recommended for patients with symptomatic peripheral arterial disease. (Class I; Level A)
Recommendation 35
Clopidogrel may be preferred as the agent of choice over aspirin in patients with symptomatic PAD. (Class IIa; Level B)
Recommendation 36
Aspirin and low-dose rivaroxaban may be considered in patients with symptomatic PAD. (Class IIb; Level B)
Recommendation 37
In patients with PAD and an indication for oral anticoagulation, the use of oral anticoagulation alone should be considered. (Class IIa; Level B)
Recommendation 38
Antiplatelet therapy is not recommended for patients with isolated, asymptomatic PAD, in general. Select asymptomatic PAD patients with a significant calculated risk for future cardiovascular events may benefit from antiplatelet therapy. (Good practice statement)
Antihypertensive therapy
Hypertension is one of the major risk factors for atherosclerotic PAD. It is defined as an office systolic blood pressure (SBP) >140 mmHg, and/or a diastolic blood pressure (DBP) >90 mmHg in the latest ESC/ European Society of Hypertension guidelines, although different thresholds have been implemented by American societies.[98] Much of the existing literature with regards to hypertension and PAD is derived from larger studies comprising of patients with known atherosclerotic CVD, with a priority focus on mortality and other MACE, but with MALE reported. Results have shown that treatment of hypertension leading to a 10 mmHg reduction in SBP, or a 5 mmHg reduction in DBP, is associated with significant reductions in all major cardiovascular events (~20%), all-cause mortality (10 - 15%), stroke (~35%), coronary events (~20%), and heart failure (~40%).[97] This has formed the primary basis for treatment of PAD patients with BP >140/90 mmHg.
Although treatment of hypertension as defined by European guidelines in PAD patients is widely recognised and implemented, treatment of PAD patients with normal or high-normal blood pressure (BP) in the range of 120 - 139/80 - 89 mmHg still remains to be properly defined. Benefit from BP-reducing therapy in this group of patients is mainly based on the total cardiovascular risk that is very high in PAD patients. Two meta-analyses'98,991 reviewing patients with predominantly cardiovascular disease with normal/high-normal BPs have reported a significant reduction in stroke risk, and reduced risk of MACE, but with no survival benefit. Although deemed marginal, the risk reduction was more pronounced in patients with CAD and those at the upper limit of high-normal BP.[99] Whether this benefit is still observed in PAD patients with/without coronary disease remains to be definitely proven in appropriate trials. However, based on current evidence, it would seem beneficial to initiate BP-lowering therapy in PAD patients with high-normal BP (130 - 139/85 - 89 mmHg).
The current ESC/European Society of Hypertension guidelines recommend BP target thresholds <140/90 mmHg in all patients, and provided that the treatment is well tolerated, treated BP values may be targeted to 130/80 mmHg or lower.'971 This further reduction in BP was shown to result in lower rates of fatal and nonfatal MACE and death from any cause in the Systolic Blood Pressure Intervention Trial (SPRINT).[100] However, intensive blood pressure control may result in greater morbidity associated with episodes of hypotension. Reducing the SBP to <120 mmHg should be avoided, as this is associated with increased incidence of cardiovascular (CV) events and death. However, a DBP target of <80 mmHg should be considered for all hypertensive patients. These BP targets are applicable to all PAD patients.
All PAD patients with hypertension should have lifestyle modifications. These includes salt restriction to <5 g per day, weight reduction, regular exercise, smoking cessation, reduction of alcohol consumption and eating a healthy balanced diet.
Most, if not all, PAD patients will need additional pharmacological drug treatment to control their blood pressure. A variety of antihypertensive agents are available mainly angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), beta-blocker, calcium channel blocker (CCB), and diuretics. All these agents have been showed to be equally beneficial with regards to clinical efficacy in a recent meta-analysis.[101] Some classes, however, may be preferred or contra-indicated according to patient's comorbidities. Studies looking at MALE endpoints with different pharmacological agents are limited. The ACEIs have been suggested to be associated with increased amputation rates in diabetics, and in patients with rest pain, with higher re-intervention rates reported, although one retrospective review of CLTI patients showed that there was no effect on limb-related outcomes.[102] Some studies have reported that diuretics may be associated with a higher amputation risk in patients with type 2 diabetes.[102] However, high-quality evidence on adverse limb events is still needed before making firm recommendations on the choice of antihypertensive medication in PAD patients.
Adding to the complexity of choosing the most appropriate initial agent is the recommendation from recent hypertension guidelines to start treatment with 2-drug combinations.[97,98] This is based on the observation that most patients in large studies required at least 2 drugs to reach their BP goals. Drug regimens with complementary activity, targeting multiple mechanisms, have been shown to be effective in lowering BP. A comprehensive review of RCTs involving 2-drug combinations can be found in the latest ESC/European Society of Hypertension guidelines.[97]- These combinations have been shown to be safe and well tolerated, with better adherence when given as a single-pill combination. The European guidelines preferred initial combination treatment includes a renin-angiotensin system blocker (either an ACEI or an ARB) with a CCB or diuretic, with adjustments made according to patients' comorbidities. It is important to note that this recommendation is based on trials involving patients of European descent for the most part, and not black African patients, who represent the dominant population in Africa. The recently published CREOLE study randomised 728 black patients from six countries in SSA and found that amlodipine plus either hydrochlorothiazide or perindopril was more effective than perindopril plus hydrochlorothiazide at lowering BP at 6 months.[103] Despite all of this, it is still to be determined which 2-drug combination is the most effective and safe in PAD patients, as none of the trials focused specifically on this group of patients and none reported adverse limb events.
Recommendation 39
Treatment of hypertension (BP >140/90 mmHg) is strongly recommended in PAD patients. Contemporary hypertension guidelines apply with respect to BP targets for select patient populations, as well as antihypertensive regimens. (Class I; Level B)
Recommendation 40
Treatment of high-normal blood pressure (BP in the range of 130 - 139/85 - 89 mmHg) should be considered in patients with PAD. (Class IIa; Level B)
Recommendation 41
Beta blockers should not be routinely prescribed in patients with PAD. This decision must be based on an indication for a ß-blocker. (Class IIb; Level C)
Intermittent claudication
Patient evaluation and diagnostic appraisal
IC is defined as fatigue, discomfort, cramping, stiffness or 'giving way' involving the muscles of the lower extremities, most commonly the calf muscles, which is consistently induced by exercise and is relieved by a period of rest (~3 - 5 minutes just by standing still).[32,33]
Symptoms of classic claudication may not always be present in patients with PAD. Studies have demonstrated that patients may present with other non-joint-related limb symptoms (atypical leg symptoms), or they may be asymptomatic, especially in diabetics with peripheral neuropathy.[104] In most patients with lower- extremity arterial disease who are asymptomatic, objective walking capacity must be assessed to unmask the arterial disease.
The lower-extremity pulses are assessed clinically, and should be documented as follows: 0 (absent pulses); 1+ (diminished pulse compared with the same anatomical reference; 2+ (normal), and 3+ (unusually bounding, e.g. with a popliteal artery aneurysm). All pulses should be palpated and recorded. Carotid, supraclavicular, iliac and groin areas need to be auscultated for bruits. The lower limbs need to be inspected for dystrophic features, foot deformities and features suggestive of CLTI. Abnormal physical findings need to be confirmed with diagnostic testing.[33]
Most claudicants will fall into Fontaine stage II (non-disabling to disabling claudication), and Rutherford clinical categories 1, 2 and 3.
The natural history of claudicants was defined in a recent meta-analysis, which reported a 5-year cumulative CV-related morbidity of 13% v. 5% in the study population.[105] Less than a quarter (21%) of the patients progressed to CLTI. The risk of limb loss in patients in the CLTI subgroup is 4 - 27%.[19,105] The overall natural history for patients with claudication is essentially benign: ~75% will improve or remain stable and only 2% will have a major amputation. It is clear from the literature that a lower threshold for interventions for claudication may lead to a higher amputation rate. It is therefore not unusual that some vascular surgeons have a simple philosophy for unrepentant claudicants, namely 'make them beg for vascular intervention'.
Patients with PAD generally have multiple risk factors for atherosclerotic PAD, and are at highrisk for CVs. These patients will benefit from evidenced-based, disease-modifying therapy that improve outcomes.
The resting ABI is the initial diagnostic test for PAD. The resting ABI has good validity as first-line testing in the diagnosis of PAD, and has a sensitivity of 64 - 84% and specificity of 84 - 99%.[104,106]
Resting Doppler pressures and ABI are not always useful in claudicants. Exercise treadmill Doppler testing can be used to assess functional status objectively, and to assist with defining the need for revascularisation.[106,107]
For patients with medically refractory, lifestyle-limiting claudication requiring revascularisation, the following investigations are indicated:
• Duplex ultrasound: reported 85 - 90% sensitivity and >90% specificity to detect >50% stenosis.
• CTA reported sensitivity and specificity >90%.
• MRA reported sensitivity and specificity of 95%.
• DSA is generally reserved for therapeutic interventions.
Recommendation 42
A good history and examination is essential in patients with claudication. Risk factor modification, optimum medical treatment and exercise therapy should be instituted prior to any consideration for revascularisation. (Class I; Level C)
Recommendation 43
Exercise Doppler with recording of ankle pressures and ABIs before and after a standardised treadmill test is useful when considering revascularisation in claudicants. (Good practice statement)
Recommendation 44
In claudicants with non-compressible crural vessels or an ABI of >1.4, toe pressures and a TBI are recommended. (Class I; Level C)
Recommendation 45
In patients with medically refractory, lifestyle-limiting claudication, vascular imaging with Duplex arteriography, CTA or MRA is mandatory prior to revascularisation. (Class I; Level C)
Management of claudication Pharmacotherapy
The treatment considerations for patients with PAD and claudication essentially aim at providing symptom relief, and reducing the risk of future CV events. Here, we evaluate the merits of pharmacotherapy for claudication.
Cilostazol
Cilostazol is a type II phosphodiesterase inhibitor, which results in smooth-muscle relaxation and inhibition of platelet aggregation. It also inhibits smooth-muscle cell proliferation. It reduces serum triglycerides and increases HDL concentrations. The exact mechanism of how it improves claudication remains unknown.
A meta-analysis of eight randomised trials reported on the effect of cilostazol on patients with IC.[108] Treatment duration ranged from 12 - 24 weeks. Cilostazol improved maximum walking distance (MWD) compared with placebo in 6 of the 8 trials. The MWD of patients taking placebo increased 21.4% over baseline, whereas patients taking cilostazol 50 or 100 mg twice daily had increases of 44% and 50%, respectively (p=0.05 v. placebo for both). Patients treated with cilostazol 50 or 100 mg twice daily had an increase in pain-free walking distance (PFWD) of 60% and 67%, respectively (p=0.05 v. placebo for both). Cilostazol improved MWD and PFWD in men and women, in old and young patients, and in patients with or without diabetes mellitus. QoL assessments revealed enhanced scores for physical well-being. Cilostazol carries an unpleasant side-effect profile of headache, diarrhoea and gastrointestinal discomfort.
Pentoxifylline
Pentoxifylline is a methlyxantine derivative. It works by improving oxygen delivery, improving blood cell deformability and viscosity of blood. The drug can cause a modest improvement in walking distance, but this does not translate to a significant improvement in the QoL for these patients. In a Cochrane review that included 24 studies with 3 377 participants, of which 17 studies compared pentoxifylline against placebo, the authors concluded that 'given the generally poor quality of published studies and the large degree of heterogeneity evident in interventions and in results, the overall benefit of pentoxifylline for patients with Fontaine class II intermittent claudication remains uncertain'. Pentoxifylline has a similar side-effect profile to cilostazol.[109
Prostaglandins
Prostaglandins have been used to treat patients with claudication. It induces vasodilation and inhibits platelet aggregation. Prostaglandin E1 (PGE1) has to be administered intravenously, and is therefore not practical in the general population. Prostaglandin I2 (Iloprost) is also available in an oral preparation (Beraprost). Both medications have demonstrated improvement in PFWD and MWD. A Cochrane review identified 18 trials with a total of 2 773 patients.[110] Individual trials reported significant increases in walking distances with administration of PGE1, and in several trials the walking capacity improved after termination of treatment. With Iloprost, the apparent clinical gains were offset by the increased withdrawal rate related to the side-effects of treatment. Beraprost sodium was associated with an increased incidence of drug-related adverse events. The review concluded that 'the overall evidence available is insufficient to determine whether or not patients with IC derive clinically meaningful benefit from the administration of prostanoids'.[110]
Statins (HMG-Co reductase inhibitors)
Statins have a generalised benefit of reduced stroke, MI and cardiac-related mortality. Statin therapy, however, also improves claudication distance.
The Scandinavian Simvastatin Survival Study (4S) reported a reduction in the risk of new or worsening IC from 3.6% on placebo to 2.3% on simvastatin (RR reduction of 38%).[1n
In a double-blinded, placebo-controlled study, 86 patients with PAD (Fontaine stage II), IC, and a total cholesterol >5 mmol/L were randomised to treatment with simvastatin (40 mg/day) or placebo for 6 months. The MWD and PFWD was significantly better in the simvastatin group compared with placebo.[112]
Vasodilators
Naftidrofuryl is a serotonin antagonist that improves aerobic metabolism in ischaemic tissue and produces peripheral vasodilation. It has been in use for >20 years in Europe. Many trials have demonstrated clinical improvement in PFWD.
A Cochrane review that included seven studies (1 266 patients) reported an absolute difference in responder rate (proportion successfully treated) of 22.3% compared with placebo (95% CI 17.1 - 27.6). The calculated NNT in this review was 4.5 (95% CI 3.6 - 5.8).[113]
Novel therapies
Gene therapy for angiogenesis is still investigational and its routine use cannot be recommended currently.
Recommendation 46
Pharmacotherapy for claudication, with the exception of statins, cannot be justified in patients who are still smoking. Smoking strategies need to be implemented successfully before considering pharmacotherapy for claudication. (Good practice statement)
Recommendation 47
Cilostazol should be prescribed to treat claudicants, pending availability. (Class IIa; Level B)
Recommendation 48
Considering the clinical benefits to claudicants, the prescription of statins is strongly emphasised. In patients intolerant to simvastatin, other statins should be considered. (Class I; Level B)
Recommendation 49
Naftidrofuryl may be considered in the treatment of claudicants, pending availability. (Class IIa; Level B)
Recommendation 50
Pentoxifylline cannot be recommended currently because of the enormous costs needed to achieve a very small clinical effect.
(Class III; Level B)
Recommendation 51
Prostanoids have no role in the treatment of claudicants, currently. (Class III; Level B)
Exercise therapy for claudication
Exercise therapy (ET) is effective for treatment of IC. It improves symptoms, QoL, and increases the MWD and PFWD. A review of 30 RCTs, which included 1 816 patients, confirmed an improvement of more than 5 minutes on a treadmill with ET compared with standard treatment.[114] PFWD and MWD were increased by 82 m and 109 m, respectively, which was sustained for a period of 2 years. ET does not improve the ABI and its effect on CVs and overall survival is not clear to date.
Supervised ET has superior results compared with unsupervised ET.[115,116] Fourteen studies including 1 002 patients compared supervised ET with unsupervised ET. These exercise programmes ranged from 6 weeks to 12 months, required at least 3 hours per week, and showed an improvement in PFWD and MWD by 180 m by supervised ET. These benefits were sustained for up to 12 months. The long-term benefit of ET remains unquantified and depends largely on compliance. Supervised ET is safe and routine cardiac screening is not required prior to the initiation of treatment.[117] It is more cost-effective than unsupervised ET.[118] Alternative modes of ET other than walking may be beneficial when indicated.[119] These may include arm ergometry, swimming and cycling.
Caution should be exercised when encouraging exercise therapy for diabetic patients. The exercise programmes should be supervised.
Attention to adequate protective and highly cushioned footwear is paramount. Regular foot inspections are advised during and after exercise to prevent ischaemia in diabetic patients.
Recommendation 52
ET for claudication is an effective modality when combined with risk-factor modification and optimum medical therapy. (Class I; Level A)
Recommendation 53
Supervised ET is superior to unsupervised ET, and should be utilised when available, practical and cost-effective. (Class IIa; Level A)
Recommendation 54
Alternative modes of ET, such as cycling or arm ergometry, may be considered where walking is not feasible. (Class IIa; Level B)
Recommendation 55
ET is safe treatment option with a very low overall complication rate. (Good practice statement)
Endovascular treatment for claudication
Endovascular management for IC is largely informed by the clinical severity of symptoms, impact of lifestyle and livelihood, location and extent of the lesions, operative risk and medical comorbidities. With improvement in endovascular techniques, an endovascular first approach has become the procedure of choice for most lesions (focal and advanced).[120]
While endovascular interventions are minimally invasive and associated with a low morbidity and few complications, shorter hospitalisation and recovery times, these procedures, especially infrainguinal interventions, are generally less durable than surgical bypass procedures. This results in an increased re-intervention rate, especially in complex lesions. Failed procedures (open and endovascular) have a potential for complications. The risks and benefits of such interventions must be carefully considered, with particular emphasis in patients with complex and/or bilateral disease. Informed consent is of paramount importance given the natural history and benign outcomes in claudicants managed with optimal medical treatment, lifestyle modification and ET.[19]
The MIMIC trial randomised patients with mild to moderate claudication (Rutherford clinical categories 1 and 2) to plain balloon angioplasty (BA) v. conservative approach in patients already receiving supervised exercise and best medical therapy in two multi-centre trials for two groups of patients: one with femoropopliteal disease, and the other with aorto-iliac disease.[121] The primary endpoints were absolute walking distance (AWD) and initial claudication distance (ICD). The trial reported that 'at 24 months, there were significant improvements in both AWD and ICD in the percutaneous transluminal angioplasty (PTA) groups for both trials. The adjusted AWD was 38% greater in the PTA group for the femoropopliteal trial (95% CI 1 - 90; p=0.04) and 78% greater in the PTA group for the aorto-iliac disease (95% CI 0 - 216; p=0.05). Further benefits were demonstrated for ABI but not for QoL'.
The CLEVER trial randomised patients with aorto-iliac occlusive disease (AIOD) to medical therapy, medical therapy with supervised exercise, or medical therapy with stent revascularisation.[122] At 6 months, changes in peak walking time were greatest with supervised ET. Stenting proved to be superior to optimal medical therapy alone. Improvement in QoL parameters was greater for stent revascularisation. After due consideration of all factors, this study suggests that patient preference plays a significant role in the approach to revascularisation.
With multisegment disease in a claudicant, treating the most proximal lesion will generally improve or alleviate symptoms, often rendering further distal intervention/s unnecessary.
Outcomes of endovascular management are driven by multiple factors. These include inflow, runoff, length of lesion, degree of stenosis, size of the vessel, calcification, location of disease and the technology used. The results of endovascular treatment are better with iliac interventions compared with femoropopliteal interventions. This may relate to the larger vessel calibre of iliac vessels and the protection from confounding extraneous forces that influence outcomes in the femoropopliteal segment. This was borne out in the MIMIC trial.
Even though advances are continually being made in this field, there is no single endovascular modality that has proven to be superior for all lesions or anatomic locations. Depending on location and type of lesion, there are benefits of using certain technologies over others. In general, balloon mounted stents are preferable in areas where high radial force and precision placement is required (ostial lesions). Covered stents are preferable in areas with high-risk of vessel rupture. Flexible self-expanding stents are better suited in tortuous vessels, and in vessels exposed to repetitive movements. More recently, drug-coated technologies (balloons and stents), and athrectomy devices, have also contributed to an already complex management treatment algorithm.
Isolated infrarenal aortic disease
A stent first approach for infrarenal aortic disease has yielded good technical success with excellent early primary patency rates and acceptable secondary patency rates.[123,124] Currently, there is no consensus on choice of stent design, although the use of covered stents may be advisable in heavily calcified lesions, and lesions at risk for aortic rupture during endovascular intervention. When performing aortic intervention, one should be cognisant not to jeopardise future open surgical options, taking care not to stent too close to the renal artery origins.
Aorto-iliac occlusive disease
Common iliac lesions can be unilateral or bilateral and are often present in conjunction with distal aortic disease. Angioplasty alone is remains an option for focal iliac artery disease but primary stenting has been shown to have better long-term patency for more extensive lesions.[125] Stenting with the aim to reconstruct the aortic bifurcation is preferable either with kissing iliac stents, or aortic stent in conjunction with kissing iliac stents (the so called Covered Endovascular Reconstruction of the Aortic Bifurcation (CERAB) technique) when iliac lesions involve the distal aorta. The use of balloon mounted covered stents in complex iliac artery lesions has shown better primary patency rates at 1-year when compared with bare metal stents (BMS).[126] BMS across the origin of the internal iliac artery is acceptable to treat the entire lesion adequately. This usually results in reasonable flow into the internal iliac artery through the uncovered stent. Where possible, the internal iliac artery should be preserved.
The external iliac artery is tortuous and hence should be best managed with a self-expandable BMS or self-expandable covered stent to avoid stent fractures. Combined Iliofemoral disease is best managed with a hybrid approach.
Common femoral artery disease
CFA lesions are often regarded as 'no stent zones'. Placing stents in the CFA is associated with a higher risk of stent fractures and stent migration due to the flexion point. Based on current evidence, endovascular management for CFA should be reserved for exceptional circumstances (i.e. hostile groins, inoperable patients). Experience in the literature currently favours percutaneous catheter-directed atherectomy of the CFA lesion combined with adjunctive drug-coated balloon (DCB) angioplasty.
Femoropopliteal disease
Currently, most femoropopliteal lesions <25 cm can be treated with endovascular options with good technical and clinical outcomes. However, long-term patency compared with open surgery is inferior, especially when comparing endovascular management to above-knee recommend endovascular management for short and intermediate-length lesions.'19-
Multiple endovascular options exist for the femoropopliteal segment. These include BA, self-expandable BMS, self-expandable covered stents, DCB, drug-eluting stents (DES) and atherectomy devices. These technologies can be used in isolation, or in conjunction with each other to achieve good technical results. As the technology has become more sophisticated, technical and clinical outcomes have improved as well, but this has also increased the cost of intervention. This cost factor needs to be considered, especially in a resource-constrained environment, when managing patients with claudication.
BA, with optional stenting ('bailout stenting with self-expandable stents') for suboptimal or complicated BA, has been shown to be an effective form of management of short and intermediate femoropopliteal lesions.[129] The RESILLIENT trial reported improved outcomes in favour of BMS over angioplasty alone (primary patency was better in the stent group: 81.3% v. 36.7%; p<0.0001) after 1-year of follow up.[130-] The Zilver PTX trial showed benefit of DES over angioplasty alone (83% v. 33%) and benefit over BMS (90% v. 73%) at 1 year.[131]- This superior patency was sustained at 5-year follow-up.[132]
The IN.PACT superficial femoral artery (SFA) and LEVANT 2 trials showed benefit of DCB over plain BA.[133,134]
Trials have failed to show benefit of the routine use of covered stents for long SFA lesions over BMS.'135- There may be a role for covered stents in heavily calcified lesions but this remains unproven. The superiority of atherectomy devices over other devices remains unproven. Current evidence on atherectomy devices lacks properly powered trials to show significant benefit.
Given the current data on endovascular management of claudication, considering the benefits of medical management and supervised exercise programmes, serious consideration must be given to the complications and long-term outcomes of endovascular management before embarking on this avenue, especially when factoring the concern that endovascular treatment may render these patients at a higher risk of future limb loss.
Recommendation 56
Endovascular management is reasonable for lifestylelimiting, medically refractory claudication in patients despite adequate ET.
(Good practice statement)
Recommendation 57
An individualised approach for endovascular therapy should be used after considering risks, benefits and expected durability of procedure. (Good practice statement)
Recommendation 58
Endovascular procedures with selective use of BMS, and/or covered stents, should be first-line therapy for endosuitable aorto-iliac occlusive disease. (Class IIa; Level B)
Recommendation 59
For significant CFA/PFA/ostial SFA lesions, atherectomy followed by treatment with a drug-coated balloon may be considered in patients where groin surgery is best avoided. (Class IIb; Level C)
Recommendation 60
Considering that the durability and clinical outcomes of endovascular interventions for femoropopliteal lesions are inferior compared to aorto-iliac endovascular interventions, the threshold for such interventions should be deliberately increased. (Good practice statement)
Recommendation 61
POBA should be the first-line option for endosuitable femoropopliteal lesions. (Good practice statement)
Recommendation 62
A technically favourable response to POBA ('responders') should be followed by treatment with a DCB. (Class IIa; Level B)
Recommendation 63
A technically unfavourable response to POBA ('non-responders') should be followed by treatment with a self-expandable DES.
(Class IIa; Level B)
Recommendation 64
Alternatively, a technically unfavourable response to POBA ('non-responders') should be followed by treatment with a DCB followed by 'spot-stenting' with a self-expandable BMS. (Class IIb; Level C)
Recommendation 65
There is no role for endovascular procedures in claudicants with infrapopliteal occlusive disease. (Class III; Level C)
Defining the role of surgery for claudication
Over the past decade, open surgery for PAD has declined, and certainly even less so for the treatment of IC. Risk-factor control and medical management have become more efficient, and evidence suggests that supervised exercise improves walking distance, and the inclination to use percutaneous balloon angioplasty and/or stenting has certainly caused a decline in open surgical procedures. Surgery is more invasive than any of these other options, with a risk of serious complications. Avoidance of surgery for claudication is not always justified, and open surgical revascularisation still offers substantial benefit, but only in carefully selected patients. The anatomical location and extension of arterial lesions have an impact on revascularisation options.
Aorto-iliac lesions
Relative indications for surgical v. endovascular approaches primarily relate to disease distribution, prior interventions performed, and overall patient risk. The location and severity of the occlusive lesions as well as the presence of any aneurysmal changes have direct implications on treatment modality. Aorto-iliac lesions are a common cause of claudication. For focal iliac stenosis/occlusion (<5 cm), endovascular therapy have good long-term patency (>90% over 5 years) with a low risk of complications.[136]
In cases of ilio-femoral lesions, a hybrid procedure is indicated, usually common femoral endarterectomy or infrainguinal bypass combined with endovascular therapy of the iliac arteries.
If an occlusion extends to the infrarenal aorta, a covered endovascular reconstruction of the aortic bifurcation may be considered instead of surgery. In a small series, 1- and 2-year primary patency was 87% and 82%, respectively.[137] With an occlusion of the aorta up to the renal arteries and including the iliac arteries, an aorto-femoral bypass graft (AFBG) is indicated in fit patients with severe lifestylelimiting, medically refractory claudication. Combined occlusive and aneurysmal disease should be treated by complete exclusion of the aneurysmal segment by either endovascular or open procedure rather than simple bypass, depending on patient risk.
In the absence of any other alternative, extra anatomic bypass procedures, femorofemoral bypass (FFB) or axillo-bifemoral bypass may be considered especially in the severely compromised patient. Their use in patients with claudication should be limited to special circumstances such as graft or stent complications, hostile abdomen, or other factors precluding an endovascular or in-line surgical approach.
Mortality (<2%) and morbidity (10%) are generally low after FFB. Long-term outcomes are quite acceptable, with patency rates in the 55 - 80% range for 5 years, although significantly inferior to in-line reconstructions.
Axillo-bifemoral bypass surgery (BS) is not commonly used in the setting of claudication currently. The presence of pre-existing stents or stent grafts in any of these segments will also influence the choice of procedure. As noted above, the presence and severity of CFA disease is a critical point that often dictates whether a purely endovascular v. an open surgical or hybrid approach is undertaken.
Femoropopliteal lesions
Femoropopliteal lesions are common in claudicants. If the circulation to the profunda femoral artery is normal, there is a good possibility that the claudication will be relieved with optimum medical treatment (OMT) and ET, and intervention is generally unnecessary. If revascularisation is needed, endovascular therapy is the first choice in stenoses/occlusions <25 cm. If the occlusion/stenosis is >25 cm, endovascular recanalisation is still possible, but better long-term patency is achieved with surgical bypass, especially when using a suitable single segment great saphenous vein (GSV). No head-to-head trials comparing endovascular therapy and surgery are yet available for claudicants. In the Zilver-PTX trial, the 5-year primary patency with conventional and DES was 43% and 66%, respectively.[132] The 5-year patency after ABK femoropopliteal bypass is >80% with GSV and 67% with prosthetic conduits.[138]
Who to intervene on?
Several studies have demonstrated the efficacy of endovascular therapy and open surgery on symptom relief, walking distance and QoL in claudicants. These interventions have limited durability and may be associated with morbidity and mortality. They should be restricted to patients who do not respond favourably to a sustained 3 - 6-month period of supervised ET, or when disabling symptoms substantially alter activities of daily living. Open surgery may be associated with longer hospital stays and higher complication rates but results in more durable patency (Fig. 1).
Recommendation 66
Bypass may be considered in average-risk claudicants who have medically refractory claudication, who do not respond to ET, and who do not have endosuitable lesions. (Class IIa; Level C)
Recommendation 67
The type of bypass procedure should be based on patient risk and anatomical pattern of disease. (Class IIa; Level C)
Recommendation 68
Considering that the durability and clinical outcomes of bypass procedures for femoropopliteal lesions are inferior compared with bypass procedures for aorto-iliac disease, the threshold for such interventions should be deliberately increased. (Good practice statement)
Recommendation 69
Axillo-bifemoral bypass procedure has the worst outcomes of all bypass procedures for aorto-iliac disease. Its utility in claudicants therefore requires extreme and compelling justification. (Class IIb; Level C)
Recommendation 70
Where feasible, common femoral endarterectomy/profundaplasty is preferred for significant CFA/PFA lesions. (Class IIa; Level C)
Recommendation 71
Bypass procedures are not indicated for claudicants with infrapopliteal disease. (Class III; Level C)
Chronic limb-threatening ischaemia
Evolving perspectives
The publication of the GVG on CLTI uses new vascular nomenclature and proposes the use of the WIfI clinical severity grading system, the global limb anatomical staging system (GLASS) anatomical classification systems, and the implementation of the patient risk, limb severity, and anatomical pattern and distribution of disease (PLAN) management approach.[8]
CLTI occurs in patients with endstage PAD, with advanced lower-limb ischaemia, who can also have chronic wounds, advanced neuropathy and associated infection. CLTI is associated with high rates of morbidity, mortality, limb loss, and diminished health-related QoL (HRQoL).[139]
Terms such as critical or severe limb ischaemia do not describe the full spectrum of this condition. CLTI includes a heterogeneous group of patients.
Making the diagnosis of CLTI entails:
• Confirmation of established PAD (absent groin or leg pulses; 1.4< ABI <0.9).
• Ischaemic rest pain. This is typically described in the mid- and fore foot at rest, often made worse with elevation of the limb and relieved by dependency. It must have been present for 2 or more weeks.
• CLTI presenting as isolated rest pain should also be associated with one or more of the following:
• Ankle brachial index (ABI) <0.4
• Ankle pressure <50 mmHg
• Toe pressure <30 mmHg
• TcPO2 <30 mmHg
• Flat pulse volume recordings or waveforms
• Ischaemic ulceration and/or gangrene affecting the foot and/ or leg.
Some patients may have normal haemodynamic parameters but still have ischaemic ulceration/gangrene due to diminished local perfusion (i.e. ischaemia in an angiosome without collateral flow).
Patients with venous ulcers, ALI, ischaemia due to emboli (including trash foot), trauma, or those with wounds related to nonatherosclerotic PAD, such as vasculitis and radiation, are excluded from the diagnosis of CLTI.
Disease staging in CLTI
An effective CLTI classification system must stratify the risk of disease while also being sufficiently detailed for research purposes. The currently used classification systems are limited by their failure to include the degree of ischaemia, not recognising the influence infections have on outcome, and focusing primarily on the anatomic features of PAD. As CLTI represents a broad range of clinical severity and anatomic PAD complexity, accurate disease staging is mandatory for designing clinical trials, identifying critical gaps in the understanding and management of the condition, and in developing effective algorithms for treatment.
The GVG on CLTI recommends the use of the SVS lower extremity threatened limb classification system,[8] which describes the type and severity of the wound, the degree of ischaemia, and the presence of foot infection - the WIfI classification.[140] The combinations of the grades of the three components are used for further classification into 4 clinical stages, which may be used to estimate amputation risk and therapeutic benefit (Figs 2 - 4, Table 4).[8]
The global limb anatomical staging system (GLASS) for CLTI[8]
An accurate assessment of limb threat and stratification of the anatomical pattern of disease is the foundation for evidencebased revascularisation (EBR) (Fig 5, Tables 5 and 6). Successful revascularisation for CLTI usually requires restoration of in-line flow to the foot. While the link between the pattern of occlusive disease, patency of the intervention and clinical success in CLTI is multifaceted, an integrated limbbased anatomic system is required to define these complex relationships. Factors that determine a successful technical outcome are also intrinsically different for BS and endovascular intervention. For BS to be successful, adequate inflow and outflow and a suitable autogenous conduit are required. In contrast, the success of endovascular intervention is largely determined by the complexity of atherosclerosis within the anticipated target arterial path (TAP), which is used to provide in-line arterial blood flow to the ischaemic tissues.
The planned TAP is the basis for the use of the GLASS framework:
• Determining the TAP is based on objective imaging. Selection of the TAP is generally based on the least diseased tibial artery providing runoff to the foot. It can also be selected based on other relevant clinical or technical factors, such as angiosome preference or avoidance of a previously instrumented vessel. The preferred TAP for endovascular intervention and preferred distal target artery for open BS may not be the same. Clinical decision-making hinges on a comparative estimate of risk and success.
• To achieve in-line flow to the foot, requires selection of a preferred femoralpopliteal (FP) and infrapopliteal (IP) pathway.
GLASS is designed to primarily correlate with endovascular outcomes. Combined with tools for stratification of patient-risk and severity of limb threat, GLASS facilitates the development of CLTI evidence-based guidelines and provides a basis for clinical practice, and will support future research but still needs further prospective validation.
In the majority of cases, CLTI is the result of multi-level disease. As a consequence, simplifying assumptions is required to develop a practical anatomic staging system that does not rely on complex lesion characterisations. In GLASS, this is achieved by:
1. The focus of GLASS is on infrainguinal disease, as existing schemes for aorto-iliac disease appear adequate. It is regular practice to separate consideration and management of aorto-iliac disease from infrainguinal disease. In GLASS, the CFA and profunda femoris artery (PFA) are considered inflow arteries, and the infrainguinal system begins at the origin of the SFA. This is justified by the distinct treatment approaches to CFA/PFA disease and the long-term patency results that are similar to those for aorto-iliac interventions.
2. With regard to vessel calcification, GLASS adopts a dichotomous, subjective scale in which severe calcification (e.g. >50% of circumference, diffuse, bulky, or 'coral reef' plaques) increases the intra-segment grade by one level. This is based on the assumption that the degree of calcification in the TAP significantly increases technical complexity (and clinical failure rates) for endovascular intervention.
3. With regard to inframalleolar disease, GLASS employs a three-level modifier to describe the status of arteries crossing the ankle (including the terminal divisions of the peroneal artery) and the pedal arch. The inframalleolar modifier is not considered within the primary assignment of overall anatomical limb staging.
GLASS makes the following assumptions:
• Restoring durable in-line flow to the affected part, particularly in patients with tissue loss, is a primary goal of revascularisation in CLTI.
• Using imaging, the clinician defines a TAP that is most likely to achieve the clinical goal of revascularisation.
• The TAP typically involves the least diseased infrapopliteal artery, and for that reason, other infrapopliteal arteries are equally or more diseased.
Multi-vessel infra-popliteal revascularisation is not considered standard practice as evidence of its role is lacking. Staging is still based on the primary infra-popliteal target.
In defining the overall anatomic stage (I - III) for the limb, GLASS combines scores (grade 0 - 4) from both the FP segment (origin of the SFA to the origin of the anterior tibial artery), and from the IP segment (origin of the tibial-peroneal trunk and the anterior tibial artery to the malleoli).
GLASS defines limb-based patency (LBP) as maintenance of in-line arterial flow through the entire length of the TAP, from the groin to the ankle. In assessing LBP, any one of the following would constitute a loss of LBP:
1. Occlusion, critical stenosis, or re-intervention of any portion of the TAP (anatomical failure); and/or
2. A drop in ABI (>0.15) or TBI (>0.10), or >50% stenosis in the TAP, with recurrent or unresolved symptoms (hemodynamic failure).
Estimating LBP following surgical or endovascular intervention is central to evidence-based revascularisation. GLASS stages are based on the likelihood of technical success and 12-month LBP following endovascular intervention to the TAP.[8]
• Stage I: average complexity: technical failure <10%; >70% 12-month LBP.
• Stage II: intermediate complexity: technical failure <20%; 12-month LBP 50 - 70%.
• Stage III: high complexity: technical failure >20%; <50% 12-month LBP.
In summary, GLASS requires the following:
1. Obtaining high-quality angiographic image including the ankle and foot.
2. A patent aorto-iliac segment. Any significant AIOD should be treated before applying GLASS. More importantly, the WIfI staging needs to be reclassified post aorto-iliac intervention.
3. Identifying the preferred TAP.
4. Determine GLASS grade for the FP segment and then for the IP segment.
5. Identifying severe calcification within the TAP (>50% of circumference, diffuse, bulky, or 'coral reef' plaques). This increases the intra-segment grade by one.
6. Combining the GLASS FP and IP grades to determine the GLASS stage.
7. Using a pedal modifier (P0, P1, or P2) to describe the infra-malleolar vessels available as outflow into the foot.
Evidence-based revascularisation - the PLAN concept
The GVG adopts a goal of evidence-based revascularisation to improve the quality of care and reduce inconsistencies in the treatment and outcomes of CLTI. The PLAN incorporates a tiered approach evaluating patient risk, limb severity, and anatomic pattern of disease. The concept lacks evidence but is considered a standard of practice.
Patient risk
This step involves assessing the patient for candidacy for limb salvage, procedural risk, and life expectancy.
CLTI is associated with advanced age, multiple comorbidities, and frailty. The goals of revascularisation include relief of pain, healing of wounds, and preservation of a functional limb. Limb salvage may incur significant morbidity and mortality, requiring multiple hospitalisations, prolonged outpatient care, and considerable health and social care costs. While the majority of patients with CLTI should be considered as candidates for limb salvage, some may require primary amputation or palliation. Predicting functional outcomes following revascularisation is challenging, particularly in frail patients. Palliative therapy rarely includes revascularisation other than when treatment of significant inflow disease is needed to improve the likelihood of successful major amputation, or when used for relief of intractable pain or wound healing.
Estimation of procedural risk and life expectancy plays a critical role in evidence-based revascularisation. Trade-offs between risk, invasiveness, haemodynamic gain and anatomic durability of the vascular intervention are commonly made. Endpoints in such decision-making include mortality, major amputation, and amputation-free survival as well as perioperative events. Predictors include advanced age, renal failure, coronary heart disease, congestive heart failure, diabetes, stroke, tissue loss, body mass index, dementia and frailty.
Limb severity
In practice, patients with CLTI present with a spectrum of disease severity. Staging of the limb, including quantitative assessment of the degree of ischaemia is used to define treatment approaches. Key factors in assessment of the limb include the degree of tissue loss, the ischaemic deficit, and the presence/severity of foot infection using the WIfI system. The WIfI clinical stage is associated with amputation risk, time-to-wound healing, and resource utilisation. Limb severity staging is therefore integral to determining the most effective revascularisation strategy.
The severity of ischaemia and benefit of revascularisation do not map in an exclusively concordant fashion with amputation risk. The need for revascularisation is infrequent in WIfI stage 1 limbs, but increases across WIfI stages 2 - 4. All symptomatic patients who have severe (WIfI stage 3) ischaemia should generally undergo attempted revascularisation. In settings of advanced tissue loss and/or infection (WIfI stage 4), revascularisation may also be of benefit in the presence of moderate ischaemia (WIfI ischaemia grades 1 and 2). Conversely, patients with lesser degrees of tissue loss/infection (WIfI stages 1 to 3) with mild to moderate ischaemia are often successfully treated with infection control, wound and podiatric care. Revascularisation can be considered selectively in these patients if their wounds fail to progress despite appropriate care, or if there are signs or symptoms of deterioration. Limb staging should be reassessed periodically, especially after the infectious component is stabilised, as this is important in guiding subsequent decisions.
ANatomic pattern of disease and conduit availability
Although secondary to the context of patient risk and limb severity, the anatomic pattern of arterial occlusive disease is an important consideration in evidence-based revascularisation. In patients who are candidates for revascularisation, imaging is required to identify the location and severity of arterial lesions, the degree of calcification, and the runoff into the foot. The overall pattern and severity of occlusive disease in the limb as described in GLASS defines the optimal strategy for vascular intervention. The quality of autogenous vein conduit (especially the GSV) is also a key consideration and should be defined early in the assessment of such patients.
An expanding array of endovascular techniques, as well as open bypass to distal tibial and pedal targets, render the majority of patients anatomically suitable for revascularisation. In general, establishing direct in-line flow to the ankle and foot is the primary technical goal of intervention in CLTI. The exception is in the treatment of patients presenting with ischaemic rest pain, where correction of inflow disease alone, or treatment of FP disease even without continuous tibial runoff to the foot, may provide sufficient arterial blood supply to the ischaemic foot in selected patients. This may also be the case in patients presenting with modest tissue loss (WIfI stage 2).
A 'no option' treatment is dependent on the clinical context, and describes either the lack of a target artery crossing the ankle (including anterior and posterior divisions of the peroneal artery) and/or absence of a suitable pedal or plantar artery target (e.g. GLASS P2 modifier). This may also be the most suitable strategy in selected patients with advanced CLTI (WIfI stages 3 and 4).
Surgical treatment for CLTI
Surgical procedures for aorto-iliac disease
Occlusive aorto-iliac disease starts at the aortic bifurcation and extends in a proximal and distal direction. Typically, there are numerous collaterals and therefore claudication is the predominant complaint in symptomatic aorto-iliac occlusive disease. CLTI related to isolated aorto-iliac disease is less common. These days, considering that patients with aorto-iliac disease and CLTI, in general, are not as fit as their counterparts more than 20 - 30 years ago (certainly in the state sector), and who do not have endosuitable lesions, extra-anatomical bypass procedures are generally more commonly employed in clinical practice than direct aorto-iliac bypass procedures.
The pioneering surgical procedures involving the aorto-iliac arterial segment was performed by Dos Santos in 1947 and 4 years later by Wylie et al.[141]-A decade later, prosthetic grafts were widely used and soon AFBG became the standard of treatment for AIOD. Open reconstruction for the aorto-iliac segment has traditionally been considered the 'gold standard' therapy.'142- Traditional open surgery is now being increasingly reserved for complex AIOD, and following failed endovascular therapy only.'143- Open surgical treatment has excellent long-term outcomes, high patient satisfaction and should remain an important, complementary treatment strategy for AIOD.[136,144]
While consideration of the disease pattern may influence the graft configuration for an AFBG, there are no clear differences for long-term outcomes between an end-to-end and an end-to-side proximal graft configuration. The former arrangement requires a shorter segment of the aorta for anastomosis and is easier to cover with retroperitoneal tissue. No clear differences have been noted between the use of Dacron and expanded polytetrafluorethylene (ePTFE). However, smaller graft sizes such as 12 χ 6 mm bifurcated grafts have been shown to have inferior patency rates.[145] Generally, aortofemoral bypass has a 10-year patency rate exceeding 80%.
Unilateral disease may be treated with an inline iliofemoral bypass or crossover iliofemoral bypass. The patency is 73% for crossover bypass and 93% for inline bypass at 5 years. No differences have been noted regarding the approach (retroperitoneal and transperitoneal). There were no differences in outcomes with the use of different graft sizes (6 - 8 mm).[146]
The patency of the outflow vessels also requires assessment and may require an adjunctive procedure.[147] In disease truly isolated to the aorto-iliac segment, with no disease of the orifices of the PFA and the SFA, the distal anastomosis may be placed at the mid-CFA position. The calibre of the SFA and PFA needs to be scrutinised on preoperative imaging and intraoperatively. The arteriotomy needs to allow the interrogation of the orifices of the two outflow arteries. The limitation of outflow is the most common reason for mid-term and long-term graft failures, and adjunctive procedures such as an endarterectomy or patch angioplasty may need to be performed to avoid such failures.
The CFA is generally regarded as a 'no stent zone'. Generally, an open CFA endarterectomy has low morbidity and may be carried out under local anaesthesia with moderate sedation. The hybrid use of endovascular therapy for the iliac occlusive disease combined with an endarterectomy and patch for CFA outflow lesions has become an attractive alternative to aortofemoral grafting.[148] Technical success is 99%, primary patency in 3 - 5 years is 90% with secondary patency at 98 - 100%.'149- The hybrid approach has lower rates of infection and surgical complications, and overall lower morbidity when compared with aortofemoral bypass.[150]
The patency rates of axillo-bifemoral bypass grafts vary between 50% and 75%. The outflow disease is a significant determinant of the patency as well as the graft configuration, axillo-bifemoral grafts have better flow rates and patency rates than axillo-unifemoral grafts. Morbidity and mortality are lower than for AFBG.[151]
While results of aorto-iliac interventions yield good outcomes, treatment of younger patients (<50 years) must be undertaken with caution and should be avoided unless there is critical ischaemia or unrelenting claudication.'152- Young patients with premature AIOD often have poorly controlled risk factors or underlying genetic or biochemical predispositions that are represented by an aggressive vascular phenotype.'152- Early intervention may accelerate the progression of disease. Medical treatment, modification of risk factors and supervised ET should be the cornerstone of treatment.
Recommendation 72
An aortofemoral bypass procedure may be considered for suitable average-risk patients with aorto-iliac disease who are not suitable for endovascular or hybrid procedures. (Class I; Level C)
Recommendation 73
Pelvic circulation and presence of aneurysmal disease must be taken into consideration when planning the type of proximal anastomosis for the aortofemoral bypass procedure. (Class I; Level C)
Recommendation 74
In patients with juxtarenal aortic occlusion, bypass surgery is to be strongly considered. (Class I; Level C)
Recommendation 75
Extra-anatomical bypass procedures should be based on patient risk, limb severity and anatomical pattern of disease. (Class I; Level C)
Recommendation 76
In patients with endosuitable iliac lesions with a significant ipsilateral CFA lesion, a hybrid approach incorporating a CFA endarterectomy-patchplasty/profundaplasty should be considered. (Class I; Level C)
Recommendation 77
Young PAD patients (<50 years) with aorto-iliac and CLTI should be counselled on inferior outcomes following direct aorto-iliac reconstructions or extra-anatomical bypass procedures. (Good practice statement)
Surgical procedures for femoropopliteal disease
CLTI has serious public health implications and, more importantly, it is associated with significant morbidity and mortality. Approximately 20% of people with CLTI will have an amputation, and 25% will die at 1 year.'22- FP disease is the most common anatomical segmental type of PAD presenting with CLTI.
Treatment strategies for CLTI in patients with FP disease include classic open surgery (generally a bypass graft with or without adjunctive procedures), endovascular procedures and hybrid procedures. The optimal first-line strategy for infrainguinal disease with CLTI is still debatable. This is largely due to scarcity of comparative trials and low-quality evidence. The cornerstone of limb salvage in CLTI involves accurate assessment of limb threat, stratification of anatomic pattern of disease, and effective revascularisation. With technological improvements in endovascular surgery positively altering clinical outcomes in the recent years, there is a growing body of interventionists that advocate an endovascular-first approach for most patients, reserving BS as a secondary option.
The Bypass v. Angioplasty in Severe Ischaemic Legs (BASIL-1) trial is the only significant multicentre RCT to date that directly compared a bypass-first strategy with an endovascular-first strategy in infrainguinal disease with CLTI.'153- After a 1-year follow up, an intention-to-treat analysis showed no significant difference between the two arms in terms of amputation-free-survival (AFS) and overall survival (OS). For patients who lived beyond 2 years, AFS and OS was better in the BS group. Furthermore, for patients who had failed endovascular interventions, the outcome of BS was inferior compared with patients who had a primary bypass. A systemic review comparing the 2 modalities, of which 3 were RCTs, showed similar mortality and amputation outcomes, but better patency in favour of BS.[154] In patients with major tissue loss, severe ischaemia or significant infections, several studies have suggested inferiority of endovascular strategy, with high rates of major amputation.[155]
Restoration of adequate blood supply in CLTI patients is the cornerstone of limb salvage. An integrated three-step PLAN-based approach facilitates decision making in the management of CLTI. Patient risk assessment quantifies periprocedural risk and life expectancy. Well-known and validated scoring systems like Finnvasc, CRAB, Prevent III, and BASIL predict perioperative morbidity and mortality at 30 days, AFS and survival up to 5 years.[156] Risk predictors include advanced age (>80 years), CAD, CVD, congestive heart failure, smoking, body mass index (BMI), dementia, and functional status. Patients are classified as low-risk when periprocedural 30-day mortality is <5%, and life expectancy is >50% at 2 years. Conversely, periprocedural mortality >5% and life expectancy <50% categorise the patient as high-risk. Limb staging integrates wound severity, ischaemia, and foot infection. The recommended classification system is the SVS Threatened Limb Classification system, the WIfI staging system. It correlates with limb salvage, amputation risk, wound healing, and identifies patients who are likely to benefit from revascularisation.[157] For infrainguinal BS, the availability of the venous conduit in the form of a good-quality GSV is crucial for long-term outcomes. The integrated approach in the decision-making for FP bypass involves patient risk and life expectancy, WIfI classification, and availability of a good-quality single-segment GSV.
For average-risk patients, BS should be considered for GLASS stage III/WIfI stages 3 or 4 patients. The indeterminate group, GLASS stage III/WIfI stage 2, and GLASS stage II/WIfI stages 3 or 4, should also be considered for BS. The selected target artery should provide continuous in-line flow to the ankle and foot. The preferred conduit is a GSV. In the absence of suitable GSV, arm vein or spliced vein can be used, though durability is inferior to a single-segment GSV. The configuration of vein graft (reversed or in situ) does not influence graft-related outcomes and remains the prerogative of the treating vascular surgeon.
Prosthetic or biological conduits should be avoided, especially in a below-the-knee FP bypass. In these patients, an endovascular approach should be considered prior to open surgical revascularisation. Heparin-bonded ePTFE may be superior to standard ePTFE.[158] Distal vein cuff as an adjunct to prosthetic grafting should be used, although evidence is limited.[159]
For high-risk patients, the endovascular approach is the preferred method. Open surgery should be considered in severely symptomatic patients and failed endovascular therapy. Decision-making should involve a multidisciplinary team and involve all stakeholders, including the patient and family.
Recommendation 78
In average-risk patients with femoropopliteal disease and CLTI, bypass surgery should be based on severity of limb threat (WIfI), anatomic pattern of disease (GLASS), and availability of venous conduit. (Class IIa; Level C)
Recommendation 79
Consider bypass surgery in select high-risk patients with CLTI, who do not have endosuitable femoropopliteal lesions or following failed endovascular treatment. (Class IIa; Level C)
Recommendation 80
A suitable single-segment GSV is the preferred vascular conduit for femoropopliteal bypass surgery. There is no justification for saving such a vein for future infrapopliteal bypass surgery or coronary artery bypass graft. (Class I; Level B)
Infrainguinal bypass procedures onto infrapopliteal target vessels
Put into current perspective, infrainguinal bypass procedures onto IP target vessels for CLTI are not commonly performed. This is attributed to the increasing enthusiasm for, and the results obtained with, single or multiple session BTK endovascular procedures. Surgical revascularisation of the lower extremity using bypass grafts to distal target arteries is an established, effective therapy for advanced ischaemia.[160] Experience, clinical judgment, creativity, technical precision, and fastidious postoperative care are required to optimise long-term results. Revascularisation onto IP target vessels is almost exclusively utilised for patients with CLTI, which occurs in <10% of all patients with PAD.[19]
Patients with CLTI who require infrainguinal bypass procedures onto IP target vessels can be considered in four groups:
Group 1: Patients with diffuse FP occlusive disease with involvement of the popliteal trifurcation and tibial vessels, with both segments not suitable for endovascular procedures.
Group 2: Patients with FP disease with involvement of the popliteal trifurcation and tibial vessels, but with a patent proximal superficial femoral artery of variable length.
Group 3: Patients with FP disease with involvement of the popliteal trifurcation and tibial vessels, but with an endosuitable SFA lesion and a patent popliteal artery.
Group 4: Patients with a patent FP segment with a diseased popliteal artery and trifurcation, and tibial vessels not suitable for endovascular procedures.
The open surgical revascularisation procedures for these patients include:
• Fem-distal bypass: here the donor vessel is generally the CFA, and the recipient artery is a tibial or peroneal artery (proximal or middle third segments). This is appropriate for group 1.
• Distally based fem-distal bypass: here the donor vessel is the mid or distal SFA. This is appropriate for group 2.
• Distal tibial (distal third tibial artery) or pedal artery bypass: here the donor vessel is invariably the popliteal artery or less commonly the CFA/distal SFA. This is appropriate for group 4.
• Hybrid procedures: here a femoral angioplasty and/or stent is followed by a distally based fem-distal bypass, or distal tibial or pedal artery bypass. This is suitable for group 3.
These patients are generally diabetic with their recognised predilection for tibial and peroneal artery involvement. Vascular calcification of variable extent is not unusual in these vessels. These vascular procedures should be regarded as major vascular procedures, considering they are generally attended by severe systemic and procedure-related complications. Distal involvement of these vessels generally correlates with similar disease in the coronary and cerebral vasculature. Wound-related complications, including surgical site sepsis is not unusual, and may require high-maintenance wound management with prolonged hospital stays. However, it is important to note that diabetes is not a risk factor for vein graft failure.[161]
While there is a general appreciation that despite the morbidity and mortality, and the disadvantages that attend these bypass procedures, a single-segment GSV bypass procedure is more durable than endovascular procedure/s. Vein graft configuration, whether reversed or non-reversed-valve ablated, or in situ, seems to have little effect on patency. Shorter vein conduits have better patency rates. Spliced vein or a prosthetic conduit with a distal vein augmentation are other alternatives for a vascular conduit; however, these have inferior outcomes compared with a single-segment GSV (3.5 mm or larger in diameter, and free of varicose or post-thrombotic involvement).
Historically, these bypass procedures have been associated with significant mortality and major amputation rates ranging between 10% and 40% at 6 months.[162-] A recent systematic review and meta-anaylsis reported that 'patients with IP disease had higher patency rates of GSV graft at 1- and 2-years (primary: 87%, 78%; secondary: 94%, 87%, respectively) compared with all other interventions. Prosthetic bypass outcomes were notably inferior to vein bypass in terms of amputation and patency outcomes, especially for BTK targets at 2 years and beyond'.[139] Some centres have reported excellent results with pedal artery bypass.[163] With extensive tissue loss in the foot, some have advocated bypass onto a wound-related artery supplying a specific angiosome/s.164 Not all infrainguinal lesions, including BTK lesions, are endosuitable. It is absolutely imperative that vascular training centres maintain a decent volume of practice with respect to these technically challenging bypass procedures to ensure adequate exposure for future vascular surgeons, especially in cases of failed, or exhausted, or lack of endo-suitability for BTK interventions. Such expertise is generally required in the multidisciplinary team approach to managing patients with diabetes mellitus and CLTI.
Recommendation 81
An evaluation for revascularisation options should be performed by an interdisciplinary care team, including a vascular surgeon, before amputation in the patient with tibioperoneal disease and CLTI, especially in diabetic patients. (Good practice statement)
Recommendation 82
Early recognition of tissue loss and/or infection and referral to the vascular team is mandatory to improve limb salvage, especially in diabetic patients. (Good practice statement)
Recommendation 83
An interdisciplinary care team should evaluate and provide comprehensive care for diabetic patients with CLTI and tissue loss to achieve complete wound healing and a functional foot. (Good practice statement)
Recommendation 84
Bypass procedures to the infrapopliteal and crural artery levels should be undertaken by experienced vascular individuals or teams, if endovascular procedures are not suitable, or following previous failed endovascular attempts. (Good practice statement)
Recommendation 85
Comprehensive vascular imaging, including a comprehensive DSA that includes the foot arch, is essential for planning such bypass procedures. (Good practice statement)
Recommendation 86
Bypass surgery is indicated for all average-risk patients with advanced limb-threatening conditions (e.g. WIfI stage 4) and significant perfusion deficits (e.g. WIfI ischaemia grades 2 and 3), whenever feasible to minimise tissue loss. (Class I; Level B)
Recommendation 87
A suitable single-segment GSV is the preferred vascular conduit for these complex bypass procedures. (Class I; Level B)
Recommendation 88
Perform ultrasound vein marking in all patients prior to bypass surgery. If an ipsilateral GSV is not suitable, the contralateral GSV or arm veins should be marked if suitable. (Class I; Level C)
Recommendation 89
A 'spliced' vein graft or prosthetic graft with a distal vein augmentation may be used, if a suitable single-segment GSV is not available. (Class
IIb; Level C)
Recommendation 90
An on-table completion angiogram is essential post bypass surgery onto infrapopliteal targets. Any technical abnormalities should be addressed immediately. (Good practice statement)
Endovascular interventions for CLTI
Endovascular procedures for aorto-iliac disease
Although AIOD is a common cause for claudication, CLI is usually the result of complex multi-level occlusive disease. Aorto-iliac occlusion/ stenosis (inflow disease) is characterised by an absent femoral pulse, blunted CFA waveform on DUS, >50% stenosis by angiography in the aorto-iliac region, and aorta to CFA systolic gradient >10 mmHg at rest.[8]- In the presence of both inflow (suprainguinal) and outflow (infrainguinal) disease, inflow disease should be corrected first. Simultaneous inflow and outflow revascularisation should be considered in patients with severe ischaemia.[8] Previous guidelines have limited endovascular therapy to short lesions (TASC-II A and B lesions), with surgery recommended for more extensive lesions (TASC-II C and D).[19] Improvements in technology and endovascular techniques, however, have resulted in endovascular therapy replacing open BS as the primary treatment for focal and advanced AIOD.[165]- Open surgery is generally reserved for patients with such extensive disease that endovascular treatment is not possible or ill-advised, and for failed endovascular interventions.[165] Although aorto-bifemoral bypass is recommended for patients fit for surgery, an endovascular first-approach should be considered in patients with extensive TASC C and D lesions, and with severe comorbidities.[166-] An endovascular first-strategy may also be considered for AIOD in patients fit for surgery, if surgery is performed by an experienced team and if such treatment does not compromise subsequent surgical options.'[137]
Aortic interventions
Stenting of aortic occlusive lesions can be performed with a 90 -100% technical success rate, with 1- and 4-year primary patency rates between 75 - 100% and 60 - 80%, respectively, and secondary patency rates between 90 -100% at 1-year and 60 - 100% at 5 years.[123,124] Lesions in the aortic bifurcation are treated with either kissing stents at the origin of the common iliac arteries or aortic stent placement down to the bifurcation followed by kissing stents into the common iliac arteries.[167] In a recent multicentre study, endovascular repair of complex aorto-iliac lesions with the kissing stent technique provided similar early and late results compared with open surgery.[168] Reconstruction of the aortic bifurcation using CERAB stents was first reported in 2015.[137] The 3-year results of CERAB in extensive AIOD reported primary and secondary patency rates of 82% and 97%, respectively.[169] Endovascular therapy for aortic occlusive disease has a reported mortality of 1 - 3%, morbidity of 5 - 20% and renal dysfunction of 2 - 10%. There is a decrease in ICU stay, blood transfusion requirements and infection rate and a quicker recovery to functional status compared with open surgery.[166]
Iliac interventions
The long-term results of endovascular management of iliac lesions compare favourably with open surgery, with 1-year primary and secondary patency rates ranging from 70 - 100% and 90 - 100%, respectively.[136] The 5-year primary and secondary patency rates are noted to be 60 - 85% and 80 - 95%, respectively.[170] Primary stenting is recommended in favour of PTA, except for very short, non-ostial iliac artery lesions.[165,166] It is important to treat the full extent of the disease and not limit coverage due to concerns of stenting over the internal iliac artery. Perfusion of the internal iliac artery can be maintained by placing uncovered stents over the origins of the internal iliac artery.[165,166]
Ilio-femoral lesions
In case of ilio-femoral lesions, a hybrid procedure consisting of endovascular management of the iliac arteries combined with common femoral endarterectomy/infrainguinal bypass procedure is indicated.[171,172]
Self-expanding stents v. balloon expandable stents
The choice of stent depends on lesion characteristics and location. A balloon expandable stent (BES) is preferred in highly calcified and ostial lesions due to greater radial strength and increased crush resistance.[166] Flexible self-expanding stents (SES) are recommended in the external iliac artery due to increased mobility of the artery and potential for kinking and stent fracture.[166] The randomised multicentre ICE trial reported that, although both SES and BES performed well in AIOD, SES had significantly lower binary restenosis on DUS (6.1% v. 14.9%) and a higher freedom from TLR (97.2% v. 93.6%) at 12-month follow-up.[173]
Covered v. bare metal stents
The 5-year results of the prospective, multicentre COBEST trial demonstrated that covered BES resulted in better primary patency than BMS in aorto-iliac disease, especially in more advanced lesions.[174] Covered stents may also provide a safety margin in the treatment of calcified common iliac lesions where rupture is a possibility.
Recommendation 91
Endovascular intervention is recommended as first-line therapy for endosuitable aorto-iliac lesions in patients with CLTI. (Class I; Level B)
Recommendation 92
An endovascular first strategy may be considered for AIOD if done by an experienced team and does not compromise subsequent surgical options. (Class IIa; Level B)
Recommendation 93
Primary stent implantation should be considered rather than provisional stenting. (Class I; Level B)
Recommendation 94
The use of BMS or covered stents is recommended for CIA/EIA occlusive disease due to improved technical success and patency.
(Class IIa; Level B)
Recommendation 95
The use of covered stents is recommended in the presence of severe calcification or aneurysmal change or where the risk of rupture may be increased. (Class I; Level C)
Recommendation 96
In patients with CLTI and multilevel disease, suprainguinal occlusive disease should be corrected first. (Good practice statement)
Recommendation 97
In patients with critical limb ischaemia and multilevel disease, consider suprainguinal revascularisation alone in patients with multilevel disease, and mild-moderate ischaemia or very limited tissue loss. (Class I; Level C)
Recommendation 98
In patients with critical limb ischaemia and multilevel disease, consider simultaneous suprainguinal and infrainguinal revascularisation in patients with severe ischaemia or high limb risk. (Class I; Level C)
Endovascular interventions for femoropopliteal disease Plain and 'designer' balloon angioplasty
The FP segment is the most involved territory in lower-extremity PAD, with involvement in 60 - 70% of patients. While most patients with PAD may be either asymptomatic or present with IC, ~5% will progress to develop symptoms of CLTI. It has been reported that the mortality rate in patients with CLTI is 20% at 6 months and 50% at 5 years, reflecting the systemic atherosclerotic burden associated with CLTI.[175] In addition to the poor OS, 6-month lower-limb amputation rates are estimated to be between 10% and 40% in patients who do not undergo revascularisation. It is therefore imperative that perioperative risk and estimated long-term survival are considered when selecting the method of revascularisation for patients presenting with CLTI.
Despite being first performed more than 50 years ago by Charles Dotter, plain old balloon angioplasty (POBA/PTA) remains the foundational element upon which endovascular intervention for PAD is centred. Its mechanism of action is that of plaque fracture with localised wall dissection and compaction to allow for luminal expansion, with a successful angioplasty being defined as having a <30% residual stenosis in the absence of any significant flow-limiting dissection. Benefits of POBA are that it is technically simple to perform, relatively inexpensive and carries a lower perioperative risk when compared with BS. The major drawback of POBA has been its inferior durability, with the FP segment demonstrating the highest incidence of restenosis across the various vascular territories of the human body.[176] This restenosis is the result of neointimal hyperplasia, induced by acute barotrauma to the vessel wall from the angioplasty balloon associated with negative remodelling.
Both technical success and durability have been shown to correlate with lesion length and lesion type, with longer lesions and/or occlusions associated with worse results. Nguyen et al.[177] in a retrospective review of 824 procedures performed in 733 patients, of whom 63% (n=517) underwent POBA, reported an actuarial 5-year primary patency of 36%, which dropped to 27% at 4 years when only patients with CLTI were considered. When stratified by TASC II classification, the mean (standard deviation (SD)) primary patency at 5 years for TASC A and B lesions was 37 (3) as compared with 12 (9) for TASC C and D lesions. Multivariate analysis confirmed that both CLTI (HR 1.63; 95% CI 1.27 - 2.10; p=0.01) and TASC II C and D lesions (HR 1.49; 95% CI 1.12 - 1.97; p=0.01) were negative predictors of primary patency.
When comparing the outcomes of POBA with the use of BMS within the FP segment, one must be cognisant of the fact that there are no prospective trials in patients with CLTI comparing POBA with BMS implantation.[139] The available data comparing POBA with BMS are comprised of predominantly patients with IC and this should be borne in mind when interpreting the outcomes data. When reviewing the results published by Nguyen et al.,[l77] 37% (n=307) of patients received BMS implantation, with only 29% being for CLTI. Primary patency for the entire cohort at 5 years was reported to be 41%, with no significant benefit of BMS over POBA. When considering patients with CLTI, 4-year primary patency was 27% for POBA v. 36% for BMS (p=0.22). There was also no significant difference in outcomes on limb salvage at 4 years and OS at 5 years. When stratified by TASC classification, there was no difference in outcomes between TASC A & B lesions; however, patients with TASC C & D lesions had improved short- and long-term primary patency (54% (BMS) v. 30% (POBA) at 1-year and 34% v. 12% at 5 years (p=0.05)). There was, however, no difference between POBA and BMS in limb salvage at 2 years, and OS at 4 years.
These findings were confirmed in a meta-analysis by Jens et al.,[178] which reviewed 15 trials comparing POBA with BMS (85% IC and 15% CLI) and revealed no significant difference in outcomes between 6 - 36 months of follow-up. The procedural cost was noted to be 57% higher in patients who received stents as compared with POBA (p=0.01).[178]
There are no published, publicly funded trials comparing POBA with DCB or DES in patients with CLTI. In a recent meta-analysis comparing DCB with POBA, most patients reported symptoms of IC.[179] The key findings of the meta-analysis were that DCBs were superior to POBA in reducing target lesion revascularisation, with reduced rates of late lumen loss and binary restenosis. There was no difference between POBA and DCB on amputation and mortality.[179] In the recently published Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia, it is suggested that POBA may be inferior to DCB angioplasty and stents for the treatment of intermediate-length SFA disease (FP grades 2 - 4) in patients with IC and possibly rest pain. However, they also reported that there are inadequate data to support a preferred approach for FP disease in CLTI.[8]
The BASIL trial has to date been the only significant RCT comparing BS with POBA. In a sub-group analysis performed on patients undergoing only FP intervention, there were 311 patients, of whom 128 underwent primary BS (n=89 vein and n=39 synthetic), and 183 had primary POBA with six receiving BMS, and the mean follow-up was 46.2 and 43.6 months, respectively. Immediate technical success was significantly better for BS (98% v. 81%; p<0.001). In the long-term, there was no difference in amputation free survival (62% v. 55%; p=0.4), OS (69% v. 63%; p=0.5) and limb salvage (85% v. 85%; p=0.8) between BS and POBA with optional stenting. However, freedom from MALE (67% v. 56%; p=0.4) and freedom from re-intervention (72% v. 63%; p=0.2) was significantly lower following BS. Resolution of rest pain and wound healing at 3 years were similar in the two groups.[180]
Plain specialty 'designer' balloons, encompassing cutting balloons, high-pressure balloons, focal-force balloons and plaque-modifying balloons were developed to provide a relatively straightforward tool to prepare a lesion. The general mechanism of action is to better concentrate the dilating force applied to the atherosclerotic plaque, leading to improved technical success (less recoil, less balloon slippage, less dissection), lower complication rates (less perforations), improved penetration of anti-proliferative drugs and ultimately increased overall patency compared with POBA. These balloons may be utilised as stand-alone therapies or prior to delivering definitive treatment (BMS, DCB or DES).
Cutting balloons are standard balloon catheters with 3 - 4 microsurgical blades or atherotomes attached to the surface of the balloon. A small RCT comparing cutting BA with POBA in short lesions (<5 cm) of the SFA, revealed a lower rate of restenosis in patients treated with a cutting balloon at 1-year (13% v. 36%; p=0.049).[181] In a larger prospective, non-randomised trial, Canaud et al.[182] were able to achieve a primary patency rate of 75.2% in patients presenting with ABK and FP lesions. They did, however, caution that these results cannot be extrapolated to potential outcomes in lesions >10 cm in length. Due to the relatively poor outcomes of POBA for the management of in-stent restenosis (ISR), cutting balloons were thought to offer a more promising alternative. However, in a randomised trial by Dick et al.,[183] there was no significant difference in the risk of recurrent ISR at 6 months between patients treated with cutting balloon angioplasty (CBA) and standard BA (65% v. 73%; p=0.73).
Unfortunately, high-quality evidence regarding the use of scoring and plaque-modifying balloons is lacking. The PANTHER registry reported on 124 calcified FP lesions treated with the AngioSculpt scoring balloon either alone, or in combination with a DEB or prior to BMS deployment.[184] They reported an overall 1-year primary patency rate of 81.5%, which did not differ significantly between the different treatment arms. Interestingly, the degree of calcification did not predict patency after 6 and 12 months. Unsurprisingly, the strongest predictor of patency was lesion length at 1-year. Excellent results were obtained in short lesions but rather disappointing in lesions >10 cm in length (53.8%). The Chocolate Bar registry is a large multicentre prospective registry that evaluated the dedicated focal force balloon (Chocolate) in 262 patients with a mean lesion length of 83.5 mm, and 23.1% of lesions were occlusions.[185] Patency at 12 months was 64.1% and freedom from clinically driven target lesion TLR was 78.5%. Interestingly, in the subgroup of severely calcified lesions, freedom from TLR was as high as 87%, indicating an increased benefit for focal force balloons in that challenging cohort.
Recommendation 99
It would be reasonable to offer POBA to a patient with a suitable venous conduit for bypass, presenting with CLTI and endosuitable femoropopliteal lesions, provided such interventions do not compromise a bypass procedure. (Class I; Level B)
Recommendation 100
POBA should be offered to patients with CLTI and endosuitable femoropopliteal lesions if no suitable saphenous vein graft is available. (Class I; Level B)
Recommendation 101
POBA may be considered in patients presenting with CLTI and femoropopliteal disease who are deemed unfit for open surgical bypass. (Class I; Level C)
Recommendation 102
Cutting balloons may be used to treat select native femoropopliteal lesions, when indicated. (Class IIa; Level B)
Recommendation 103
Focal force or plaque-modifying balloons may be considered in the treatment of focal and calcified femoropopliteal lesions. (Class IIb; Level C)
Drug-coated balloon treatment
The success of infrainguinal revascularisation procedures (i.e. POBA and BMS) is hampered by medium-term restenosis associated with neo-intimal hyperplasia and negative remodelling. The femoropopliteal artery segment is subject to multiple intrinsic and extrinsic mechanical forces and, as such, it is well known that BMS may not be ideal due to the potential risk of stent fracture and deformation.[1861] DCB was developed to achieve long-term patency without leaving an implant behind in an effort to increase future durable treatment options available to clinicians.[187] Local drug delivery of an anti-proliferative drug from balloon catheter systems to the site of arterial injury has been attempted repeatedly over the years with limited success in drug uptake and retention.[188] DCBs achieve the short-term transfer and long-term retention of paclitaxel to the arterial wall by different biological mechanisms. Accessibility of the drug at the site is critical to combat the body's response to the procedural trauma of angioplasty. Recent formulations have been designed that achieve delivery of therapeutic doses of the anti-proliferative drug paclitaxel to arteries with higher efficiency and longer tissue retention.'1891 These formulations succeed through formation of a drug reservoir in the arterial wall enabling release after the initial angioplasty procedure. These formulations have become the cornerstone of several DCB technologies that have found an initial, broad therapeutic application in infrainguinal revascularisation.
Several first-in-man randomised trials and registries using firstgeneration DCBs in FP lesions have shown favourable technical outcomes in terms of late lumen loss (LLL), restenosis rate, and freedom from TLR when compared with POBA.[190] DCBs have shown efficacy in the endovascular treatment of de novo lesions in the FP segment. Several RCTs have shown superior endovascular outcomes of DCBs compared with POBA, and in some cases, with outcomes almost comparable with BMS, and the advantage of a non-implant strategy. The randomised multicentre IN.PACT SFA trial reported that clinically driven TLR rates were significantly lower with DCB when compared with those achieved with POBA (2.4% v. 20.6%; p<0.001) at 1 year.'1911 Primary patency at 360 days calculated by Kaplan-Meier estimates was 89.8% for the DCB group and 66.8% for the POBA group (p<0.01). In the LEVANT I trial, which incorporated a 'blinded follow-up' in contrast to previous trials, the primary patency at 12 months, defined as freedom from both restenosis and TLR, was 65.2% for the DCB and 52.6% for plain angioplasty, demonstrating superior efficacy (p=0.015).[192] The freedom from clinically driven TLR in the DCB group was similar to the control group (87.7 v. 83.2%; p=0.208) at 12 months. In this study, both the safety (freedom from death, amputation or re-intervention) and efficacy endpoints were met. However, the lack of clinical efficacy of DCB expressed by a clinically driven TLR rate similar to the control group at 12 months is of concern. In both studies, no device-specific side-effects were reported, and no major amputation occurred. Furthermore, there were no safety concerns regarding wash-off of some of the anti-proliferative drugs into the distal vasculature.'193,1941 Only a few registries are available that included a large population of patients with CLTI with treatment of longer lesions. These registries, albeit non-randomised, all show better patency outcomes with DCBs as compared with POBA.
ISR has been reported to occur in up to 40% of FP lesions treated with BMS within 1 year. Moreover, the risk of ISR increases with increasing lesion length. As the population with FP stenting continues to increase, occurrence of ISR has become a clinically relevant problem. The treatment of ISR in the FP artery is one of the major remaining challenges of endovascular therapy because treatment modalities such as PTA and CBA have failed to provide durable results; hence, the shift to DCBs. The data of the randomised, controlled drug-eluting-balloon v. PTA for the Femoral Artery In-stent Restenosis (FAIR) trial reported on 119 patients with ISR up to 20 cm in length, and a mean lesion length of 8 cm in both groups (DCB v. PTA).[195] The primary endpoint was the 6-month restenosis rate, which was in favour of DCB when compared with PTA (15.4% v. 44.7%; p=0.002). At 1 year, restenosis rates were 29.5% and 62.5%, respectively (p=0.004), and freedom from clinically driven TLR at 390 days was 90.8% and 52.6% (p=0.0001), respectively, in favour of DCBs.
There has been some concern regarding the use of DCBs and the increased mortality in DCB patients. A recent meta-analysis of the randomised DCB trials in the FP segment does report an association between the use of DCBs and increased mortality.'1961 This meta-analysis by Katsanos et al.,[196] found that POBA shows an all-cause mortality of 8.1% v. 14.7% (RR 1.93) in DCB at 5 years.[196] Intriguingly, the cause of the increased mortality in DCB-treated patients could not be identified. The methodology behind the meta-analysis remains highly debatable, and the individual trialists have failed to reproduce these findings. As such, the FDA has issued a 'black-box' warning leaving the use of DCBs at the discretion of interventionalists with informed consent advised to patients.
Future directions
Various DCBs are available on the market, with no known 'class effect' of this technology across these various products. Head-to-head comparison trials of DCBs available on the market are required to assist in product choices. The DCB evidence is hinged on claudicants with short SFA lesions and as such, RCTs in CLTI population are awaited to assist in real-world extrapolations of their clinical efficacy.
Recommendation 104
In the endovascular treatment of de novo femoropopliteal lesions following optimal vessel preparation ('responders'), DCBs are recommended as the final definitive treatment option. In view of the still-to-be resolved mortality data conflict with paclitaxel-coated balloons, such treatment needs to administered with caution, with informed patient consent until recommended otherwise in future guidelines. (Class IIa; Level B)
Recommendation 105
There is currently no clear-cut evidence to suggest that DCB use is independently associated with death, to absolutely preclude its use until further evidence recommends otherwise in future guidelines. (Good practice statement)
Recommendation 106
DCBs are recommended as an adjunct to the endovascular treatment of in-stent restenosis. (Class IIa; Level C)
Debulking strategies
Debulking atherectomy theoretically allows for a more uniform angioplasty result at lower pressures with less vessel barotrauma, improved luminal gain, a decreased risk of vessel wall recoil and flow-limiting dissection requiring a stent. It also potentially disrupts the calcium barrier and optimises both drug transfer and delivery in drug-coated or drug-eluting technologies. Endovascular atherectomy devices can be divided into four categories according to the mechanism used for atheromatous plaque removal: directional, rotational, orbital and laser atherectomy devices.
There are no data regarding the comparison of different atherectomy devices in PAD patients. Each device presents unique features with discrete advantages and disadvantages.
Directional atherectomy
In directional atherectomy, plaque is removed by guiding the cutting device of the catheter directly to the plaque. Rotation of the catheter tip to the preferred direction allows targeted atherosclerotic plaque removal. Examples include the SilverHawk, TurboHawk and Hawk 1 systems. These devices do not have an aspirating mechanism and excised plaque is collected and compacted within the nose cone. Once the nose cone is full, the device needs to be retrieved and emptied before further use. Disadvantages include possible arterial wall trauma and long procedure times.
The Pantheris device is an over-the-wire catheter that has optical coherence tomography technology to enhance directional atherectomy efficacy and safety. The catheter uses a side cutter and a nose cone like the previously mentioned devices but also uses an apposition balloon, which enables ocular computer tomographic (OCT)-guided depth modification of atherectomy. The image guidance allows avoidance of damage to the normal vessel wall
Distal embolisation remains an issue with all these devices and use without an appropriate protection filter is not advisable.
The TALON registry is a large, prospective, multicentre study documenting outcomes with the SilverHawk device in 601 patients (748 limbs) with claudication or CLI.[197] Procedural success was 97.6%, and the 6- and 12-month rates of freedom from TLR was 90% and 80%, respectively.[197]
The DEFINITIVE LE is a multicentre prospective, real-world registry of 800 patients with infrainguinal lesions of up to 20 cm treated with SilverHawk. Primary patency was 78% at 1-year and was similar between diabetics and nondiabetics. Peri-procedural adverse events were embolisation (3.8%), vessel wall perforation (5.3%) and abrupt vessel occlusion (2%).[198]
Zeller et al.[199] reported long-term results of a prospective single centre registry using SilverHawk in FP lesions in 84 patients (100 limbs). Primary patency according to DUS was 84% in de novo lesions, 54% for native vessel restenosis and 54% for ISR at 12 months. Distal embolisation occurred in 6% of the cases.
Shammas et al.[2001 published a two-centre RCT comparing primary BA v. SilverHawk directional atherectomy with adjunctive BA in 58 patients. Patients had IC or CLI. During follow-up, TLR (11.1% v. 16.7%) and target vessel revascularisation (TVR) (11.1% v. 21.4%) were similar between the 2 groups. Atherectomy plus BA resulted in significantly less bailout stenting (27.6% v. 62.1%; p=0.017). Macro-embolisation was, however, significantly higher in the atherectomy arm (64.7% v. 0%; p<0.001).[200]
Rotational atherectomy
In rotational atherectomy, plaque is excised by a concentrically rotating specially designed tip. As a result, luminal gain usually matches the size of the tip. Systems include the Pathway Jetstream PV atherectomy system, the Peripheral Rotablator system, the Phoenix Rotational Atherectomy system, and the Rotarex S device. The Jetstream system has active debris aspiration and is indicated for both acute thrombus removal and atherectomy of chronic lesions. The Phoenix system is a single-use catheter without capital equipment. Excised plaque is mechanically transported within the catheter using an Archimedes screw. Micro- and macro-embolisation is possible with all these systems and filter protection is recommended.
The Pathway PV trial is a multicentre, prospective registry that investigated rotational atherectomy in FP and infrapopliteal lesions in 172 patients with either IC or CLI. Technical success was 99%. Clinically driven TLR rates were 15% and 26% at 6 and 12 months, respectively. The restenosis rate detected with DUS was 38.2% at 1 year.[201]
Mehta et al.[202] published a retrospective series investigating CFA rotational Jetstream atherectomy with adjunctive BA and provisional stenting v. plain BA in 167 patients, and follow-up at 42.5 months. Patients in the PTA only group had significantly less patency. The CFA provisional stent group achieved 100% primary patency rate.[202]
The EASE study investigated 148 lesions in 128 patients using the Phoenix atherectomy device. Technical success was 95.1% and 6-month freedom from TLR and TVR were 88.0% and 86.1%, respectively.[203]
Orbital atherectomy
The Diamondback 360° Peripheral Orbital Atherectomy system is an atherectomy mechanism based on the high-speed rotational spin on the shaft and the orbital rotation of a specially designed debulking, diamond-coated crown. The debulking area increases with the increasing rotational speed of the crown. There is no aspirating mechanism and theoretically the small particles created are not hazardous. Distal embolisation can still occur, and a peripheral protection filter is still advised.
A multicentre RCT comparing orbital atherectomy plus BA v. BA alone in 50 patients, and 65 calcified FP lesions, showed significantly less stenting in the atherectomy arm (5.3% v. 77.8%; p<0.001), but no significant superiority in outcomes at 1 year (freedom from TLR 81.2 v. 78.3%; p>0.99).[204]
Laser atherectomy
Laser atherectomy devices (Turbo-Elite, Turbo-Power and TurboTandem devices) use excimer laser technology to ablate atheroma. Ultraviolet radiation removes 10 μιη of atheroma with each pulse of energy. Laser atherectomy is indicated in de novo stenoses and ISR. Micro- and macro-embolisation have been described.
A RCT compared excimer laser atherectomy (ELA) plus BA v. BA alone in 250 patients with femoropopliteal ISR and presenting with either CLI or IC.[205] The ELA device resulted in superior procedural success (93.5% v. 82.7%; p=0.01), and freedom from TLR (73.5% v. 51.8%; p<0.005), and fewer procedural complications and 30-day major adverse event rates (5.8% v. 20.5%; p<0.001). The ELA device was also associated with a 52% TLR reduction.[205]
A 2014 meta-analysis summarised outcomes of percutaneous transcatheter atherectomy in the FP segment.[206] The study included 6 RCTs comprising 287 patients (328 lesions) treated with atherectomy or BA. Technical success, bailout stenting, distal arterial embolisation, and 9-month primary patency were similar between the two groups.[206]
Ramkumar et al.[207] analysed the clinical outcomes by endovascular treatment type in 16 838 patients. The 5-year rate of MALE was 38% in patients receiving atherectomy v. 33% for PTA and 32% for stenting (p<0.001). Controlling for unmeasured confounding using instrumental-variable analysis, patients treated with atherectomy experienced outcomes like those of patients treated with PTA, except for a higher risk of any amputation (HR 1.51; 95% CI 1.08 - 2.13).
However, atherectomy patients had a higher risk of major amputation (HR 3.66; 95% CI 1.72 - 7.81), any amputation (HR 2.73; 95% CI 1.60 - 4.76), and MALE (HR 1.61; 95% CI 1.10 - 2.38) compared with stenting patients.[207]
The DEFINITIVE AR trial is a multicentre, industry-sponsored RCT that compared SilverHawk/TurboHawk atherectomy plus DCB (n=48) v. DCB alone (n=54) in 102 patients.[208] There was significantly lower flow-limiting dissection in the atherectomy plus DCB arm (2% v. 19%; p=0.01) and the need for bailout stent was only 4.1%. The 1-year freedom from restenosis was 93.4% for the combined approach and 89.6% for the DCB arm (p>0.05) using DUS.[208]
Based on contemporary data, atherectomy can be effectively and safely used in both FP and infrapopliteal disease. It appears to significantly decrease the need for stenting, and this facilitates future endovascular or open surgical options. It also potentially allows avoidance of stenting in hostile arterial segments such as flexion points.
Despite the advantages of plaque removal, percutaneous atherectomy has not significantly reduced restenosis rates compared with standard endovascular therapy. Real-world evidence varies, with some reports of improved or equivalent outcomes compared with traditional treatments such as PTA or stenting. RCTs of atherectomy lack long-term outcome evaluation and are underpowered to appropriately evaluate atherectomy performance against other endovascular treatments. The long-term outcome of atherectomy remains unknown despite its widespread use. Another major disadvantage is the risk of distal embolisation and therefore the mandatory need for distal filter protection.
Recommendation 107
Percutaneous endovascular atherectomy should only be considered in select cases, as it has not been shown to be superior to standard endovascular techniques and is associated with greatly increased costs. (Class IIb; Level B)
Accessory devices for endovascular intervention
A wide array of devices are available, enabling interventionalists to treat longer and more complex lesions in the peripheral arterial circulation. Unfortunately, there is only limited evidence comparing these devices with one another, or with standard treatment protocols. Examples of these devices include crossing and re-entry devices, EPD, intravascular ultrasound (IVUS) and mechanical thrombectomy devices (MTD). The main driver for the use of these devices is to increase the scope of lesions that can be treated endovascularly, in other words, to avoid BS and its complications. Another aim is to try and improve on the fairly average to poor results of PTA and stenting in some complex lesions and to decrease complications such as perforations and dissections. These devices are typically advocated for use in chronic total occlusions (CTO), flexion areas, heavily calcified lesions and at bifurcations. A drawback of all these devices is that they add considerable cost and complexity to the intervention.
Crossing and re-entry devices
Up to 40% of patients presenting for endovascular intervention have CTO. The treatment of long complex lesions can be challenging and time consuming and risk vessel perforation, dissection, distal embolisation with worsening ischaemia, loss of collateral vessels and distal bypass targets, formation of arteriovenous fistula, increased radiation exposure and an increased contrast load.'209- The CTO lesion often has a rigid fibrous cap at both ends with the distal cap often tapered and softer, with varying amounts of lipids, organised thrombus and extracellular matrix in the centre. Endothelialised micro-channels that traverse the occlusion increase the likelihood of passage with low-profile hydrophilic catheters.[209] Standard guidewire technique to cross a CTO include a combination of a hydrophilic guidewire with a low-profile support catheter to cross the lesion intraluminally. The guidewire can also be passed in the subintimal plane and techniques to facilitate re-entry into the true lumen are well described.'210- Other techniques to cross CTOs include retrograde and transcollateral crossing techniques.[209]
Several CTO crossing devices are available when standard techniques fail and can be guided fluoroscopically or with adjunctive intravascular imaging. Fluoroscopically-guided devices include the Frontrunner (blunt microdissection device), Crosser (high-frequency vibrational energy device), Wildcat (crossing catheter with distal spiral flutes that can be rotated), Viance catheter (distal blunt tip with forward push and fast spin) and TruePath (diamond-coated distal tip on hydrophilic wire with spin). The Ocelot catheter is OCT-guided and uses high-resolution cross-sectional images of the vessel with infrared light to aid crossing of CTO. The use of IVUS to cross lesions has been described. Technical success in crossing CTOs after failed guidewire ranging between 65% and 95% have been described for these devices. However, poor results (41% for crossing) as well as perforations have also been reported.[209,211]
Re-entry devices (RED) are available to link the subintimal space to the distal true lumen. All of these have a puncture needle and a method to orientate the needle in the direction of the true lumen for guidewire passage after successful puncture. Fluoroscopically-guided devices include the Outback catheter, Enteer (part of Viance system with flat-shaped balloon to orientate in the subintimal space), and Offroad (which was previously called conical-shaped positioning balloon (SPOT)). The Pioneer catheter orientates the needle toward the true lumen with IVUS guidance. Successful re-entry ranging between 70% and 95% has been reported. However, complications such as bleeding and pseudoaneurysm have also been reported.[209]
Embolic protection devices
Distal embolisation after peripheral intervention can be a devastating complication resulting in limb loss. The incidence of distal embolisation during peripheral intervention ranges from 50 - 100% depending on the mode of detection (filter inspection v. Doppler signals). Clinically significant embolisation requiring further treatment occurs in 2 - 11 % of cases, with an estimated 2% being severely limb-threatening.'212- Embolisation occurs during all steps of peripheral intervention, but are more frequent during stent implantation, and especially during atherectomy and laser procedures.[213] In the PROTECT registry, 100% of atherectomy cases had embolic material in the filter basket, which was larger than 2 mm (macro-emboli) in 90% of cases.'214- Attempts to identify patients at high risk of clinically significant embolisation has yielded conflicting results. Karnabatidis et al. [215] identified lesion length, increased vessel diameter, acute thrombosis and total occlusion as risk factors, but Mendes et al.[216]failed to show an association with lesion length, TASC score, runoff score, treatment type or indication (only occlusion was identified as a risk factor). Medical comorbidities such as diabetes and end-stage renal disease may place patients at a higher risk.'217-Acute and subacute lesions, especially when treated with catheter-directed pharmacomechanical thrombectomy (CD-PMT), or PTA after thrombolysis, seem particularly prone to embolisation. This is often treated by aspiration thrombectomy, or further thrombolysis, or CD-PMT, with adverse clinical sequelae being rare.'218- Techniques to limit embolisation during CD-PMT have been described. Restenosis lesions, thrombosed or occluded stents and bypass grafts, patients with prior history of embolisation during endovascular intervention, and patients with prior amputations are also mentioned as risk factors.'212- The successful use of embolic protection device (EPD) for aorta-coronary SVG and carotid interventions has created interest to use these devices in the lower extremities and several studies have shown that it can be done safely.'219,220-Drawbacks include costs, technical difficulty in delivering the devices, the need for long device wire lengths, vessel dissection or thrombosis, vasospasm, filter occlusion, entanglement, and tear or separation.[22] This has led to conflicting recommendations ranging from very aggressive use when any of the above risk factors are present,[222] to selective use when there is poor runoff or lesions appearing complex and vulnerable to fragmentation,[223] and not recommending its use due to clinically significant events being rare.[213]
Intravascular ultrasound (IVUS)
Use of IVUS in the peripheral circulation has been proposed on the grounds that it allows accurate measurement of lesion length, vessel diameter, treatment result such as stent apposition, and presence and severity of dissection. It can also reduce contrast load and radiation dose, and assist with re-entry to the true lumen. Drawbacks include cost, longer procedure times and current paucity of evidence. Correct sizing for stents in the SFA is important since excessive oversizing leads to decreased patency. The use of IVUS post PTA or stenting of the SFA in one study demonstrated a residual stenosis >70 % in 79% of angioplasty patients, and in 54% of stented patients.[224] Analysis of propensity score-matched patients (in a retrospective multicentre database) revealed higher primary, primary-assisted and secondary patency for SFA stenting in patients where IVUS was used.[225-
Recommendation 108
Crossing and re-entry devices may be considered in the treatment of CTOs after standard endovascular techniques have failed. (Class IIa; Level B)
Recommendation 109
Embolic protection devices may be considered when an atherectomy device is used (especially in patients with limited outflow) for lesions appearing complex and vulnerable to fragmentation, for ISR, for acute and subacute thrombosis (de novo, stent, or graft) if pharmaco-mechanical thrombectomy is used, or when balloon angioplasty is being considered after catheter-directed thrombolysis. (Class IIa; Level C)
Recommendation 110
Due to limited evidence for the use of IVUS, no recommendation can be made currently. Its use should be limited to very select and motivated cases. (Good practice statement)
Bare metal stents
Endovascular recanalisation is less invasive than surgery and has evolved to become the primary method of treatment for most TASC A - C lesions.[19] Currently, there is no evidence to support the use of primary BMS in the FP segment. Contemporary practice recommends the use of BMS as a bailout option following a suboptimal or complicated BA (major flow-limiting dissections, significant recoil, etc.). BMSs are more durable than plain BA. However, primary patency rates diminish remarkably beyond 2 years.
Results of PTA alone to recanalise peripheral arteries are often hampered by vessel-wall recoil and trauma causing dissection. It is possible that stent placement can prevent this. Stent design must address multiple challenges related to specific anatomical and pathological features of the SFA, the popliteal artery, plague morphology, mechanical forces such as elongation, compression and torsion, and finally restenosis caused by neointimal hyperplasia[187,226]
The UK National Institute for Health and Care Excellence (NICE) 2012 guidelines recommended the use of PTA as a primary treatment in SFA lesions <10 cm long with bailout stenting. Multiple RCTs have compared the effectiveness of PTA v. PTA and BMS and the results of these trials are against this recommendation.[227] The authors included 11 trials with 1 387 participants and a 2-year follow-up. They concluded that there was a short-term gain in primary patency; however, there was no sustained benefit from primary stenting of lesions of the SFA compared with optional stenting post angioplasty after 24 months.[227] Anti-platelet therapy and inclusion criteria regarding affected arteries between trials showed marked heterogeneity. Additionally, the 'Palmaz' balloon expandable stent was used in the five trials, which is not optimal for this application.
There are two registries (SUMMIT and COMPLETE SE) with newer-generation self-expanding stents which showed improved rates of primary patency, with low to zero rates of stent fractures and improved patient-reported outcomes.[228,229]
In recent years, multiple trials have shown superiority of DES over BMS. The possibility of using thin-strut self-expanding BMS with DCBs was also reported.[230] However, this enthusiasm was tempered by the systematic review and meta-analysis of RCTs in December 2018, which reported significantly higher mortality associated with paclitaxel-coated balloons and stents.[196]
The efficacy of stenting of the popliteal artery is unclear, as most studies report on stenting of the SFA and popliteal artery proximal to the knee joint. The Supera stent, which is a self-expanding interwoven nitinol stent, was proposed for use in the popliteal artery across the knee joint, and in long SFA and popliteal lesions due to a design which provided unique flexibility and resistance to fracture. It is well known that all current endovascular technologies show worse results when arterial lesions get longer. Supera seems to have consistent primary patency rates in 1 year regardless of lesion length.[231-
The Tigris stent, with the dual-component design which is supposed to be dedicated to address challenges encountered in the management of FP artery occlusive disease, has the distinct advantage of extremely accurate deployment and long-axis adaptability with absence of foreshortening or elongation.[232-
Recommendation 111
Bare metal stenting of femoropopliteal lesions may be considered in select patients following suboptimal or complicated BA. (Class IIa; Level B)
Recommendation 112
The Supera stent might be used selectively in complex and calcified femoropopliteal lesions, or when stenting is required across the knee joint. (Class IIa; Level B)
Drug-eluting stents
Drug-eluting technology in the coronary circulation came onto the scene with the RAVEL trial, which brought out the paradigm shift in interventional cardiology.[233] This was further supported by large-scale randomised trials, which confirmed the superiority of both paclitaxel-eluting and sirolimus-eluting stents, compared with both PTA and BMS.[234] Despite these remarkable and exciting developments, these were not translated to the peripheral arterial system.
The complex anatomy and mechanical forces in the SFA pose a challenge in the performance of the stents, including DES. The mechanical forces such as torsion, compression, elongation, and flexion put a significant stress in this arterial segment. Further challenges are brought out by long segments of stenosis/occlusions and extensive plaque burden, in contrast to the coronary circulation.
The SIROCCO studies (I and II) compared sirolimus-eluting self-expanding nitinol stents to BMS. In the drug-eluting arm, the restenosis rates were 18.4% and 22.9% for DES, compared with 12.8% and 21.1% for BMS at 18 and 24 months, respectively.[235] The STRIDES trial was a single arm study of evorolimus-eluting nitinol stent that reported a 1-year patency rate of 54.6%.[236] The STRIDES authors compared their results with the identical BMS in the VIENNA trial, which demonstrated a 63% patency at 1 year.[237] These disappointing results led to disillusionment about drug-eluting technology in PAD.
Besides the complex mechanical forces in the FP segment, factors like time course to restenosis, drug dosage and elution kinetics play a significant role in the success of DES. Non-resorbable polymers in the DES may induce inflammatory and thrombotic reactions, leading to late restenosis and thrombosis.[238] The polymer configuration, coated v. free, is tested in the ongoing IMPERIAL trial, which compares a polymer-coated paclitaxel-eluting stent (Eluvia) v. a polymer-free paclitaxel-coated stent (Zilver PTX), and demonstrated similar outcomes in terms of primary patency and major adverse events at 12 months after treatment of patients for femoropopliteal artery disease (published as an interview with Dr WA Gray in Vascular Disease Management: Volume 16 - Issue 12 - December 2019). Paclitaxel, once incorporated in the target artery and locally circulating macrophages interferes with the turnover of microtubules and inhibits smooth-muscle proliferation and migration, which are key elements of neointimal hyperplasia.[239]
The Zilver PTX DES has been investigated in the RCTs and large prospective multicentre registries, comparing it to BMS and PTA for lesions up to 14 cm in length in the SFA and proximal popliteal artery. The 1-year primary patency after primary stenting was superior in the DES group compared with PTA group (83% v. 33%). In the subgroup which needed bailout stenting because of suboptimal PTA or flow-limiting dissection, provisional DES was superior to provisional BMS (89.9% v. 73%) in 1-year primary patency rates.[240] DES had a superior primary patency over BMS, with 2-year primary patency of 83.4% v. 61.1%, and 5-year primary patency of 66.4% v. 43.4%.[131,132]
The limitations of most studies are that they are mostly industry sponsored, with a large percentage of IC patients compared with patients with CLTI. Most trials are underpowered to determine differences in clinically relevant outcomes like limb-salvage, and rather they report on patency or LLL.
Recommendation 113
DES are preferred to BMS following select cases with suboptimum or complicated femoropopliteal BA. (Class IIa; Level B)
Covered stents
Surgical bypass with a vein graft has long been considered the gold standard for FP revascularisation. Covered stents (stent graft/ endograft/endoprosthesis) are considered an acceptable alternative in high-risk patients, long-length lesions or when there's a lack of a suitable vascular conduit. Earlier studies on covered stents to treat FP lesions reported on the safety and efficacy results in small observational case series. In one series, the primary and secondary patency rates were 90% and 95% at 30 days (n=59), 67% and 81% at 1 year (n=58), 57% and 80% after 3 years (n=49), and 45% and 69% after 5 years (n=32). However, 92% of the study population were claudicants.[241]
Currently, most of the reported literature studies and data on the use of covered stents in the FP segment relate to the use of a self-expandable heparin-bonded, ePTFE-lined nitinol endoprosthesis. The body of evidence supporting the use of these covered stents in treating long lesions (>15 cm) and chronic occlusions of the FP segment continues to evolve.
A single arm, prospective multicentre VIPER study enrolled 113 patients and treated 119 limbs with Rutherford clinical category 3 - 5 patients with TASC C and D FP lesions.[242] More than 80% of the study population were claudicants. The mean lesion length was 19 cm (56% were occlusions). The mean (SD) Rutherford clinical category improved by 2.4, and the ABI increased from 0.6 (0.2) to 0.9 (0.19) (p=0.0001) at 12 months. The primary and secondary patency rates were 73% and 92%, respectively. Aggressive stent graft oversizing >20% was associated with a reduced primary patency rate (p=0.047). The stent graft performance was not influenced by stent graft diameter or lesion length (>20 cm or <20 cm).
The Japanese multicentre VIABAHN study was a similar study design.[243] One hundred and three patients were enrolled (97% were claudicants). The average lesion length was ~21.8 cm, and 65.7% were total occlusions. The primary patency-surgical rate was 92.1% (95% CI 84.8 - 96.0), and freedom from TLR was 93.1% (95% CI 86.1 - 96.7). No deaths or major amputations were reported in this cohort of patients.
The prospective, randomised multicentre VIASTAR TRIAL compared stent graft (VIABAHN) with BMS in patients with symptomatic FP disease.[244] More than 80% of patients were claudicants (Rutherford clinical category 2 and 3). The mean (SD) lesion length was 19.0 (6.3) cm in the VIABAHN group v. 17.3 (6.6) cm in the BMS group. The primary patency rates for VIABAHN and BMS were 63.1% and 41.2% (p=0.04) at 24 months, respectively. The patency rates for lesions >20 cm were significantly better in the VIABAHN group (65.2% v. 26.7%; p=0.004) compared with the BMS group. Moreover, the freedom from TLR was better in the VIABAHN group (79.4% v. 73.0%; p=0.37) compared with the BMS group.
The RELINE study compared VIABAHN with standard BA in the treatment of ISR.[245] This was a prospective, randomised multicentre trial. Eighty-three patients were enrolled into this study, and ~87% were claudicants. The technical success was 100% for the VIABAHN group and 81.8% for the angioplasty group (p=0.002). The 12-month primary patency rate was 74.8% for the VIABAHN group and 28.0% for the angioplasty group (p<0.001). Nine patients required bailout stenting after failed angioplasty.
A prospective randomised, multicentre, Dutch study compared FP bypass with heparin-bonded-ePTFE endografts.[246] One hundred and twenty five patients were treated, 63 in the endoluminal and 62 in the surgical group (42 venous and 20 prosthetic). Approximately two-thirds of the patients were claudicants. The Rutherford clinical categories 4 and 5 was 32.2% in the surgical arm, and 38.1% in the endoluminal treatment arm (p=0.55). Mean lesion length was 23 cm in both groups, and predominantly TASC D lesions were treated. The 30-day and 1-year results were published: 'there were no significant differences in the Rutherford category between groups at any time point. At 30 days, the endoluminal group showed a greater improvement in QoL scores. At 1 year, these differences had largely disappeared and no differences in primary (endoluminal: 64.8%; surgical: 63.6%), assisted primary (endoluminal: 78.1%; surgical: 79.8%), secondary patency (endoluminal: 85.9%; surgical: 83.3%), and target vessel revascularisation (endoluminal: 72.1%; surgical: 71.0%) were observed. Limb salvage rate was 100% in both groups'. A subgroup analysis of the patients with CLTI was not performed.
With the rapid advances in peripheral balloon and stent technology, endovascular outcomes are steadily improving. The indications for covered stents, however, based on current performance in patients with FP disease and CLTI, are extremely limited. Despite this, certain situations like residual stenosis or dissections may necessitate the use of selective stenting and the risk of future ISR.
ISR (>50% reduction in diameter angiographically) due to negative remodelling has always been the Achilles heel of BMS. Restenosis rates as high as 40% have been reported after 1 year. ISR can occur at any point along the length of an uncovered stent and probably accounts for the reduced patency rates when treating longer lesions. With the use of covered stents, neointimal hyperplasia only occurs at the ends of the stent ('edge stenosis'). This is a factor that accounts for the superior patency rates seen when treating longer lesions. Other possible contributing factors to improved patency rates reported are the heparin-bonded luminal layer, and long stent lengths up to 250 mm, which avoid the use of a second device and stent overlap.
Long-segment endoluminal management of patients with femoral and proximal occlusive disease is technically safe and effective in the short and intermediate term for covered stents. Much of the results, unfortunately, have been reported in predominantly claudicant populations. Whether similar results can be reproduced in CLTI patients remains speculative. In clinical practice, these stent grafts may provide a useful bailout option in patients with complicated FP BA.
Guidelines are continuously evolving as newer technologies, improved physician experience and latest trial data become available. It must therefore be individualised for each patient on a case-by-case basis considering clinical indication, operative risk, angiographic findings, desired outcomes and resource availability.
Recommendation 114
Self-expandable covered stents are generally not considered as first-line treatment for treating femoropopliteal lesions. Balloon expandable covered stents are not indicated to treat femoropopliteal lesions. (Good practice statement)
Recommendation 115
Self-expandable covered stents may be considered following suboptimum BA for long calcified femoropopliteal lesions. (Class IIa; Level B)
Recommendation 116
Self-expandable covered stents may be considered in the treatment of ISR. (Class IIa; Level C)
Recommendation 117
Self-expandable covered stents may be considered to treat vessel wall rupture following femoropopliteal BA. (Class I; Level C)
Recommendation 118
Self-expandable covered stents may be considered in treating long-segment femoropopliteal lesions as an alternative to a bypass procedure in a high-risk patient with CLTI. (Class IIb; Level C)
Below-the-knee (BTK) interventions
Plain balloon angioplasty and debulking strategies
Infrapopliteal or below-the-knee (BTK) PAD has been historically implicated in CLTI. Open surgical techniques previously dominated the treatment of CLTI in this context. However, several limitations of surgery allowed the endovascular approach to gain traction and momentum. Subsequently, the technologies available for BTK interventions have increased exponentially, especially in the past decade. Despite these significant therapeutic advances in the infrapopliteal region, sparse data exist regarding comparative outcomes between endovascular and surgical interventions, as well as outcomes comparing different endovascular techniques. Numerous percutaneous approaches are available, although no single device or combination of devices has demonstrated clear superiority. This lack of clarity is attributed to the limitations of the clinical evidence, including variation in outcomes that have been studied across clinical trials, the paucity of studies exclusively looking at patients with CLTI, and a lack of direct comparisons of devices. Additionally, many clinical trials have been conducted at a single centre, or in only a few centres by experienced operators, limiting their generalisability to the broader population.
Balloon angioplasty
The traditional endovascular treatment algorithm supports the use of POBA and BMS as a bail-out option (provisional/secondary stenting) in cases of residual stenosis or flow-limiting dissection. However, tibial arteries often have medial calcification, limiting POBA efficacy due to vessel recoil, dissection, and high rates of TVR.'2471 BA, however, remains the first-line interventional mainstay of endovascular treatment for infrapopliteal disease as recently re-emphasised by the 2019 GVG.'81 Progress in balloon technology has resulted in improved crossing profiles, longer shafts, and balloon lengths. In addition, tapered balloons are available which help approximate the natural vessel taper of tibial vessels. In addition to platform peripheral balloons, coronary balloons are frequently used in the treatment of infrapopliteal disease and particularly in distal, inframalleolar, and vessels which have improved crossing ability as compared with their peripheral counterparts.'2481
As the boundaries of therapy have advanced, so have the shortcomings of POBA in complex lesions. BA can incur several initial technical complications, such as flow-limiting dissection leading to occlusion, or elastic recoil leading to technical failure. It also has a high short-term restenosis rate due to negative remodelling and neointimal proliferation. While the development of stent technology has sought to address these major concerns, several BA systems based on innovative, disruptive technology have emerged, combining the features of conventional balloon dilation with advanced physical and microsurgical capabilities. These include cutting, scoring and nitinol-caged balloons. Limited data suggest that these specialised balloons may result in fewer dissections, but long-term efficacy data compared with POBA are lacking.
Mustapha et al.[249] recently published the largest known meta-analysis in patients treated with POBA for infrapopliteal atherosclerotic lesions, including 52 studies with almost 7 000 patients. They reported that POBA can be successfully performed in 91% of cases with only a 5.6% incidence of flow-limiting dissection. They also reported that 9.1% of BTK interventions required provisional stent placement. They reported moderate success for POBA in patients with infrapopliteal arterial disease with 1-year outcomes of primary patency of 63.1%, repeat revascularisation rate of 18.2%, major amputation rate of 14.9%, and all-cause mortality of 15.1%. Comparing 1-year outcomes from this meta-analysis with a 2008 meta-analysis by Romiti et al.'2501 that included studies published between 1990 and 2006, primary patency was 63% v. 58%, major amputation was 15% v. 14%, and all-cause mortality was 15% v. 13%. This is concerning, as these findings suggest that outcomes of POBA for infrapopliteal disease have not changed over the last decade, despite newer techniques, approaches, and available technologies. It could seem that POBA technology has reached a therapeutic plateau.
Adjunctive therapies
While BA remains the mainstay of therapy for infrapopliteal occlusive disease, BA alone is limited by vessel recoil, dissection, and high rates of restenosis and occlusion. For these reasons, a number of adjunctive therapies have been developed to improve the early and potentially long-term results of infrapopliteal endovascular interventions.
Cryoplasty
Cryoplasty delivers pressurised liquid nitrous oxide at -10°C for 20 seconds via a balloon catheter, followed by a passive warming cycle. Cryotherapy is thought to create a uniform rigidity in the wall to alter collagen fibrils and to induce smooth-muscle cell apoptosis -this produces a uniform mechanical load on the balloon-vessel wall interface that will decrease significant plaque dissection, limit recoil in the vessel, and decrease intimal hyperplasia. In the 2009 BTK Chill trial, 108 patients were treated with cryoplasty, with a primary endpoint of technical success defined as <50% residual stenosis.[251] This was attained in 97.3% of procedures. The AFS was 85.2% at 1 year. Controversies continue to exist on the cost-benefit of cryoplasty v. POBA, and there are limited data to support the routine use of cryoplasty in the treatment of PAD.[252]
Atherectomy
Atherectomy'253- or catheter-based plaque debulking technologies, have in recent years been studied as an adjunctive therapy to POBA to improve early and late results.
The potential benefits of atherectomy include increased vessel compliance, removal of eccentric plaque, reduction of neointimal hyperplasia, and reducing the rate of dissection after subsequent BA.[254] A number of atherectomy devices have been utilised as an adjunctive therapy to PTA in an effort to modify the atherosclerotic plaque. These include laser, directional, rotational and orbital atherectomy. Some studies suggest that it is associated with increased costs and resource utilisation, and that its cost-effectiveness remains controversial.'255-
Data on the use of atherectomy for BTK intervention are extremely limited. A subgroup analysis of the DEFINITE-LE trial appeared promising. The primary patency of 189 lesions (mean lesion length 58 mm) in 145 patients treated with stand-alone directional atherectomy was 84% at 1 year. Freedom from major amputation was 97% (94% in patients with CLI) at 1 year.[198] The OASIS trial of 201 lesions (mean lesion length 30 mm) in 124 patients reported a 90% technical success rate, and an improvement of 78% in the Rutherford clinical category.[256] Although both trials reported positive outcomes, the short lesion length, the short follow-up period, and the lack of standardisation of the primary outcomes in the OASIS trial confounded clinical interpretation of these studies.
In their analysis of a multicentre registry, Khalili et al.[247] suggested that the use of atherectomy in BTK intervention may decrease the need for repeat interventions at 1 year by 59%, coinciding with a numerically lower target-limb minor amputation rate. Furthermore, despite concerns for prolonged procedural times with atherectomy, they demonstrated a shorter procedure duration, similar contrast utilisation, and comparable complication rates. As such, atherectomy may prove to be important in BTK revascularisation as an adjunctive therapy to PTA or DCB angioplasty, pending adequately powered randomised trials with relevant clinical outcomes.
Intravascular lithotripsy
Intravascular lithotripsy (IVL) is an emerging technology developed to tackle vascular calcifications, and BTK disease is heavily burdened by the presence of intimal and medial calcification. The IVL device induces lithotripsy of calcific plaques using circumferential pulse pressure waves emitted by a balloon catheter. This is the only technology that addresses both intimal and medial calcification, and does so with minimal vessel injury because of its ability to selectively differentiate calcium from soft tissue.
The Disrupt BTK study is a prospective, non-randomised, multicentre, feasibility and safety trial that enrolled 20 patients (mean (SD) age 79.0 (9.6) years; n=14 men) at three participating sites.'257- Fifteen patients had Rutherford category 5 ischaemia, and all patients had moderate to severe BTK arterial calcification. The IVL catheter delivery was successful in 19 patients. There were no major adverse limb events at 30 days. There was a 46.5% acute reduction in diameter stenosis of target lesions. Vascular complications were minimal, with only one arterial dissection reported, and two stents placed. None of the subjects experienced thrombus formation, abrupt closure, distal embolisation, or perforation. The early results of this pilot study demonstrate that calcified, stenotic infrapopliteal arteries can be safely and successfully treated with IVL. IVL efficacy at mid-and long-term follow-up remains to be defined. Future studies should definitely address the mid- and long-term efficacy of IVL to allow further expansion of this novel technology in clinical practice.
Future directions
As the optimal endovascular treatment modality for BTK lesions remains to be determined, POBA remains a reasonable primary endovascular approach for anatomically suitable infrapopliteal disease. Current evidence is inadequate to support other, more expensive techniques. Contemporary studies of POBA in infrapopliteal arteries reveal suboptimal short-term and 1-year clinical outcomes. However, significant heterogeneity for main outcomes hinders interpretability and generalisability of current findings. Procedural failures and loss of patency remain therapeutic challenges to be solved in this expanding patient population. In summary, POBA currently remains the standard of care for the endovascular treatment of BTK disease in CLI. Specialised cutting, scoring and nitinol-caged balloons as well as adjunctive modalities including atherectomy, cryoplasty and IVL show promise as complementary treatment modalities to PTA. However, more large-scale, multicentre, prospective studies are needed to elucidate their exact role in the armamentarium of BTK endovascular treatment.
Recommendation 119
POBA remains the primary endovascular approach for anatomically suitable infrapopliteal disease. (Class IIa; Level B)
Recommendation 120
Specialty BA (cutting balloons, scoring balloons, etc.) may be considered in select cases to prevent dissections; however, long-term efficacy data compared with traditional angioplasty are lacking. (Class IIb; Level C)
Recommendation 121
Cryoplasty may be considered as an adjunct to plain balloon angioplasty but there are insufficient data to support its routine use. (Class IIb; Level C)
Recommendation 122
Atherectomy may be considered as an adjunct to plain balloon angioplasty, but given the clinical equipoise and higher costs, it is imperative to determine any additional clinical benefit afforded by its use. (Class IIb; Level C)
Recommendation 123
Intravascular lithotripsy may be considered as an adjunct to plain balloon angioplasty for heavily calcified tibial lesions. (Class IIb; Level C)
Drug-coated balloons
Endovascular therapy has evolved as the preferred first-line treatment modality for BTK segment of PAD in contemporary clinical practice.[258] This is mostly reserved for patients with CLTI as a limb salvage modality. A previous meta-analysis reported a primary patency rate of 50%, a secondary patency rate of 60%, and a limb salvage rate of up to 80% (up to 36 months follow-up) after infrapopliteal POBA.[250] DCB therapy, which has shown good outcomes in the FP segment, has been applied to the BTK segment to improve patency rates.
The Debellum trial showed favourable outcomes with the use of DCBs in the BTK segment, with lower LLL in the DCB group of 50 patients.[259] In a randomised study of DCB v. POBA, the DEBATE-BTK trial (132 patients) reported a statistically significant reduction in binary restenosis at 12 months for the DCB group compared with POBA.[260] Both restenosis (27% v. 74%; p= 0.001), and TLR (18% v. 43%; p=0.003), were reduced at 1 year. Moreover, vessel occlusion was 17% v. 55% (p<0.001), complete wound healing occurred in 86% v. 67% (p=0.01) of the patients, and there was no significant difference in major limb amputation. Unfortunately, a much larger randomised study (358 patients), the IN.PACT-DEEP study failed to show better DCB efficacy, with a higher associated amputation rate in the DCB group.[261] The IN.PACT-DEEP trial compared the performance of the IN.PACT Amphirion DCB with POBA in a 2:1 randomisation protocol in 358 patients with pre-specified primary endpoints for efficacy (TLR and LLL), and safety (all-cause death, major amputations and TLR). All patients were analysed at 1-year follow-up for their clinical endpoints whereas a sub-cohort of patients with lesions <10 cm in length underwent an angiographic control for assessment of the technical endpoints. Significant baseline differences were noted between the DCB and POBA cohorts including mean lesion length (10.2 v. 12.9 cm; p=0.002), impaired inflow (40.7% v. 28.8%; p=0.035), and previous TLR (32.2% v. 21.8%; p=0.047). There was no difference in the primary efficacy endpoints in DCB v. POBA. Clinically driven TLR (CD-TLR) rates of 9.2% v. 13.1% (p=0.291), and mean (SD) LLL rates of 0.61 (0.78) v. 0.62 (0.78) mm (p=0.950) were reported. The primary composite safety endpoint was 17.7% v. 15.8% (p=0.021), which met the non-inferiority hypothesis. However, a safety signal driven by major amputations at 1 year was observed in the DCB arm (8.8% v. 3.6% for POBA; p=0.080). As a consequence, the IN.PACT Amphirion DCB product was withdrawn from the market.
In the Lutonix Global (BTK) registry, the study's primary safety endpoint of MALEs at 30 days was 98.6% (95% CI 96.6 - 99.4) in 346 patients treated with BTK DCBs. The investigators highlighted a 94.8% rate of freedom from amputation at 12 months.[262] There is currently widespread opinion to suggest that DCB is likely to substantially improve the success of endovascular procedures for BTK disease. However, the recent results of the IN.PACT-DEEP study that alarmed in terms of safety and efficacy suggest that a more cautious approach to the use of DCBs in the BTK segment is recommended.
Ongoing reports from 'real-world registries' still suggest good technical and clinical outcomes with DCBs in the BTK segment. The 5-year outcomes of the IN.PACT-DEEP DCB trial have just been published during the writing of this guideline.[263] There was no difference in the freedom from CD-TLR in 5 years (70.9% v. 76.0%; p=0.406), and in the incidence of the safety composite endpoint of major amputations and all-cause mortality (59.8% v. 57.5%; p=0.309) in the DCB and PTA groups. There was no significant difference in limb salvage rates between the two groups. The major amputation rate was 15.4% in the DCB group compared with 10.6% in the PTA group (p=0.108). The 5-year results demonstrated that 'given the recent concern regarding a late mortality signal in patients treated with paclitaxel-coated devices, additional analyses from this study showed no increase in all-cause mortality with DCB angioplasty (39.4%) compared with PTA (44.9%; p=0.727)'. The predictors of mortality in this study included age, Rutherford category >4, and previous revascularisation but not paclitaxel by dose tercile.
Recommendation 124
An individualised and tailored utility of DCBs in the BTK segment is advocated until further evidence is available. (Class IIb; Level C)
Recommendation 125
There is currently insufficient evidence to suggest that DCBs are associated with limb loss in BTK interventions. (Class IIa; Level B)
Bare metal stents, drug-eluting stents and covered stents
Infrapopliteal arterial stenting has expanded the armamentarium of percutaneous interventional strategies for CLTI. It is generally considered in the setting of CLTI with tissue loss involving the foot (ischaemic ulcer or gangrene).
Infrapopliteal BA with bailout stenting utilising BMS has long been considered the standard of care. Recently, some studies have indicated the superiority of balloon expandable DES in focal infrapopliteal lesions. Most of the experience with infrapopliteal stenting has involved only short-length lesions to date.
While stent trials generally address conventional technical outcome measures (primary patency, technical success rate, clinically driven target-vessel revascularisation or TLR, binary restenosis, and LLL), not many have reported on clinically relevant outcomes such as wound reduction rates, wound closure rates, minor and major amputation rates, independent ambulation, and HRQoL. Of concern is the lack of reporting on angiosome-based revascularisation which clearly impacts on clinical outcomes. Supporting medical therapy following infrapopliteal stenting and details regarding wound management are not reported frequently either.
The safety and efficacy of infrapopliteal stenting reported in earlier observational studies has been validated prospectively in RCTs. To date, there have been 6 RCTs involving infrapopliteal stenting. Two RCTs compared BA with BMS.[264,265] Two RCTs compared BMS with DES.[266,267] Two RCTs compared BA with DES.[268,269]
A recent network meta-analysis of RCTs reported on the 12-month follow-up data comparing infrapopliteal treatment modalities for CLTI.[270] They identified 11 RCTs employing BA, BMS, DES atherectomy devices, and DCBs. They included the 6 stent trials mentioned earlier. They reported that DES significantly increased primary patency compared with BA (OR 2.42; 95% CI 1.57 - 3.74) and BMS (OR 3.86; 95% CI 2.24 - 6.65). They also reported that DES significantly increased the technical success rate compared with BA (OR 11.78; 95% CI 1.42 - 7.59), with no significant effects identified in the rest of the comparisons. No significant differences were identified in TLR and major amputation. The modality of infrapopliteal treatment did not significantly alter CD-TLR rates or major amputation rates. In their overall analysis, they concluded that in terms of technical success and major amputation, DES was considered the most effective treatment at the 12-month follow-up.
A recent Cochrane review compared angioplasty to stenting for infrapopliteal arterial lesions in CLTI.[271] Their objective was to determine the efficacy and safety of PTA alone v. PTA with provisional stenting of infrapopliteal arterial lesions for patients with CLTI. They included seven trials with 542 participants, of which only one trial truly compared angioplasty to angioplasty with provisional stenting. They reported no clear differences in short-term patency at 6 months between infrapopliteal arterial lesions treated with PTA with provisional stenting v. those treated with PTA alone. Furthermore, there were no clear differences between the groups relating to periprocedural complications, major amputation rates, and mortality.
A recent systematic review and meta-analysis of eight RCTs and two cohort studies compared infrapopliteal DES with control therapy (BA or BMS).[272] A total of 927 patients were included (DES n=484 patients v. control treatment n=443 patients). The objectives were to analyse the 6-month, 12-month and 3-year performance of DES v. control treatment. The study reported that DES significantly decreased the risk of restenosis at 6 months and 12 months after therapy, but not at 3 years (here benefits were reported only for sirolimus-eluting stents). Similar results were noted for restenosis and event-free survival in favour of DES (benefits were reported for both sirolimus- and everolimus-eluting stents). The results of the meta-analysis indicated no significant difference in overall mortality between the two groups at 6 months, 12 months, and 3 years. The study showed that DES therapy significantly increased the rate of wound healing. There were no significant differences in terms of decreased risk of limb amputation at the endpoint of the studies with DES compared with control therapy.
A systematic review and meta-analysis comparing DEB/DES to BA (PTA)/BMS identified nine studies (n=707 in the DEB/DES group and n=606 in the PTA/BMS group).[273] They concluded that DES may decrease the risk of CD-TLR, restenosis rate and amputation rate without any impact on mortality. They could not identify any obvious advantage for DEB in the treatment of infrapopliteal disease.
A more recent systematic review and meta-analysis identified
7 RCTs enrolling 801 randomly assigned patients, comparing DES with control treatment (PTA, BMS and DCB) for infrapopliteal disease.[274] The midterm results favoured DES with respect to rates of primary patency, re-intervention, Rutherford class improvement and major amputation for the treatment of atherosclerotic disease of infrapopliteal arteries compared with control therapy, with no effect on patient survival. They also reported that sirolimus-coated stents were more effective than paclitaxel-coated stents.
Future directions
The role of stents in infrapopliteal interventions needs to be better defined with larger, good-quality studies. The role of self-expandable stents, especially for long infrapopliteal lesions remains to be defined for clinical practice.
Recommendation 126
Infrapopliteal primary or bailout stenting should only be performed in patients with CLTI and tissue loss. (Good practice statement)
Recommendation 127
For short infrapopliteal lesions (arbitrarily up to 40 mm, not requiring more than two DESs, not requiring complex trifurcation interventions), DES should be considered as first-line therapy.
(Good practice statement)
Recommendation 128
For longer lesions plain balloon angioplasty with provisional ('bailout-out') stenting should be first-line therapy, especially if the popliteal trifurcation is involved. (Class IIa; Level B)
Recommendation 129
For 'bailout stenting' post plain balloon angioplasty, a DES is preferred to BMS. (Class IIa; Level B)
Antithrombotic therapy post lower-extremity revascularisation
Vascular interventions have evolved dramatically over the past few decades from BS to aggressive endovascular interventions. A variety of antiplatelet and anticoagulant drugs have been used, either as stand-alone therapy, or as combination therapy to prevent procedure-related restenosis, or early and late occlusions. Oral antiplatelet drugs include[91, 275]
• Amino salicylic acid such as aspirin (irreversibly inhibits cyclo-oxygenase 1 and 2 enzymes, which decreases the formation of thromboxane A2).
• Thienopyridine derivatives (clopidogrel, ticlopidine and prasugrel) and cyclopentyltriazolopyrimidines (ticagrelor) - these are all adenosine diphosphate (ADP) receptor P2Y12 antagonists.
• Inhibitors of platelet adenosine uptake (dipyramidole).
• Phosphodiesterase III inhibitors (cilostazol).
• Thromboxane blockers (icotamide).
• Protease-activated receptor-1 antagonist (vorapaxar).
Intravenous antiplatelet agents which inhibit glycoprotein IIb/IIIa receptors (abciximab, eptifibatide and tirofiban) are commonly used during percutaneous coronary interventions (PCI), but have not been extensively used or studied following peripheral arterial interventions.[276]
Aspirin and clodiprogel are the most commonly prescribed antiplatelet agents following peripheral endovascular interventions. There is only limited evidence that antiplatelet drugs prevent re-occlusion at 6 months.[277] Dual antiplatelet therapy is often used based on evidence extrapolated from coronary intervention trials at 1 - 6 months. However, there are no large randomised trials providing any evidence base for dual antiplatelet therapy (DAPT) following lower-extremity endovascular interventions.[8, 275]
The Clodiprogel and Aspirin in the Management of Peripheral Endovascular Revascularisation (CAMPER) study compared aspirin and clodiprogel (300 mg as loading dose 12 hours before intervention, and 75 mg daily thereafter) with aspirin alone in patients with claudication undergoing endovascular intervention.[278] The trial was stopped due to poor enrolment, but did show a statistically significant platelet inhibition with DAPT.
The MIRROR study compared aspirin with DAPT post endovascular FP intervention, and showed statistically significant reduction of TLR in the DAPT arm at 6 months in spite of clodiprogel resistance in 30% of patients,[279] but this benefit was lost at 12 months (clodiprogel was stopped at 6 months).[280]
A meta-analysis by Katsanos et al.[91] showed significant reduction in leg amputations (but more severe bleeding) after DAPT with aspirin and clodiprogel. However, a Swedish[281] study reported reduced amputation rates for diabetic patients with CLTI undergoing FP stenting (not for PTA or subintimal BA), but not for non-diabetic patients. A retrospective analysis of 57 041 patients showed a statistically significant survival benefit for patients discharged on both aspirin and a thienopyridine derivative after a revascularisation procedure (both endovascular and BS) for CLTI (but not for claudicants) at 1 year, and this benefit was sustained at 5 years.[282] A meta-analysis by Weem et al.[283] reported a lack of evidence for the benefit of DAPT after revascularisation, but did not show any increased risk of bleeding.
Current evidence is conflicting and does not take into account lesion complexity, primary or recurrent interventions, bleeding risk, dose and timing of antiplatelet agents, resistance against drugs and mode of presentation. The GVG offers a class II, level C recommendation to consider DAPT (with aspirin and clopidogrel) after first percutaneous intervention for 1 month, and for 1 - 6 months after repeated intervention.[8] In general, trials looking at superficial femoral artery DES used 2 months of DAPT,[241] and 6 months if infrapopliteal DES were used.[284] The use of DAPT for longer than 1 month should also be considered if the patient has a coronary indication for DAPT such as recent acute MI or coronary intervention.[32]
Evidence is limited on antiplatelet therapy after vascular intervention in patients with lower-extremity artery disease (LEAD), on oral anticoagulation (OAC) for other indications (e.g. atrial fibrillation/prosthetic heart valves). Due to an increased bleeding risk, the combined ESC/European Society of Vascular Surgery guidelines recommend only OAC after surgery (Class IIa/Level C) in these patients. A single antiplatelet agent is recommended after percutaneous intervention in patients with a low bleeding risk compared with the occlusion risk with an OAC. Only an OAC should be prescribed in these patients if the bleeding risk is high compared with the occlusion risk (Class IIa/level C recommendation). Triple therapy (OAC + DAPT) is discouraged except for BTK stenting and complex lesions in patients at high risk for thrombosis. Gastric protection with proton pump inhibitors is also advised.[32]
There is conflicting evidence whether antiplatelet agents improve infrainguinal vein graft patency, even though its efficacy was supported in a recent meta-analysis.[285] A Dutch[286] trial found warfarin to be more effective at preventing vein graft occlusion than aspirin at the price of more bleeding. A north American[287] trial found no benefit for aspirin and warfarin compared with aspirin alone, even though the benefit of aspirin and warfarin has been shown in patients with suboptimum venous conduits.
Antiplatelet agents have a beneficial effect on the patency of prosthetic grafts and are superior to warfarin alone.[285,286] Although the Dutch trial did not support the use of warfarin in prosthetic grafts, a single centre retrospective American study showed statistically significant improved prosthetic graft patency in patients on therapeutic warfarin, with low-flow documented in prosthetic grafts (midgraft velocity <45 cm/s).[288] The CASPAR trial showed improved graft patency for patients with BTK or crural prosthetic bypass grafts on DAPT for 6 - 24 months. No such benefit was reported for vein grafts over aspirin alone in this study. No survival benefit was reported for any of the groups. Although bleeding was more frequent in the DAPT group, there was no difference in severe bleeding between groups.[289]
The Voyager PAD trial randomised 6 564 patients post intervention (endovascular or open surgery) to either low-dose rivaroxaban 2.5 mg twice a day and aspirin, or aspirin alone (concurrent clodiprogel use was allowed for 6 months, but not long-term at the discretion of the interventionalists). A statistically significant lower incidence of the composite outcome of ALI, major amputation, MI, ischaemic stroke or cardiovascular death was noted in the rivaroxaban and aspirin group (absolute reduction of 2.6% at 3 years) at the cost of increased major (but not fatal or intracranial) bleeding. Of note, >30% of patients discontinued rivaroxaban (or the placebo) early.[290] It is important to note that this dose of rivaroxaban is not currently available commercially in SA.
Cilostazol has antiplatelet, antiproliferative and vasodilatory effects and has been shown to improve walking distance in claudicants. There is conflicting evidence regarding its effect on patency post revascularisation. A meta-analysis reported improved primary patency and reduced ISR in the FP segment.[291] The CABBAGE trial showed no benefit for cilostazol following infrapopliteal PTA in patients with CLTI.[292] Current evidence remains inadequate to make any meaningful recommendations. Antiplatelet agents other than aspirin and clodiprogel have been studied in patients with LEAD, but not extensively post intervention, and as such very few recommendations can be made currently.
Recommendation 130
DAPT with aspirin and clodiprogel should be considered after peripheral endovascular intervention for a period of 1 month (up to 6 months depending on lesion complexity, bleeding risk, recurrent intervention and device utilised). (Class IIa; Level C)
Recommendation 131
Antiplatelet monotherapy should be prescribed after a peripheral bypass procedure. Anticoagulation monotherapy should be considered in high-risk vein bypass grafts. (Class I; Level C)
Recommendation 132
DAPT should be considered for a period of 6 - 24 months after prosthetic bypass grafting onto a below-knee popliteal or infrapopliteal target vessel. (Class IIa; Level B)
Recommendation 133
In patients requiring oral anticoagulation for another indication, no antiplatelet therapy should be prescribed after a bypass procedure. A single antiplatelet agent may be added for a limited duration after endovascular intervention if the thrombosis risk is high, and bleeding risk is low. (Class IIa; Level C)
Surveillance strategy following revascularisation procedures
Following lower-extremity revascularisation, follow-up of vascular patients is generally considered central to the detection of recurrent vascular disease that can lead to even further morbidity and mortality. All vascular interventions have a potential for failure which must be timeously identified and managed appropriately to provide the most durable results. The primary goal of follow-up is to detect failing vascular interventions at an early stage, when they can be addressed more safely and effectively, even before they become clinically evident. The optimal methods and frequency for follow-up are not clearly defined for lower-extremity vascular interventions. The challenge is to develop a follow-up plan for each patient that will achieve the desired goals while minimising expenses, risks, and disruption of the patient's lifestyle.
According to the latest guidelines from the Society for Vascular Surgery, the term surveillance implies the routine, planned use of serial objective testing to evaluate the current status of a vascular intervention.[293] Surveillance is, therefore, generally performed in asymptomatic patients and is based on the assumption that significant abnormalities may not be detected by clinical monitoring alone, and may employ some form of vascular testing (such as Doppler pressures) or imaging (such as DUS). Diagnostic testing, on the other hand, refers to the use of various physiological or imaging methods in a patient who has signs or symptoms suggestive of a problem with a previous vascular intervention.
The simplest approach to follow-up is clinical monitoring with a periodic vascular history and physical examination. Surveillance by clinical follow-up alone may be insufficient to detect restenosis, as patients may remain asymptomatic until the target vessel or vascular conduit has occluded.
A number of non-invasive physiological testing modalities are available for utilisation in a surveillance programme. The most basic of these is the determination of the ankle pressures and the ABI. The ABI measurement alone has limited value - it is unable to provide the exact anatomic location of the culprit lesion, it has significant limitation in diabetics with calcified vessels, and there is a variability of correlation when there is a drop in ABI (>0.15) with lesion severity.[8] Other modalities include segmental limb pressures, pulse volume recordings, plethysmography and transcutaneous oxygen pressure measurements. None of these modalities have managed to establish a routine role in the setting of a surveillance programme.
Imaging modalities include DUS, CTA or MRI/MRA, with and without contrast-enhancement (CE-MRA), and DSA. Modalities such as CTA, MRI/MRA/CE-MRA, and DSA are not reasonable for surveillance because of the invasiveness, costs, access limitations, exposure to ionising radiation, contrast toxicity, and potential risks from the procedure itself.
DUS imaging on the other hand, has been the mainstay of surveillance vascular imaging for decades. DUS provides anatomic information using direct visualisation of the vessel, as well as physiological information based on spectral waveforms, and velocity measurements and velocity ratios. The combination of peak systolic velocity (PSV) and velocity ratio (Vr) measurements offers a high positive predictive value for identifying moderate to severe restenosis when it is correlated with angiography.[8]
Surveillance following surgical revascularisation
Infrainguinal bypass procedures using vein conduits are performed routinely on patients with PAD. However, vein grafts can develop stenotic lesions that can lead to graft thrombosis and recurrent symptoms of lower-extremity ischaemia. Such lesions are identifiable in 25 - 30% of vein bypass grafts within the 1st year.[294]
The purpose of a vein graft surveillance programme would, therefore, be to identify these stenotic lesions as they develop and intervene timeously to prevent graft thrombosis. A thrombosed vein bypass graft is difficult to salvage. When successfully salvaged, assisted primary vein graft patency is improved, the need for redo-bypass grafting is reduced, and limb salvage in the CLTI population is improved.[8] The best protocol for vein graft surveillance remains an unresolved issue. A DUS scan immediately after infrainguinal vein bypass was not recommended in the 2007 Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) document.[19] Instead, a clinical surveillance programme (palpation of limb pulses, measurement of ABI) was proposed in the immediate postoperative period, and conducted every 6 months for at least 2 years. There are supporting outcome data demonstrating asymptomatic development of Duplex-detected vein graft stenoses in one-quarter of patients, graft failure when stenotic lesions were not repaired, and assisted primary patency rates >80% with vein graft salvage strategies at 3 - 5 years.[8]
With DUS-informed reintervention, the 5-year assisted-primary patency rate for vein grafts approaches 80%, nearly identical to the primary patency of a graft that never developed stenoses. Such patency results are superior to the reported 1-year secondary patency of 20 - 35% after treatment of thrombosed vein grafts, and thus lends weight to the argument for intervention prior to the development of an occlusion.[295] Recognising the severe consequences of lower-extremity vein graft failure and the challenges of restoring patency once thrombosis has occurred, many vascular surgeons have elected to use some form of DUS surveillance in their patients. The rationale for this approach is also based on the non-invasive nature and relatively low cost of a DUS surveillance program compared with other imaging modalities.[296]
Ihlberg et al.[297Î9S] performed a RCT comparing DUS surveillance of vein grafts with simple clinical follow-up. Primary patency, assisted primary patency, and secondary patency rates were no different between the two groups. Of note, very few graft revisions were necessary in either study. Another prospective RCT performed by Lundell et al.[299] showed a significant benefit of intensive DUS surveillance. Assisted primary and secondary patency rates were 78% and 82% in surveyed grafts compared with 53% and 56% in the group where the vein grafts were not surveyed at 3 years.[299]
Davies et al.[294] reported the results of the Vein Graft Surveillance Randomised Trial (VGST), a prospective randomised trial from the UK, in which 594 bypasses were randomised to either clinical surveillance, or combined DUS and clinical surveillance. The investigators found no differences in primary, primary-assisted, and secondary patency rates between the two surveillance strategies at 18 months. The authors reported that the trial provided conclusive evidence of the suspicions that limb salvage is not improved by DUS-based surveillance protocols.
Golledge et al.[300] performed a systematic review of 6 649 vein grafts comparing DUS with clinical surveillance, and found that the total number of deaths, occluded grafts, and number of occlusions were significantly greater in those not undergoing surveillance after 30 days. However, there was no difference in limb salvage between the two groups.
In an attempt to clarify the utility of DUS surveillance for infrainguinal autogenous vein bypass grafts, a systematic review and meta-analysis of the current literature on this topic was funded by the Society for Vascular Surgery.[296] DUS surveillance was not associated with a significant change in primary, assisted-primary, and secondary patency rate or mortality compared with ABI combined with clinical examination. Surveillance with DUS was associated with a non-significant reduction in amputation rate (OR 0.70; 95% CI 0.23 - 2.13). The systematic review demonstrated that the evidence supporting routine DUS surveillance of infrainguinal vein grafts is dependent on low-quality evidence. The review also concluded that considering the opportunity for early intervention offered by DUS, the non-invasive nature and low cost of this approach, DUS can be incorporated in surveillance protocols of lower-extremity vein grafts that can be individualised on the basis of the settings and resources.
Based on this review, the Society of Vascular Surgery recommends clinical examination, ABI, and DUS for infrainguinal vein graft surveillance immediately postoperatively, and follow-up at 3, 6, and 12 months, and at least annually thereafter. They also suggest that more frequent surveillance should be considered when abnormalities are identified on DUS or when alternative vein conduits other than a single-segment GSV were used.[293]
The recent Global Vascular Guidelines on the Management of Limb-Threatening Ischaemia also recommended observation of patients who have undergone lower-extremity vein bypass for CLTI on a regular basis for 2 years with a clinical surveillance program consisting of interval history, pulse examination, and measurement of resting ankle and toe pressures. They advise the addition of DUS only when available.[8]
With regards to the threshold values at which intervention should be contemplated, it is generally accepted that a focal increase in PSV can be used to calculate Vr, defined as the PSV at the site of a stenosis divided by the PSV in a normal vessel segment proximal to the stenosis. Increased risk for graft thrombosis is informed by an increase in PSV of 180 cm/s - 300 cm/s, and a Vr of 2.0 - 3.5. The highest risk for graft thrombosis is conferred by an increase in PSV >300 cm/s, Vr >3.5, graft flow velocity <45 cm/s, and a drop in ABI >0.15.[293]
The latest guidelines from the Society for Vascular Surgery recommended the various thresholds for stratification of risk for thrombosis of infrainguinal vein graft (Table 7).[293] These guidelines were also supported by the recent Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischaemia that recommended additional imaging in patients with lower-extremity vein grafts who have a decrease in ABI >0.15 and recurrence of symptoms, or a change in pulse status. They also further recommended intervention for DUS-detected vein graft lesions with an associated PSV >300 cm/s, a Vr >3.5, or a low mid-graft velocity <45 cm/s.[8]
Methods for postoperative surveillance for both aorto-iliac and iliofemoral prosthetic reconstructions are even less well defined in the literature. High flow rates and large-calibre conduits contribute to high patency rates. As a result, there are no studies describing or supporting long-term surveillance benefits for these bypass grafts. The extra-anatomic femoral-femoral and axillo-bifemoral bypasses generally result in patency rates that are consistently lower than those of AFBG, and yet still there is very little literature supporting surveillance.'293-
The Society of Vascular Surgery guidelines recommend clinical examination and ABI with or without the addition of DUS after AFBG, and iliofemoral, femoral-femoral and axillofemoral bypass grafts. They suggest that these evaluations should be repeated every 6 and 12 months initially, and then annually as long as there are no new signs or symptoms suggestive of ischaemia.[293]
Evidence as to the efficacy of prosthetic infrainguinal graft surveillance programmes is even more inconclusive. In one study,[302] 69 patients with infrainguinal prosthetic bypasses were assessed by DUS after 4 weeks and every 3 months thereafter (total follow-up was 3 years). The DUS examination appeared to be of limited value, with 12 of 14 failing grafts not correctly predicted. In a retrospective analysis of 118 above-knee prosthetic grafts, most bypass occlusions occurred without detection of the lesions.[302] A quarter of patients developed a graft-related stenosis detected by DUS. The DUS surveillance of prosthetic grafts does not reliably detect correctable lesions that precede failure as it does in vein bypass grafts. Instead, surveillance may serve as a predictor of graft thrombosis by the detection of mid-graft velocities <45 cm/s. Prosthetic grafts with low velocity may benefit from warfarin to improve patency, which may justify surveillance.[8]
The latest GVG did not recommend the addition of DUS in the follow-up for patients who undergo lowerextremity prosthetic bypass for CLTI.[8] The Society for Vascular Surgery recommends clinical examination and ABI, with or without the addition of DUS in this group.[293] They also advised that this evaluation should be performed in the early postoperative period, at 6 and 12 months and thereafter annually as long as no new signs or symptoms of ischaemia develop.
Surveillance following endovascular revascularisation
Endovascular interventions have superseded lowe-extremity bypass in the treatment of PAD. Yet, while peripheral interventions continue to increase, and new advances in endovascular technology seemingly appear every day, the optimal management of patients following endovascular intervention is not well defined. Despite the high initial technical success rates of endovascular interventions, early failure of these minimally invasive procedures is common.
In a study by Bui et al.,[295] the natural history of target lesion restenosis in endovascular interventions was markedly different from that observed in vein grafts. After endovascular treatment, the tendency to develop restenosis was much greater, but lesions appear more likely to stabilise or regress than those found in vein grafts. In contrast to vein grafts, limbs with severe restenosis after endovascular intervention were less frequently thrombosed and were often patent at the time of clinical deterioration compared with vein grafts. Such patients usually present with restenosis rather than occlusion and can be retreated. BA of stenotic lesions is associated with a higher procedural success rate, as well as better long-term patency than the recanalisation of occlusions. Bui et al.[295]- concluded that the currently accepted DUS threshold criteria for reintervention after autogenous vein graft placement are not applicable in patients undergoing endovascular therapy.
To date, there are inadequate data demonstrating clinical benefit of a DUS surveillance programme after endovascular intervention for CLTI. Still, there are likely to be subgroups of patients who may benefit more than others from close surveillance and early reintervention. This is yet to be defined.
The GVG recommended follow-up for patients who undergo endovascular interventions for CLTI by means of clinical examination and non-invasive testing in the form of ankle and toe pressures only.[8] They do, however, recommend additional arterial imaging after endovascular intervention for failure to improve (wound healing ischaemic rest pain) or in the event of recurrence of symptoms. They suggest reintervention for patients with DUS-detected restenosis >70% (Vr >3.5, PSV >300cm/s) if symptoms of CLI are unresolved, or on a selective basis in asymptomatic patients.
The Society for Vascular Surgery guidelines on the other hand recommend clinical examination, ABI and DUS examination be performed within 1 month of intervention to establish a baseline, with 3 - 6 months' follow-up consisting of clinical examination and ABI, but admit that the evidence for this recommendation is low.[293]
Recommendation 134
Follow-up of vascular patients post revascularisation is essential to detect recurrent disease that can lead to even further morbidity and mortality. (Class IIa; Level C)
Recommendation 135
All vascular interventions have a potential for failure which must be identified timeously and managed appropriately to provide the most durable results. (Good practice statement)
Recommendation 136
Surveillance in the form of clinical examination and ABI (with or without the addition of DUS) after aortobifemoral, iliofemoral, femoral-femoral and axillofemoral bypass may be considered. These evaluations should be performed in the early postoperative period, repeated at 6 and 12 months, and then annually as long as there are no new signs or symptoms. (Good practice statement)
Recommendation 137
After infrainguinal venous bypass, it is reasonable to perform surveillance in the form of clinical examination and ABI, with the addition of DUS. These evaluations should be performed in the early postoperative period, repeated at 3, 6 and 12 months, and then annually as long as there are no new signs or symptoms. (Class IIa;
Level B)
Recommendation 138
Patients who have a decrease in ABI >0.15, recurrence of symptoms, or a change in pulse status should be considered for vascular imaging.
(Class IIa; Level B)
Recommendation 139
DUS-detected vein graft stenoses with an associated PSV >300 cm/s, a Vr >3.5 or low mid-graft velocity (<45 cm/s) should be considered for intervention. (Class IIa; Level B)
Recommendation 140
Surveillance in the form of clinical examination and ABI, with the addition of DUS after endovascular intervention, may be considered. These evaluations should be performed in the early postoperative period, repeated at 3 and 6 months, and then annually. (Class IIa; Level B)
Recommendation 141
DUS in addition to clinical examination and ABI is not recommended for routine surveillance after infrainguinal prosthetic bypass. (Class IIa; Level C)
Strategies for vein bypass graft salvage
Up to 80% of infrainguinal vein graft stenoses are solitary and focal in nature. Multiple focal synchronous or metachronous lesions are found in 15 - 20% of cases. Long diffuse lesions are usually uncommon, and account for <5% of the cases.'303- There are multiple treatment options available, including endovascular and open surgical options, to treat a failing vein graft. The choice of strategy depends on location of the occlusive lesion, the length of the lesion, early (<6 months) v. late lesions, and the patient's comorbid profile. AFS has been reported to be better for late-onset compared with early-onset vein graft failure.'304-
Endovascular options include POBA, generally employing high-pressure balloons and CBA. Focal lesions that develop after 6 months respond favourably to angioplasty.'305- Endovascular treatment of early-onset lesions shows inferior results when compared with open surgical revision. Data indicate that cutting balloons yield superior results compared with POBA, and are equivalent to open surgical revision.[306-] The utility of DCB angioplasty does not confer any benefit over standard BA.'307- BA of mid-graft lesions has been reported to achieve primary-assisted patency of 65% at 5 years, while primary-assisted patency of 53% at 3 years has been reported for endovascular treatment of distal anastomotic lesions.[308] A study by Patel et al.[304-] showed primary, primary-assisted, and secondary patency rates of 32%, 73% and 73%, respectively, at 3 years for endovascular treatment of failing vein graft with lesions involving proximal anastomoses, mid-graft and distal anastomoses of distal bypass vein grafts.
Open surgical strategies include interposition graft repair, patch repair and proximal or distal anastomotic transposition. Patch repair yields results that are equivalent to segmental interposition graft repair. '309- There is a recent trend towards utilising open surgical strategies as secondary options for endovascular treatment failures, or in cases of early-onset graft-threatening lesions.
Recommendation 142
Endovascular treatment of failing vein graft lesions is recommended considering that it compares favourably with open surgical modalities. (Good practice statement)
Recommendation 143
High-pressure/cutting balloon angioplasty of vein graft lesions is recommended as these modalities show superior results compared with standard BA. (Class IIa; Level B)
Recommendation 144
Stent technology may be considered for anastomotic vein graft stenoses in select cases where surgery is best avoided. (Class IIa; Level B)
Recommendation 145
Vein graft salvage strategies have superior outcomes when treating lesions that develop later (after 6 months) compared with early lesions. (Class IIb; Level C)
Strategies for target lesion revascularisation
TLR specifically refers to re-intervention on native artery lesions previously treated by endovascular therapy. These reinterventions may be technical or CD-TLR.
Target lesion revascularisation
TLR is defined as either repeat percutaneous or surgical revascularisation for a previously endovascularly treated native artery lesion. If a stent was placed, ISR can be anywhere within the stent or 5 mm beyond the stent edge proximally or distally.
Clinically driven-target lesion revascularisation
CD-TLR is a reintervention on a lesion after the development of ischaemic symptoms and a finding of >50% stenosis on imaging.
These terms have been borrowed from the cardiac literature, where non-invasive imaging is not feasible and thus patients are generally followed up with a coronary angiogram in a pre-specified protocol. They can be misleading as TLR based on imaging only and without a clinical indication may represent unnecessary interventions. Thus, it is an anatomical outcome. Also, without objective parameters for assessing lesion relevance, it is subject to bias even when clinically driven. Without follow-up, objectively documenting resolution of clinical symptoms, these endpoints are of limited value.
The role of drug eluting technologies
The most effective strategy to treat significant target lesions after endovascular therapy is controversial. The treatment strategy for managing SFA lesions has moved away from primary stenting to DCBs as primary treatment, and limited use of bailout stenting in non-responders and those with flow-limiting dissections. There are no trials presently reporting results on the use of DCBs after restenosis of previous DCB or POBA treatment. The literature on DCBs in restenosis of the SFA is focused on ISR after stenting. The concept is borrowed from the cardiac literature where DCBs for ISR have demonstrated decreased TLR rates. There are three randomised trials examining this in the SFA literature.[195,310,311] The PACUBA trial compared DCB with POBA for treating ISR.[310]- The mean (SD) lesion length was 17.3 (11.3) cm in the DCB group, and 18.4 (8.8) cm in the PTA group. The 1-year primary patency rates were 40.7% (95% CI 0.26 - 0.64) and 13.4% (95% CI 0.05 - 0.36; p=0.02) in the DCB and PTA groups, respectively. There was no significant difference in freedom from CD-TLR (p=0.11) or clinical improvement by >1 Rutherford-Becker category (p=0.87) between the two groups.
The FAIR trial compared DCB v. POBA for ISR. Recurrent ISR (by DUS) was lower in the DCB group than the POBA group (45% v. 15%). This benefit was sustained at 12 months (63% v. 30%).[195] The TLR rates at 6 months (96% v. 81%) and 12 months (91% v. 53%) were significantly less, but this was not clinically driven and was based on a >50% restenosis.
The ISAR-PEBIS trial found a decrease in angiographic diameter (44.33% v. 65.33%), and a decrease in TLR rates (19% v. 50%; p=0.007) in favour of the DCB group.[311] However, the numbers were small (n=36 and n=34), and the interventions were reported as clinically driven, but no details of this were provided, and the primary endpoint was angiographically defined.
A meta-analysis of these three trials concluded that there is an advantage of DCBs for technical endpoints such as TLR and binary restenosis, but insufficient evidence for clinical endpoints. They further concluded that the certainty of evidence was low due to the small number of studies, participants included and the potential bias in study design; thus, requiring further RCTs to adequately investigate.[312]
Recommendation 146
DCBs can be used to prevent recurrent ISR. (Good practice statement)
Recommendation 147
DCBs can be used to prevent MALE for ISR. (Class IIa; Level B) Recommendation 148
For restenosis following native artery POBA or DCBs, lesions should be treated as de novo lesions. (Class IIa; Level C)
Recommendation 149
Infrainguinal bypass surgery is strongly recommended for ISR in average-risk patients with CLTI and a suitable vein graft. (Class IIa; Level C)
Managing the 'young' PAD patient
PAD is uncommon in the young (arbitrarily defined as age <50 years). Symptoms may be acute or chronic in nature, with the majority of patients presenting with claudication. The most common aetiology includes premature or accelerated atherosclerosis (generally >70% of cases in clinical practice). Other aetiologies include vasculitides, hypercoagulable states, thrombo-embolism, and entrapment syndromes in both the non-athletic and athletic populations.[313,314] Universal consensus in managing such diverse pathology is lacking. The disease rarity has prompted a high index of suspicion in young patients who present a diagnostic challenge in clinical practice. Some of the patients may have intact palpable pulses, therefore, dynamic provocation testing is required. Optimal medical therapy and definitive intervention is guided by the awareness and understanding of the common and uncommon causes of limb ischaemia in the young PAD patient.
Precocious and accelerated atherosclerosis[313, 315]
Patients with precocious and accelerated atherosclerosis manifest with recognised risk factors for atherosclerosis (predominantly smoking and dyslipidaemia) and sometimes HIV-related vasculopathy. Optimum medical treatment includes lifestyle changes, ET and pharmacotherapy, similar to conventional patients with PAD. Interventions with proven benefits entail smoking cessation, antiplatelet and lipid-lowering therapy. Exercise programmes and cilostazol may have a role in improving walking distance. IC may be managed medically, which may be inadequate in patients with an active lifestyle. Revascularisation includes endovascular and surgical techniques. The BASIL trial demonstrated similar AFS at 2 years for surgery and BA.[153] The TASC provides guidance reliant on anatomical suitability for the utilisation of endovascular and surgical procedures in the iliac and SFA.[316] Studies are required to assess the durability of the respective interventions.
Precocious atherosclerosis is generally encountered in young patients <40 years of age. Long-standing type 1 diabetes mellitus, familial hypercholesterolaemia, familial hyperhomocysteinaemia, early-onset endstage renal failure (ESRF), and early-onset retroviral disease patients on long-standing antiretroviral therapy (ART) have been associated with precocious atherosclerosis. A single centre study reported that HIV occlusive disease patients, with CLTI, had advanced retroviral disease and a mean age of 44 years.[317] They reported a 30% major amputation rate in this cohort.[317]
Non-atherosclerotic disorders in young PAD patients Popliteal entrapment syndrome'315,3181
Popliteal entrapment syndrome pathology usually requires surgical correction to address the underlying mechanical obstruction with catheter-directed thrombolysis reserved for acute presentations with a poor run-off as a result of thromboembolic events. Definitive treatment avoids long-term complications of stenosis, thrombosis and aneurysm formation. More recently in patients with functional popliteal entrapment syndromes, botulinum toxin A has been advocated for therapeutic relaxation in the restrictive gastrocnemius muscle area. The 81% success rate makes this an attractive modality in delineating patients who may benefit for gastrocnemius surgical release procedures.
Chronic exertional compartment syndrome[315'316] Conservative measures such as discontinuing sport and resting delays surgery. Fasciotomy remains the definitive treatment for chronic exertional compartment syndrome (CECS).
Adductor canal syndrome[319]
Surgery is indicated in symptomatic individuals and entails removal of the fibrous bands with vessel patch angioplasty or SFA bypass procedure.
Iliac-artery endofibrosis[315, 319]
First-line treatment comprises conservative measures such as reduction of cycling activity and posture variation. Percutaneous BA provides temporary relief and has a high restenosis rate. Stenting is contraindicated as its durability is complicated by stent fractures. Surgical options are variable and includes the following:
• External iliac arteriolysis.
• Vessel redundancy with kinking requires resection and primary anastomosis.
• Segmental vessel endofibrosis may be treated with endofibrosectomy or endarterectomy.
Cystic adventitial disease'[315]
Conservative measures are first attempted with ultrasound or CT-guided drainage of the cyst. Failure to achieve resolution is followed by surgical intervention which comprises cyst enucleation. Interposition grafting is indicated for thrombosis and degenerative vessel wall changes.
Thromboembolic disease[315]
Therapy comprises treating the underlying thromboembolic cause, anticoagulation, surgical exploration with a view to thrombo-embolectomy, thrombolysis and bypass procedures depending on the clinical scenario encountered.
Buerger's disease[315-320]
Smoking cessation remains the cornerstone of therapy. Medical therapy comprises antiplatelets, anticoagulants, vasodilators (calcium channel blockers), pentoxifylline and cilostazol (phosphodiesterase type III inhibitor). These agents may aid by increasing PFWDs. Prostaglandin analogues have been prescribed for severe ischaemia. Intravenous iloprost has been effective for symptom relief, accelerating resolution of distal trophic changes, and reducing amputation rates. Reported results have been superior to lumbar sympathectomy. Surgical and percutaneous revascularisation have been futile owing to the distal nature of the disease. Surgical procedures are technically challenging with poor patency rates. Novel stem cell therapies have demonstrated promising results.
Takayasu's arteritis'[315]
Steroids are indicated as first-line therapy for active disease, with surgery reserved for vascular complications of Takayasu's disease involving the lower extremities. BS has yielded good long-term results. Endovascular intervention is indicated for focal lesions.
Hypercoagulable states
Hypercoagulable disorders are largely underappreciated, and their estimated prevalence is 13 - 50% according to a retrospective study.[321] The most common hypercoagulable abnormalities for arterial disease are antiphospholipid syndrome and hyperhomocysteinaemia. Young patients with hypercoagulability have worse outcomes when subjected to lower-limb revascularisation. In a retrospective cohort study with 91 patients <50 years, 55% had a documented hypercoagulable state.[321] The majority, who presented with ALI had a higher amputation rate (50%; n=6/12).There was a trend towards increasing perioperative thrombosis and a poor 3-year graft patency rate. Similar results are obtained with surgical or endovascular interventions. In some patients, catheter-directed thrombolysis was associated with worse outcomes. Strategies that guide best medical and surgical practice directed against the high rate of limb loss in this group of patients are yet to be defined. Hypercoagulable disorders should be considered as a differential in a young patients with limb ischaemia and with failed early revascularisation.
Persistent sciatic artery
Asymptomatic lesions in the absence of mural thrombi and aneurysms can be treated medically. Symptomatic persistent sciatic artery with aneurysmal transformation may be managed surgically or endovascularly with stent grafts/coils. Surgical exploration with a view to ligation or excision is an option; however, there is a risk of sciatic nerve damage. A bypass procedure may be required in the event of the superficial artery being incomplete.
Future directions
Despite technological advances, endovascular procedures have a limited role in the management of limb ischaemia in young patients. A greater understanding of these rare aetiologies is required to better understand the disease entity.
Recommendation 150
The rarity of clinical scenarios in young patients with PAD has resulted in a hiatus in the literature pertaining to therapy. At present, experience is anecdotal and is confined to high-volume centres with published case reports with limited data. As a result, the feasibility and durability of the various therapeutic options are difficult to evaluate in the longer term. Novel and minimal treatment options have been proposed in addition to traditional medical and surgical approaches. (Good practice statement)
Interventions for lower-extremity acute limb ischaemia
ALI is caused by an abrupt decrease in arterial perfusion to the limb. It is defined as the presence of symptoms of acute ischaemia <14 days in duration.[19] Causes for ALI include embolism, thrombus on a preexisting arterial lesion (acute on chronic), bypass graft thrombosis, thrombosed popliteal artery aneurysm, etc. ALI is a medical emergency that carries a significant risk of amputation and mortality. Patients should be evaluated urgently by a vascular surgeon, or if one is not available, by a general surgeon with expertise in vascular problems.
As soon as the diagnosis of ALI has been made, the patient should be systemically anticoagulated using unfractionated heparin (80 - 100 IU/kg). Appropriate analgesia should be administered.[19] Definitive treatment will depend on the clinical stage of ALI (Table 8).
For viable limbs (stage I), revascularisation, when indicated, should be performed on an urgent basis. Revascularisation for threatened limbs (stage IIA and IIB) should be performed on an emergency basis (i.e. within 6 hours). Primary amputation is indicated in patients with stage III (non-viable) limbs.[32] Revascularisation modalities include open repair (OR), thrombolytic therapy with catheter-directed thrombolysis (CDT), catheter-directed pharmaco-mechanical thrombolysis (PMT/CD-PMT), mechanical thrombectomy (MT) and thrombus aspiration (TA). The treatment strategy will be determined by the presence of neurologic deficit which mandates immediate intervention, ischaemic time, patient risk factors, available facilities, and expertise. A technique that will restore arterial flow most rapidly should be selected.'321 Surgery (OR) has been the standard treatment for ALI for many years. Fogarty embolectomy is extremely successful in the management of ALI due to embolic occlusion of an artery. It is less successful in cases of acute native vessel thrombosis. Advances in technology and endovascular techniques have made interventional treatment a viable and appealing option.[19,32]
Endovascular management of ALI include CDT, MT, TA, and PMT. Systemic thrombolysis has no role in the treatment of patients with ALI.[331]
Endovascular management has various potential advantages over open surgery:
• Patients with ALI are often high-risk patients with a high incidence of associated perioperative morbidity and mortality following emergency surgery.
• Endovascular therapy is less invasive. This is useful in high-risk patients.
• Balloon embolectomy often results in incomplete clearance of thrombus, especially from tibial vessels.
• Lytic therapy restores patency to outflow vessels.
• Thrombolysis identifies underlying/pre-existing arterial lesions that can be treated endovascularly or with open surgery.
A Cochrane review based on the initial randomised trials comparing CDT with OR (STILE, TOPAZ, Rochester), found no significant difference between surgery and CDT in limb salvage or mortality at 30 days, 6 months, and 12 months.[322] However, CDT was associated with a higher complication rate including stroke (1.3% v. 0%) and major bleeding (8.8% v. 3.3%). Since the publication of the original trials, thrombolytic therapy and techniques have evolved, and current techniques differ significantly from those used in the original trials.
Wang et al.[323] reviewed the literature from 1992 - 2014, comparing contemporary surgical and endovascular revascularisation (CDT and/or PMT). They recommend initial treatment of ALI with endovascular therapy because of equivalence in short-term outcome (limb survival and AFS), and lower morbidity and mortality rates achieved with endovascular intervention.
Taha et al.[324] compared endovascular treatment (CDT and PMT) with surgery in ALI stage IIa patients. They found the results to be comparable as far as limb salvage rates were concerned, but overall mortality rates were significantly higher at 30 days (13.2% v. 5.4%), 1 year (33.8% v. 12.9%), and 2 years (40.5% v. 18.7%) in the surgery group.[324]
There are various PMT and mechanical thrombectomy devices, two of which are available in SA (Angiojet from Boston Scientific and Rotarex from Straub Medical). The addition of thrombolytic therapy to mechanical thrombectomy (Angiojet) has the potential of accelerated thrombus removal with decreased lytic times, decreased dosage of lytic therapy, and potentially fewer bleeding complications. Various studies compared CDT with PMT and found that the clinical success rate, complication rate and mortality rates were similar between the two modalities.[325,326] However, PMT had a higher technical success rate, decreased duration of thrombolysis, with a trend towards decreased amputation rate and a statistically significant decrease in length of hospital stay.[325.326] Results of the PEripheral use of Angiojet Rheolytic thrombectomy with a variety of catheter Lengths (PEARL) registry confirmed that PMT yields superior results compared with CDT, with higher rates of procedural success (88% v. 74%), and a higher AFS at 12 months (87% v. 72%).[327] The authors concluded that PMT causes rapid reperfusion to the extremity with reduced procedure time and with an acceptable risk profile.
Thrombolytic therapy (CDT and PMT) has been applied successfully in ALI stages I, IIA and IIB, and proved to be effective in restoring flow in native artery thrombosis, in-stent thrombosis, graft thrombosis and thrombosed popliteal artery aneurysms.[323-327] Where contraindications to lytic treatment exist, the thrombus can be removed with aspiration thrombectomy or mechanical thrombectomy.[328,329] After successful thrombus removal, any pre-existing arterial lesions should be managed by either endovascular technique/BS or a hybrid procedure. Lower-extremity fasciotomies should be performed to prevent post-reperfusion compartment syndrome in patients with longstanding ischaemia of >4 - 6 hours.[329]
Future directions
The only prospective randomised trials available were conducted >20 years ago. Thrombolytic management has changed significantly since then with regards to lytic agents used and endovascular techniques. New trials are required comparing contemporary endovascular techniques with OR and comparing CDT with PMT.
Recommendation 151
Systemic anticoagulation with unfractionated heparin should be administered as soon as the diagnosis of ALI has been made. (Class I; Level C)
Recommendation 152
Transfemoral Fogarty balloon embolectomy is recommended for acute embolic occlusion causing ALI. (Class I; Level B)
Recommendation 153
CDT is effective for patients with salvageable limbs that are not imminently threatened (categories I and IIa), and where no contraindications to thrombolytic therapy exist. (Class I; Level B)
Recommendation 154
Systemic thrombolysis should not be used in patients with lower-extremity ALI. (Class IIa; Level A)
Recommendation 155
PMT may be considered in patients with categories I and IIa ALI. (Class I; Level B)
Recommendation 156
PMT is recommended for patients with IIb ALI. (Class I; Level B) Recommendation 157
Any pre-existing underlying arterial lesion unmasked by thrombolytic therapy should be corrected by either endovascular interventions, or open repair, as indicated. (Class I; Level B)
Recommendation 158
Four-compartment fasciotomy is recommended after revascularisation in cases with significant ischaemia. (Class I; Level C)
Predicting outcomes for limb salvage in patients with CLTI
The evidenced-based revascularisation procedures for PAD include BS, endovascular interventions or hybrid procedures. The benefits of 'situational perfusion enhancement', e.g. BA for an ischaemic ulcer, as an alternative to definitive therapy remain to be defined. Traditional endpoints of revascularisation (graft patency, OS, AFS) need to be expanded to reflect more tangible benefits (perioperative mortality, MALE, MACE, major reintervention rates, minor reintervention rates, independence and ambulation, surgical and ischaemic wound healing, and cost efficacy). The goals of revascularisation (representing the ideal result) are to preserve life and limb, to maintain function (independence, ambulation and employment), to relieve ischaemia (pain and wound healing) and to minimise the frequency and magnitude of repeat interventions (safety and cost-efficacy).
What has evolved from the literature is that not all patients benefit from revascularisation. Patient recovery after infrainguinal bypass grafting for limb salvage in one large series with a mean follow-up of 42 months revealed an OS of 49% despite a graft patency rate of 77% and a limb salvage rate of 87%, 73% still ambulant, 70% still independent, 54% repeat operations, mean wound healing time (surgical and ischaemic wounds) of 42 months, wound non-healing rate of 22; and overall major amputation rate of 23%.[330]- Only 14.3% of the patients achieved the ideal result.[330]
Traditional reporting standards systematically underestimate the expenditure of effort required to attain limb salvage. In a study by Goshima et al.,'331- 48.9% of patients with infrainguinal bypass procedures required reoperation within 3 months, 49.3% required readmission in 6 months, wound healing exceeded 3 months in 54% of the patients, and the mean 5-year mortality rate was 45 - 50%. The authors concluded that tissue loss was a significant risk factor for re-operation, and that a significant portion of patients spend the remainder of their lives attending to their ischaemic limb needs.
A large series using patient-orientated outcome measures (revascularisation procedure patent until wound healing, limb salvage for 1 year, maintenance of ambulation for 1 year and survival for 6 months) following lower-extremity bypass and endovascular intervention for ischaemic tissue loss had an overall clinical success rate of 40.9% with better results for BS v. endovascular treatment (44.3% v. 37%; p=0.06).'332- Independent predictors of failure (irrespective of treatment) were impaired ambulatory status at presentation, diabetes mellitus, end-stage renal failure, presence of gangrene and prior vascular intervention. The probability of failure is cumulative, ranging from 35.4% if no independent factors are present to 92.8% failure rate if all 5 independent predictors were present.
Clinical presentation definitely influences outcomes following revascularisation. In one large series, the results were uniformly better for claudication compared with rest pain and tissue loss (p=0.001).'333-It cannot be emphasised strongly enough that the blending of clinical categories when reporting on outcomes needs to be aggressively discouraged (we should not be comparing apples with oranges by including pears!).
Any risk factor, however favourable, cannot overcome a poor technical result. Technical, anatomical and procedural factors trump all others.'334- Factors independently influencing endovascular interventions are multilevel interventions, tibial interventions, poor tibial runoff scores, anatomical lesion stratification, and FP stenting. Factors affecting bypass grafting include infrainguinal prosthetic grafts, small-calibre vein grafts, non-GSV grafts and spliced vein grafts. The GSV orientation does not influence patency or limb outcomes. The GSV length influences primary, but not secondary patency.
The influence of ethnicity and gender on revascularisation still remains elusive. A review of US registry and PREVENT III trial data suggests that young black females may be at the highest risk. These patients are generally diabetic or may have renal impairment.'335-Tight blood pressure control, blood sugar control and lipid-lowering strategies may influence cardiovascular event rates, but do not influence the natural history of lower-extremity revascularisation.'334-
The reduced graft patency rates associated with continued smoking are well described. Well-controlled epidemiological studies have not conclusively implicated novel biomarkers or emerging risk factors in the causal pathway of restenosis to date.'334-
The Lower Extremity Grading System (LEGS) has been proposed to rationalise revascularisation modality or amputation in patients with PAD.'336- It utilises five objective criteria (angiographic findings, clinical presentation, functional status, comorbidities and technical factors). A total score of 0 - 9 favours surgery, 10 - 19 favours endovascular interventions, and >20 favours major amputation. The LEGS score has been validated retrospectively and prospectively, and its utility in treatment planning needs to be encouraged.[336,337]
The PIII CLI risk score is derived from the PREVENT III study.[338] It employs five binary variables to predict 1-year AFS, each with a weighted score: dialysis-dependent ESRF (scores 4), presence of tissue loss (scores 3), age >75 (scores 2), haematocrit <30 (scores 2) and history of advanced CAD (scores 1). A total score of 0 - 3 is associated with a 1-year AFS rate of 86%, whereas a total score of 8 or more is associated with a 1-year AFS rate of 45%. The PIII CLI risk score has been internally and externally validated.[338,339]
The FINNVASC risk score has been proposed to predict 30-day postoperative outcomes in patients undergoing lower-extremity revascularisation.[340] It is based on four variables, each assigned 1 point (diabetes, CAD, gangrene and urgent operation). The FINNVASC score has been validated, with a lower 30-day mortality and amputation rates associated with lower FINNVASC scores (0 - 2) compared with higher FINNVASC scores (3 - 4).[341] Patients with a FINNVASC score of 3 - 4 have a 30-day mortality of 12.8%,
an amputation rate of 25.5% and a combined mortality/amputation rate of 35.9%.).[341] This group also reported that patients with a FINNVASC score of 3 - 4 and a creatinine level >150 μmol/L have a 1-year AFS of 53.1% v. 12.5% for creatinine levels <150 μmol/L.
The BASIL trial developed a survival prediction model to facilitate clinical decision making.[342] Despite reporting better outcomes in patients with BS who survive beyond 2 years initially, this report concluded that 'patients in the BASIL trial were at high risk of amputation and death regardless of revascularisation strategy', and 'it may thus be possible to define the clinical and anatomical (angiographic) characteristics of CLI patients who are likely to live for >2 years after intervention'. To confound things further, a recent analysis of the BASIL study concluded that women had similar short-term, but better long-term outcomes after revascularisation, and that female gender is an independent risk factor for outcomes following revascularisation as well as development of symptomatic PAD.[343]
The CRAB and EVRICA predictive models have also been previously published.[344,345]
The GVG on CLTI have been recently published.'8- In this document, with regard to anatomic classification, risk stratification, and predictors of limb salvage, the almost uniform answer to the question - how satisfied are you with the present systems - was 'somewhat satisfied'. 'There was strong support for a new approach to patient and limb risk stratification and for a new anatomic classification system'. Whether these recommendations translate to better predictive abilities with respect to revascularisation remains to be defined in clinical practice.
Future directions
While various risk predictive models exist, the ability of these models to predict the absolute futility of revascularisation or the utility of a specific revascularisation modality in a given patient remains elusive. The utility of these predictive models needs to be validated using the recent guidelines on CLTI. The role of HIV/AIDS in risk prediction models remains undefined. The role of these predictive models needs to be validated in SSA, especially in SA, considering the cosmopolitan composition of its population.
Recommendation 159
More intensive treatment planning is essential prior to revascularisation. PLAN needs to be considered before proceeding to revascularisation. (Class Ic; Level C)
Recommendation 160
Predictive scoring systems should be utilised in decision making. (Class I; Level B)
Recommendation 161
Revascularisation procedures should be considered only in patients who are independent and ambulatory. (Class I; Level B)
Recommendation 162
Operative and endovascular strategies are complementary and not competitive, and evidence-based revascularisation needs to be individualised. (Class IIa; Level C)
Endo-first strategy for infrainguinal disease in patients with CLTI? Appraisal of the current evidence
More than two decades of progressive utility and technical advances have consolidated the appeal of endovascular procedures for patients with CLTI and infrainguinal disease. The advantages of endovascular interventions over bypass graft surgery for infrainguinal disease merit consideration:
• All these procedures can be performed under local anaesthesia and some sedation, in a cathlab or hybrid facility. Theatre resources, with all their current failings and frustrations, are not required.
• Post-procedure high care is not required.
• These procedures are minimally invasive, requiring only an access sheath (>90% from the groin), and a guiding sheath or catheter for intervention.
• Blood loss is minimal.
• Procedural times are generally shorter than BS.
• Post-procedure systemic complications are markedly reduced following endovascular procedures.
• BS is attended by the considerable baggage of surgical site sepsis and wound healing considerations.
• Length of hospital stay is shorter with endovascular procedures.
• Most of the complications of endovascular procedures can be addressed without surgery (e.g. repeat endovascular interventions for restenosis, stents for major dissections, covered stents for perforation, etc.).
The only limitation afflicting infrainguinal endovascular procedures is its long-term durability compared with infrainguinal BS with a good-quality single-segment GSV graft.
Not all patients are suitable for infrainguinal BS. High-risk patients are at a considerable risk for perioperative MACE and MALE such as graft occlusion, major amputations, and mortality. Patients with diabetes and truncal obesity are at an increased risk of surgical site sepsis and wound healing complications, especially groin surgical wounds. Patients may be admitted for weeks or months in hospital in a dedicated wound care nursing facility to address their wound-related complications. The QoL is severely impaired in these patients, not to mention the overall costs of treatment.
It is not surprising then that infrainguinal endovascular procedures have surpassed the tag of competitive treatment strategy for CLTI in recent years. It is now universally accepted as a useful complementary treatment strategy for infrainguinal revascularisation.
It is debatable whether an 'endovascular intervention-first' strategy is mandated for patients with CLTI and infrainguinal disease. Supporting evidence generally comprises case series, registries, and device-specific trials, which are generally industry-sponsored with considerable patient selection and reporting bias. Another confounding consideration is that not all infrainguinal anatomical lesions are endosuitable.
Whether an 'endo-first' strategy is superior to a 'bypass-first' strategy requires that the following variables are comparable in a well-designed RCT: patient risk, limb severity, suitable GSV, and an infrainguinal endosuitable anatomical lesion.
The only RCT that has even come close to such a comparison is the BASIL trial that involved 27 centres in the United Kingdom. A total of 452 patients with CLTI were enrolled, 228 were randomised to a bypass-first revascularisation strategy, and 224 were randomised to a BA-first revascularisation strategy. The primary endpoint was AFS. An interim analysis reported that at 6 months, AFS and OS were similar after the two treatment strategies. BS was associated with more morbidity, as was expected. There was no difference in the HRQoL between the groups. BS was one-third more expensive than BA.[153] The trial ran for 5.5 years, and follow-up was complete when patients reached an endpoint (major amputation or death). Follow-up was 100% at 3 years, and 54% >5 years. An intention-to-treat analysis reported that at the end of the follow-up period, mortality was 56% (n=250), 38% were alive without a major amputation (n=168), 7% were alive with a major amputation and four patients were lost to follow-up. Overall, there was no significant difference in AFS or OS between the two strategies.[346 ]However, for those patients who survived for at least 2 years after randomisation, a bypass-first revascularisation strategy was associated with a significant increase in subsequent OS (HR 0.61; 95% CI 0.50 - 0.75; p=0.009), and a trend towards improved AFS (HR 0.86; 95% CI 0.5 - 1.07; p=0.108).
In an analysis based on treatment received, BA had a higher immediate technical failure rate (20% v. 2.6% for BS).[347] A quarter of the grafts used were prosthetic. The outcome of vein bypass was better for AFS (p=0.003) but not OS (p=0.38) compared with prosthetic bypass. Survival was significantly worse after bypass following a failed BA than after bypass as a first revascularisation attempt for AFS (p=0.006) but not for OS (p=0.06). Most BA patients ultimately required a BS. Bypass with vein offered the best long-term AFS and OS. Prosthetic grafts performed worse than BA. A recent analysis reported sustained benefits for a bypass-first strategy, primary bypass (PB), in the long-term, compared with secondary bypass (SB) following a failed BA-first strategy.'180- At a median of 7 years, PB was associated with a better AFS (HR 1.58; p=0.04), limb salvage (PB 85% v. SB 73%; p=0.06), and OS (PB 68% v. SB 51%; p=0.06).
Results comparing the two groups with respect to HRQoL, resource utilisation and cost-effectiveness were reported.[348,349] There was no significant difference in HRQoL between the two groups. BS had a lower reintervention rate at 1 year compared with BA. The mean admission costs were higher for BS at 1 year. At the end of the follow-up period, however, there was no significant cost difference. The admission rates and overall length of hospital stay between the two groups were similar. They reported that 'The probability that BSX (BS) was more cost-effective than BAP (balloon angioplasty) was relatively low given the similar distributions in HRQoL, survival, and hospital costs'.
A recent analysis reported that the 30-day outcomes were similar between men and women.[343] However, 'at three years, female sex was associated with significantly better AFS (HR 0.65; 95% CI 0.47 - 0.89; p<0.01), OS (HR 0.66; 95% CI 0.46 - 0.95; p=0.02) and freedom from MALE (HR 0.74; 95% CI 0.57 - 0.96; p=0.02)'.[343]
BS for CLTI in patients with infrainguinal disease is a well-established treatment strategy with proven durability, and sustained long-term outcomes in average-risk patients who have a suitable vein graft. The challenge remains the patients at risk for significant procedure-related perioperative morbidity. Better patient selection may improve HRQoL, resource utilisation and cost-effectiveness.
Challenges confounding the 'endo-first' strategy have always related to durability, which impacts negatively on the HRQoL, reintervention rates, overall costs and cost-effectiveness. Newer catheter-based technologies attempting to resolve these deficiencies for infrainguinal revascularisation represent an ever-shifting goal post that may yet salvage an 'endo-first' treatment strategy. But at what cost? And will the benefits be sustained long-term?
Quite clearly more good-quality trials are needed. There are three trials currently underway that are designed to test, and hopefully rationalise, the merits of and indications for a 'bypass-first' revascularisation strategy v. an 'endo-first' revascularisation strategy for patients with infrainguinal PAD and CLTI.[350-352]
Limb-salvage strategies for non-reconstructible disease
The therapeutic goals in treating CLTI include improving survival, relieving ischaemic foot pain, healing areas of ulceration, preventing major amputations, improving functional status, and improving QoL.
Although the optimal treatment of CLTI is undoubtedly revascularisation, unfortunately, a significant proportion of patients are not suitable for revascularisation for anatomical or physiological reasons. Whereas major amputation may be the only option for these patients, there is clearly a group of 'no option' CLTI patients who may benefit from certain alternative treatment strategies. These include interventional treatment modalities, pharmacotherapy, pain control and wound management, and biological and regenerative therapies.
Spinal cord stimulation
Spinal cord stimulation (SCS) is achieved with the use of a device that stimulates sensory fibres through electrodes implanted in the epidural space. This modality promotes activation of cell signalling pathways that cause the release of vasodilatory substances, leading to a decrease in vascular resistance and relaxation of vascular smooth-muscle cells. This has been shown to result in increased capillary flow and density of perfusing capillaries, higher skin temperatures and local TcPO2, normalisation of pulse wave morphology, and improved skin nutrition. In addition, SCS suppresses sympathetic vasoconstriction and pain transmission.
A Cochrane review[353] concluded that SCS offered a modest positive effect on pain relief and an 11% reduction in the amputation rate compared with medical treatment at 1 year. They stress, however, that the positive benefits should be weighed against the high cost and possible complications. They concluded that SCS is not a cost-effective treatment of CLTI.[353] The ESVS practice guidelines state that the benefit of SCS is unproven, with insufficient evidence to recommend its use in the treatment of CLTI.[8,353]
Lumbar sympathectomy
Sympathetic denervation of the lumbar sympathetic ganglia is performed either through open or laparoscopic retroperitoneal access or through percutaneous chemical blockade. A Cochrane systematic review was unable to find any RCTs that evaluated the effect of lumbar sympathectomy (LS: open, laparoscopic, or chemical) compared with no intervention in CLTI associated with non-reconstructible PAD. Overall, there is no evidence to suggest that LS reduces the risk of major amputation in patients with CLTI.[354]
Intermittent pneumatic compression
The mechanism of action of intermittent pneumatic compression (IPC) in patients with CLTI remains indeterminate. Arterial flow, PSV, end-diastolic velocity, and pulse volume are all increased with IPC.[355] Two controlled studies and several case series have been published regarding IPC, but there is no robust evidence from high-quality trials. This modality ameliorates rest pain, reduces minor amputations, and adjourns major amputations. However, it does not significantly reduce the incidence of inevitable major limb loss.[355]
Venous arterialisation
A recent meta-analysis reported that venous arterialisation could be a valuable treatment option in selected patients with 'no option' CLTI. Unfortunately, there are currently no data robust enough to support any recommendation on how to appropriately select patients for this procedure.[356]
Prostanoids
Prostanoids act by inhibiting the activation of platelets and leukocytes, and by promoting vasodilation and vascular endothelial cytoprotection through antithrombotic and profibrinolytic mechanisms.
A 2018 Cochrane paper reviewed 33 prostanoid studies with various formulations, doses, and administration routes. As a group, however, prostanoids did not have a significant impact on amputations or mortality. Prostanoids were associated with a statistically significant increase in side-effects including headache, facial flushing, nausea, vomiting, and diarrhoea. The authors of the Cochrane systematic review concluded that there was no strong evidence supporting the efficacy and safety of prostanoids in patients with CLTI on the basis of a high-quality meta-analysis of homogeneous, long-term RCTs. A subgroup analysis of the Cochrane meta-analysis, however, suggested that iloprost appeared to reduce major amputation, and fared better with rest pain and ulcer healing.[357]
There are no data supporting the use of prostanoids to reduce the risk of major amputation in CLTI patients in whom revascularisation is not possible.[8]
The ACC/AHA guidelines recommend that parenteral administration of PGE1 or PGE2 may be considered to reduce pain, and to improve ulcer healing in CLTI, but that the beneficial effect is likely to occur only in a small subset of patients.[8,358-
Naftidrofuryl
A Cochrane review of eight RCTs evaluated the use of naftidrofuryl in CLTI patients. The studies were found to be of low methodologic quality, with varying levels of severity of CLTI, varying duration of treatment, and different measures of effect, precluding a meaningful pooling of results. There is currently insufficient evidence to support the use of naftidrofuryl in the treatment of CLTI.[359]
Pentoxifylline
The benefits of pentoxifylline in CLTI remain ill-defined. There is currently a lack of consistent evidence to recommend the use of pentoxifylline in the treatment of CLTI.[8,360]
Hyperbaric oxygen therapy
There are numerous plausible mechanisms for hyperbaric oxygen therapy (HBOT) to have a therapeutic role in CLTI. Overall, despite the ongoing controversy, there may be a role for the use of HBOT to accelerate ulcer healing in diabetic patients with non-healing neuropathic ulcers, and low-grade ischaemia, who have failed to respond to conventional wound care. However, HBOT does not prevent major limb amputation and should not be used as an alternative to revascularisation in patients with CLTI.[361,362]
Pain management
Management of ischaemic pain is challenging in patients with 'no option' CLTI, and optimal pharmacological therapies have not been established. Optimising neuropathic ischaemic pain control appears to be a cornerstone of management. No recommendations of pharmacological agents can be made currently, but a number of novel approaches to manage pain have shown positive results and require further investigation. These include the use of intravenous lidocaine for short-term relief of ischaemic pain. The benefit of ketamine in varying pain states still exists, but its use in CLTI on the current level of evidence is not supported.[263] The role of other agents such as pregabalins, duloxetine and amitriptyline needs to be researched. Current pain control in clinical practice includes simple analgesics, oral opioids and amitriptyline.
Biologic and regenerative therapy in 'no option' CLTI
Biologic and regenerative therapy include gene therapy and stem cell therapy. There have been promising early safety and efficacy trial data for both gene and cellular therapies in patients with CLTI. Despite these early promising results, no phase 3 trials have shown this therapy to be effective. Still, current trial design has improved, and there are multiple phase 3 clinical trials that are either actively enrolling or are in early stages of development. These involve potentially disruptive technologies that, if proven effective, could dramatically alter how patients with 'no option' CLTI are cared for in the future. Until further evidence is available, these therapies should be considered investigational.[8]
Future directions
There is enormous scope for future studies to address therapeutic strategies for 'no option' CLTI patients. These need to be actively encouraged to address the needs of these patients who do not have a desperate indication for expedited major amputations.
Recommendation 163
Operative and endovascular strategies are complementary and not competitive, and evidence-based revascularisation needs to be individualised. (Good practice statement)
Recommendation 164
Consider SCS in carefully selected patients where revascularisation is not possible to reduce the risk of amputation and to decrease intractable ischaemic rest pain. (Class IIb; Level B)
Recommendation 165
Lumbar sympathectomy is not recommended for limb salvage in CLTI patients in whom revascularisation is not possible. (Class I; Level C)
Recommendation 166
Intermittent pneumatic compression therapy may be considered in carefully selected patients (e.g. intractable rest pain, non-healing minor tissue loss) in whom revascularisation is not possible. (Class IIb; Level C)
Recommendation 167
Venous arterialisation procedures cannot be recommended currently.
(Good practice statement)
Recommendation 168
Prostanoids may be used selectively for patients with non-reconstructable disease with intractable rest pain and/or non-healing minor tissue loss. (Class IIb; Level C)
Recommendation 169
Naftidrofuryl, pentoxiphylline, cilostazol and vasodilators cannot be recommended currently for the treatment of 'no option' CLTI. (Good practice statement)
Recommendation 170
Hyperbaric oxygen therapy is not recommended currently for managing patients with CLTI (WIfI ischaemia grade 2/3) and non-reconstructable disease. (Class I; Level C)
Recommendation 171
Continue to provide optimal wound care until the lower-extremity wound is completely healed, or the patient undergoes an amputation.
(Good practice statement)
Recommendation 172
Optimum pain management is absolutely essential in 'no option' CLTI patients. The expertise of pain management clinics or specialists should be engaged were feasible. (Good practice statement)
Recommendation 173
Restrict use of therapeutic angiogenesis to 'no option' CLTI patients who are enrolled in a registered clinical trial. (Class I; Level B)
Managing the septic vascular graft, stent, or stent graft
Prosthetic grafts, stents, and stent grafts (covered stents or endografts) are indispensable in the management of PAD. The use of vascular prosthetic grafts has led to a significant improvement in the QoL but have also been accompanied by a concomitant increase in the incidence of vascular graft infections (VGIs). The entity of VGI is rare yet represents a grave complication of vascular surgery. Infections are associated with a high mortality rate, a high amputation rate of affected extremities, and a possibility of reinfection. VGIs can be divided into 2 categories:[364]
• Extracavitary, mainly located in the groin.
• Intracavitary, located in the abdomen and/or thorax.
The incidence of intracavitary aortic graft infections is 0.2 - 5.0%.[366] The incidence of extracavitary graft infections can be as high as 6%.[364,367] Intracavitary aortic graft infections have a higher mortality (24 - 75%) compared with extracavitary graft infections (17%).[368] However, extracavitary graft infections are associated with a high morbidity, with an amputation rate of up to 40%[364,365,368]
Early VGIs occur within the first 4 months after graft placement surgery, whereas late VGIs occur after 4 months. However, early VGIs mostly occur within the first 2 months postoperatively. Early VGIs are usually caused by virulent organisms, such as Staphylococcus aureus, Escherichia coli, Proteus spp., and Pseudomonas aeruginosa. Late infections are usually caused by low-virulence bacteria such as S. epidermidis. Procedure-specific risk factors include incision in the groin area, wound infection, redo surgery, and emergency procedures.
Prosthetic graft infections can be classified by time of appearance after implantation, relationship to postoperative wound infection (Szilagyi classification), and the extent of graft involvement (Bunt's classification).[368] The Samson classification provides guidance for selection of imaging techniques, options for medical and surgical management, management of complications, and prognosis (Table 9).[369]
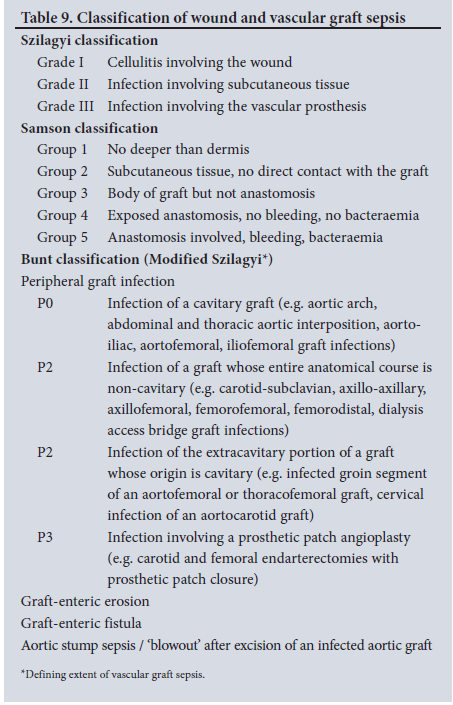
The AHA guidelines recommend ultrasound as the initial imaging modality of choice when suspecting a VGI.[364] Ultrasound examination allows evaluation for pseudoaneurysm formation or any fluid collection. When ultrasound examination findings are indeterminate, CTA or MRI can be considered. When these two modalities also prove to be unhelpful in confirming a diagnosis of VGI, a positron emission tomography (PET)/CT scan, or indiumlabelled white blood cell study scan can be done. A PET/CT is useful in the setting of late infections, where the symptoms are nonspecific and non-localising, and where all other diagnostic modalities have provided no evidence of a focus of infection. In a prospective cohort study involving 34 patients suspected to have a VGI, Sah et al.[370] reported on the diagnostic accuracy of 18-fluoredeoxyglucose (FDG) PET/CT in VGIs.[370] The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of FDG-PET/CT were 100%, 86%, 96%, 100%, and 97%, respectively.
In the setting of an aorto-enteric fistula and gastrointestinal bleeding, patients should undergo esophagogastroduodenoscopy to look for erosions, ulcers, or thrombi. When the infected vascular graft is intrathoracic, imaging findings should be combined with blood culture results and echocardiography.[364]
Management
The optimal management of prosthetic VGIs involves partial or total excision of the graft, debridement of the infected surrounding tissues, restoration of blood flow (in-situ or extra-anatomical bypass), and finally, appropriate antibiotic therapy.[364,365,368]
Empiric antibiotic therapy should be parenterally administered, with targeted activity against the organisms expected to be grown in culture. Initial therapy involves broad-spectrum Gram-positive coverage (accounting for methicillin-resistant S. aureus (MRSA)), and broad Gram-negative coverage (accounting for Pseudomonas spp.). Vancomycin or linezolid can be used for Gram-positive coverage and the antipseudomonal β-lactams can be used for initial Gram-negative coverage.[365] Once the antibiotic susceptibilities become available, directed antibiotic therapy can be instituted. In patients with penicillin allergy, fluoroquinolones can substitute for ß-lactams.[371] There are no clinical trials that have evaluated the optimal duration of antibiotic therapy after a VGI. However, there is consensus that a minimum 6 weeks of parenteral antibiotic therapy is necessary. In cases of partial graft excision or graft preservation, patients may be placed on indefinite antibiotic therapy.[365]
Antimicrobial therapy alone, with or without debridement, is reasonable for patients with Samson group 1 or 2. These infections should be treated as soft-tissue infection that does not involve the graft. This is generally the norm for select early graft infections. All patients with Samson group 3 and 4 should have meticulous debridement of all infected material and tissue. Usually, multiple surgical debridements are necessary to enable wound coverage with a muscle flap, and in some cases, a vacuum-assisted closure device. Deep surgical specimens should be sent for culture and susceptibility in general.
For Samson group 3, reports indicate better outcomes in select patients treated with graft preservation rather than graft resection, and in-situ reconstruction. Calligaro et al.[372] reported that patients with early-onset Samson group 3 (<2 months postoperatively) were more likely to have successful graft preservation than patients with later onset of infection. Late-onset Samson group 3 was more likely to have occluded grafts or disrupted anastomosis and could be considered for graft resection and in-situ reconstruction rather than preservation.
In-situ reconstruction methods in the setting of low-virulence sepsis employs rifampin-bonded or silver-coated synthetic vascular grafts, cryopreserved or fresh arterial allografts, and autogenous venous grafts.[373] The selection of a specific conduit must be individualised and is somewhat dependent on the personal experience of the vascular surgeon. There is no consensus about which specific material should be chosen for reconstruction.[368]
Extra-anatomic revascularisation followed by graft excision was long considered the preferred surgical approach for Samson group 3 or 4 because of a theoretical decreased risk of infection attributable to avoiding graft preservation, or in-situ reconstruction of a new graft in an infected area with high virulence sepsis. However, this procedure is associated with significant morbidity, including persistent infection at the site of vascular stump ligation, blowout of the aortic stump with potentially life-threatening haemorrhage, as well as the risk of infection in the new graft. In addition, thrombosis can occur within the extra-anatomic graft, resulting in lower-extremity amputation.
For Samson group 3 or 4, extra-anatomic revascularisation followed by graft resection is reasonable only for patients with infection caused by MRSA, P. aeruginosa, or multidrug-resistant microorganisms, or for patients for whom graft preservation or in-situ reconstruction has failed. For Samson group 5, extra-anatomic revascularisation followed by graft excision is reasonable.[374]
There is no consensus about the procedure of choice for the surgical management of intra-abdominal vascular graft infections. The choice of therapy should be individualised for each patient and depends in part on personal preferences, experience of individual surgeons, and availability of equipment and resources. For patients who have life-threatening bleeding or sepsis, emergency surgery is necessary. The most important goals are surgical control of bleeding, drainage of abscess, control of sepsis, and haemodynamic stabilisation. Patients can be categorised into those who need emergency surgery to control bleeding and sepsis, and those who do not require emergency surgery. There could be fewer surgical options available in an emergency. For patients who cannot be stabilised long enough to select the most appropriate surgical option, endovascular bridge therapy might be the only realistic option.[364-368]
Aorto-enteric fistula is a serious complication of aortic graft infections. Standard treatment of abdominal VGIs complicated by aorto-enteric fistula is a staged procedure that consists of an axillofemoral bypass through a non-infected field, graft removal, and closure of the aortic stump. In-situ repair of an aorto-enteric fistula with rifampin-soaked grafts is another option. The surgical technique consists of excising the infected graft, repairing the intestinal defect, placement of the in-situ rifampin-soaked graft, and finally covering the graft with omentum. The patients are then treated with long-term oral antibiotics. This technique can be used in patients with limited infection, but not in those patients with large abscesses and excessive purulence.[364,368]
Thoracic VGI involve a synthetic arterial allograft used to treat aortic aneurysm, dissection, or repair of the aorta damaged from blunt trauma. Unlike intra-abdominal VGI, there are fewer surgical options for successful management. The use of a cryopreserved or fresh arterial allograft for treatment of intrathoracic VGI is reasonable.[364]
Antibiotic prophylaxis
The aim of antibiotic prophylaxis is to achieve serum and tissue concentrations of the antibiotic at a level above the minimum inhibitory concentration for organisms likely to have colonised the surgical site to prevent surgical site infections. The recommended prophylactic antibiotics are cefazolin and cefuroxime. Antibiotics should be administered within 60 minutes of the incision, and additional dosing is warranted if the surgical procedure persists for >two half-lives of the antibiotic administered (2 - 5 hours for cefazolin and 3 - 4 hours for cefuroxime). Antibiotic prophylaxis should be discontinued 24 hours after the end of surgery, because prolonged prophylaxis duration does not decrease the risk of postoperative infections and has been associated with increased resistance should a surgical site infection occur. In patients allergic to β-lactams, it is recommended to give either vancomycin or clindamycin. With vancomycin use, the infusion should begin 120 minutes before the incision and an additional dose is recommended 6 - 12 hours after prolonged surgery. With clindamycin, a second dose is needed after 3 - 6 hours.[364,368]
Future directions
There remain a lot of grey areas in the management of graft sepsis mainly with regards to conservative v. aggressive surgical management due to lack of randomised trials. More reporting will also assist with future management of this rare yet grave complication.
Recommendation 174
DUS should be considered as the initial imaging procedure of choice in suspected peripheral graft sepsis. (Class I; Level C)
Recommendation 175
CTA or MRI should be considered when intra-abdominal or intra-thoracic vascular graft infection is suspected. (Class I; Level C)
Recommendation 176
In patients with gastrointestinal bleeding and suspected of having a secondary aorto-enteric fistula, an oesophago-gastroduodenoscopy and CTA are recommended. (Class I; Level C)
Recommendation 177
In patients with suspected VGI and indeterminate CTA or MRI findings, a PET/CT scan, or indium white blood cell study may be considered. (Class IIa; Level C)
Recommendation 178
For Samson group 1 and 2, a trial of antimicrobial therapy with or without surgical debridement for 2 or 4 weeks is reasonable.
(Class IIa; Level C)
Recommendation 179
For Samson group 3 or 4 with low-virulence graft sepsis, antimicrobial therapy for 4 to 6 weeks is reasonable. After the initial therapy, a course of oral antimicrobial therapy for 6 weeks to 6 months may be considered. (Class IIa; Level C)
Recommendation 180
In patients with intrathoracic VGI, those who cannot tolerate extensive reconstructive surgery, or those with in-situ repair using a synthetic graft, lifelong suppressive antimicrobial therapy may be considered. (Class IIa; Level C)
Recommendation 181
For Samson group 3, early VGI (<6 weeks post surgery), it is reasonable to consider graft preservation treatment rather than graft excision and reconstruction. (Class IIa; Level C)
Recommendation 182
For Samson group 5 and Samson group 3 or 4, VGI caused by MRSA, Pseudomonas spp., or multidrug-resistant microorganisms, or for patients for whom graft preservation or in-situ reconstruction has failed, it is reasonable to perform extra anatomic revascularisation followed by graft excision instead of graft preservation or in-situ reconstruction. (Class IIa; Level C)
Recommendation 183
In patients who have no aortoenteric fistula, graft excision and in-situ reconstruction with cryopreserved, arterial allograft, or venous autograft or rifampin-bonded prosthetic graft, or silver-impregnated prosthetic graft is reasonable. (Class IIa; Level C)
Recommendation 184
In patients with aortoenteric fistula, and in patients with infection caused by MRSA, Pseudomonas spp., or multidrug-resistant microorganisms or those with extensive intra-abdominal abscess or perigraft purulence, extra-anatomic bypass revascularisation, followed by graft excision may be considered. (Class IIa; Level C)
Recommendation 185
Perioperative administration of a β-lactam antibiotic to prevent wound infection is reasonable for patients who undergo clean vascular graft surgery for a period <24 hours. (Class IIa; Level C)
Lower-extremity amputations
CLTI is associated with a reduced life expectancy, impairment of ambulation, and a high risk of limb loss. An important aspect of clinical care in CLTI is preservation of ambulation. Revascularisation is the best method for achieving functional limb salvage.[8,375,376]
Although most patients require a single procedure to accomplish limb salvage, many, particularly diabetics, will need minor amputations to remove distal necrotic or infected tissue to achieve a well-healed and functional extremity. Minor amputations of the foot include digital and ray amputation of the toe, and transmetatarsal amputation of the forefoot (TMA).
There are, unfortunately, some situations in which an aggressive attempt at limb salvage would either be unlikely to succeed, pose too great a physiological stress on the patient, or would be of limited value because of other causes of limb dysfunction.
For these patients, a major amputation would be the best alternative.
Primary amputation
This may be defined as a lower-extremity amputation without an initial open or endovascular attempt at limb salvage. The four major goals of primary amputation are:
• Relief of ischaemic pain.
• Removal of all lower-extremity diseased, necrotic, or grossly infected tissues.
• To achieve primary stump healing.
• Preservation of independent ambulatory ability for patients who are capable.
Indications for primary amputation include:
• Non-reconstructible arterial disease, as confirmed by vascular imaging studies.
• Non-salvageable foot (associated with advanced necrosis or sepsis precluding salvage of a TMA).
• Non-functional lower extremity, e.g. patient with a fixed flexion deformity of the knee.
• Severe comorbid conditions or limited life expectancy.
• Multiple surgical procedures needed to restore a viable lower extremity.
Level of amputation
Selection of the appropriate level of amputation that will heal primarily is vital for prosthetic rehabilitation and mobility.
Assessment of preoperative tissue perfusion may make it possible to lower the level of amputation, although there is no accurate method to predict the optimal level of amputation.
Laser Doppler flowmetry, thermography, skin perfusion pressure, fluorometric quantification of a fluorescein dye, TcPO2, and indocyanine green fluorescence angiography have all been investigated. However, there is no single best method of evaluating tissue perfusion that can accurately predict the wound healing potential, or failure at the site of amputation.
Ankle-level amputations are no longer recommended in patients with PAD.
Fate of the contralateral limb
The contralateral limb faces a variable risk of amputation (2.2 - 44%), with a lower risk if the index amputation is a minor amputation. The main reason for amputation is disease progression. For this reason, continued follow-up of these patients, at least annually after an index amputation with attention to the contralateral limb, is important.
Rehabilitation, prosthesis
Once the decision to amputate has been made, a prosthetist should be involved with the surgical team to determine the optimal level of amputation that will ensure the best opportunity for healing, survival, and maximum functional mobility.
Recommendation 186
Minor foot amputations should be staged or otherwise, should be sparingly limited, and designed to preserve adequate foot function. (Class IIa; Level C)
Recommendation 187
Major amputations should be reserved for patients who are not suitable for revascularisation and who have medically refractory intractable rest pain, or non-salvageable foot (progressive foot necrosis, or advanced foot sepsis). (Class IIa; Level C)
Recommendation 188
Tissue perfusion studies should be used preoperatively to lower the level of amputation in candidates who are suitable for rehabilitation. (Class IIa; Level C)
Summary
PAD, in its current definition, is an established circulation disorder involving the lower limbs. Most cases are associated with atherosclerosis ('hardening of the arteries'), which leads to narrowing and/or blockages in the leg circulation. The risk factors for PAD are well recognised, and are predominantly lifestyle-related: smoking, diabetes mellitus, hypertension, hypercholesterolaemia, obesity, lack of exercise, gout, etc. It is not surprising then, that the population at highest risk for PAD are >50 years old.
Most individuals in the population do not have symptoms, or have vague leg symptoms that are not disabling and often ignored. The only time people in the community seek medical attention is when they have lifestyle-limiting exertional leg symptoms (cramping, stiffness, 'giving way', etc.), or when there is a significant chance of losing that limb (critical foot pain, leg ulcers or gangrene).
PAD has huge implications with respect to individual outcomes relating to life, limb and livelihood. People in the community living with PAD have a shorter life expectancy than people without PAD (~10 year difference). The life expectancy is even worse if they develop critical leg symptoms (~25% will not survive for 1 year). More than 70% of these deaths are related to heart attacks, strokes and other vascular complications.
In the community, we can reduce the risk of limb loss, improve life expectancy, and improve the QoL by addressing modifiable risk factors such as smoking, regular exercise, reducing weight with appropriate diets, regular medical leg circulation checks by the primary treating physician, appropriate foot care, and by implementing evidence-based drug treatment that protects predominantly against heart attacks and stroke, such as aspirin and statins. These health failings at the community level need to be addressed as a matter of urgency. People with PAD at risk of limb loss should be identified early, and referred for an evaluation by a vascular specialist.
Most of the people with PAD with exertional symptoms (>75%) will improve with exercise programmes, and by addressing and implementing strategies mentioned previously. There is no urgency in vascular treatments to address the circulation in these patients, such as balloon dilatation, stents or BS. Actually, these vascular treatments may be attended, ultimately, by more harm than good. These treatment options should be reserved for the small subset of patients who do not respond to lifestyle modification, medical treatment, and ET described previously.
Patients with critical leg symptoms related to PAD will need to have their lower-limb circulation evaluated as a matter of urgency by a vascular specialist. The decision to proceed with vascular treatments to improve the lower-limb circulation is based on the patient's fitness and life expectancy, the limb severity and whether the vascular imaging tests support such treatments. Not all patients will be suitable for vascular treatments. These include high-risk medical patients, extensive gangrene or sepsis of the foot, and where the vascular imaging tests report that such vascular treatment options are not feasible. These patients may be best served by palliative pain control, or by a major amputation.
When indicated, PAD patients with critical limbs can have their circulation improved by various vascular procedures. These include open surgical bypass procedures, and minimally invasive procedures generally performed through a puncture of the groin vessel that dilate or stent narrowings or blockages in the circulation. There are advantages and disadvantages with both these approaches, and vascular treatment needs to be individualised. Which approach is suitable will depend on where the circulation problem is, how extensive the circulation problem is, the patient's fitness and life expectancy, the availability of a patient's surface vein for bypass, availability of resources and expertise to perform these procedures. Sometimes both these approaches may be used ('hybrid' procedure).
The latest minimally invasive technologies to treat critical limbs offer the promise of durability to rival that of BS. However, the evidence for their efficacy, and sometimes safety, is disappointingly limited in patients with critical limbs. But this evidence will improve in the years to come, especially with the current high-quality trials and studies. Hopefully, the current practice guideline principles formulated to address the huge gaps in knowledge, standardisation for evaluation, and clinical applications in PAD patients, will go a long way in the future to rationalise treatment strategies for people living with PAD.
Even with successful vascular treatments to improve circulation in patients with critical limbs, the overall benefits will not be realised until we properly supervise post-procedure wound and foot care, address the risk factors for PAD, implement evidence-based medical treatments, encourage ET, reduce obesity and improve patient fitness. The health community support in addressing these issues cannot be overstated.
Declaration. None.
Acknowledgements. None.
Author contributions. Equal contributions.
Funding. The process of developing this practice guideline for PAD was totally funded by the Vascular Society of Southern Africa (VASSA). No funding was solicited, nor offered, from any vascular device or pharmaceutical company, locally or internationally. Vascular device and pharmaceutical companies were deliberately excluded from any process involved in the development of this practice guideline on PAD.
Conflicts of interest. None.
References
1. Kung J, Miller RR, Mackowtak PA. Failure of clinical practice guidelines to meet Institute of Medicine standards. Two more decades of little, if any, progress. Arch Intern Med 2012;172(21):1628-1633. https:// doi.org/10.1001/2013.jamainternmed.56 [ Links ]
2. Murad MH. Clinical Practice guidelines: A primer on development and dissemination. Mayo Clin Proc 2017;92(3):423-433. https://doi.org/10.1016/j.mayocp.2017.01.001 [ Links ]
3. Guyatt G, Oxman AD, Akl EA, et al. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64(4):383-394. https://doi.org/10.1016/j.jdinepi.2010.04.026 [ Links ]
4. Guyatt GH, Oxman AD, Kunz R, et al. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64(4):395-400. https://doi.org/10.1016/j.jclinepi.2010.09.012 [ Links ]
5. Balshem H, Helfand M, Schünemann HJ, et al. Rating the quality of the evidence. J Clin Epidemiol 2011;64(4):401-406. https://doi.org/10.1016/jjclinepi.2010.07.015 [ Links ]
6. Guyatt GH, Oxman AD, Vist G, et al. Rating the quality of evidence - study limitations (risk of bias). J Clin Epidemiol 2011;64(4):407-415. https://doi.org/10.1016/j.jdinepi.2010.07.017 [ Links ]
7. Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary. J Am Coll Cardiol. 2011;57(8):1002-1044. https:/doi:10.1161/CIR.0b013e31820d8d78. [ Links ]
8. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischaemia. J Vasc Surg 2019;69(6S):3S-125S. https://doi.org/10.1016/j.jvs.2019.02.016 [ Links ]
9. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71:510-515. https://doiorg/10.1161/01.cir.71.3.510 [ Links ]
10. Selvin E, Hirsch AT. Contemporary risk factor control and walking dysfunction in individuals with peripheral arterial disease: NHANES 1999 - 2004. Atherosclerosis 2008;201(2):425-433. https://doi.org/10.1016%2Fj.atherosclerosis.2008.02.002 [ Links ]
11. Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20(2):384-392. https://doi.org/10.1093/ije/20.2.384 [ Links ]
12. Thorogood M, Connor M, Tollman S, et al. A cross-sectional study of vascular risk factors in a rural South African population: Data from the Southern African Stroke Prevention Initiative (SASPI). BMC Public Health 2007;13(7):326. https://doi.org/10.1186/1471-2458-7-326 [ Links ]
13. Guerchet M, Aboyans V, Mbelesso P, et al. Epidemiology of peripheral artery disease in elder general population of two cities of Central Africa: Bangui and Brazzaville. Eur J Vasc Endovasc Surg 2012;44(2):164-169. https://doi.org/10.1016/j.ejvs.2012.05.019 [ Links ]
14. Ganta N, Soherwadi S, Hughes K, Gillum RF. Burden of peripheral arterial disease in sub-Saharan Africa and the Caribbean 1990 to 2015. Vasc Endovascular Surg 2018;52(7):520-526. https://doi.org/10.1177/1538574418784709 [ Links ]
15. Johnston LE, Stewart BT, Yangni-Angate H, et al. Peripheral arterial disease in sub-Saharan Africa. A Review. JAMA Surg 2016;151(6):564-572. https://doi.org/10.1001/jamasurg.2016.0446 [ Links ]
16. Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral arterial disease in 2000 and 2010: A systematic review and analysis. Lancet 2013;382(9901):1329-1340. https://doi.org/10.1016/s0140-6736(13)61249-0 [ Links ]
17. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286(11):1317-1324. https://doi.org/10.1001/jama.286.11.1317 [ Links ]
18. Gr0ndal N, S0gaard R, Lindholt JS, et al. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65 - 74 years from a population screening study (VIVA Trial). Br J Surg 2015;102(8):902-906. https://doi.org/10.1002/bjs.9825 [ Links ]
19. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007;33; S1-S75. https://doi.org/10.1016/j.jvs.2006.12.037 [ Links ]
20. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295(2):180-189. https://doi.org/10.1001/jama.295.2.180 [ Links ]
21. TASC Working Group. Management of peripheral arterial disease (PAD). Trans-Atlantic Inter-Society Consensus (TASC). Eur J Vasc Endovasc Surg 2000;19(Suppl A):S1-S250. [ Links ]
22. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary: A collaborative report from the American Association for Vascular Surgery/ Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). J Am Coll Cardiol 2006;47(6):1239-1312. https://doi.org/10.1161/circulationaha.106.174526 [ Links ]
23. Criqui MH, Vargas V, Denenberg JO, et al. Fronek ethnicity and peripheral arterial disease: The San Diego population study. Circulation 2005;112(17):2703-2707. https://doi.org/10.1161/circulationaha.105.546507 [ Links ]
24. Dormandy JA, Murray GD. The fate of the claudicant - prospective study of 1969 claudicants. Eur J Vasc Surg 1991;5(2):131-133. https://doi.org/10.1016/s0950-821x(05)80676-0 [ Links ]
25. Fowkes FG, Low LP, Tuta S, Kozak J, AGATHA Investigators. Ankle-brachial index and extent of atherothrombosis in 8 891 patients with or at risk of vascular disease: Results of the international AGATHA study. Eur Heart J 2006;27(15):1861-1867. https://doi.org/10.1093/eurheartj/ehl114 [ Links ]
26. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for report dealing with lower extremity ischaemia: Revised version. J Vasc Surg 1997;26(3):517-538. https://doi.org/10.1016/s0741-5214(97)70045-4 [ Links ]
27. Lepäntalo M, Mätzke S. Outcome of unreconstructed chronic critical leg ischaemia. Eur J Vasc Endovasc Surg 1996;11(2):153-157. https://doi.org/10.1016/s1078-5884(96)80044-x [ Links ]
28. De Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: Systematic review and metaregression analysis. Stroke 2009;40(4):1105-1113. https://doi.org/10.1161/strokeaha.108.532218 [ Links ]
29. Hansen KJ, Wilson DB, Craven TE, et al. Mesenteric artery disease in the elderly. J Vasc Surg 2004;40:45-52. https://doi.org/10.1016/j.jvs.2004.03.022 [ Links ]
30. Bageacu S, Cerisier A, Isaaz K, Nourissat A, Barral X, Favre JP. Incidental visceral and renal artery stenosis in patients undergoing coronary angiogram. Eur J Vasc Endovasc Surg 2011;41(3):385-390. https://doi.org/10.1016/j.ejvs.2010.11.014 [ Links ]
31. Barba A, Estallo L, Rodriguez L, Baquer M, Vega de Céniga M. Detection of abdominal aortic aneurysm in patients with peripheral artery disease. Eur J Vasc Surg 2005(5);30:504-508. https://doi.org/10.1016/j.ejvs.2005.05.011 [ Links ]
32. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: A report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016;135(12):686-725. https://doi.org/10.1161/cir.0000000000000471 [ Links ]
33. Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55(3):305-368. https://doi.org/10.1093/eurheartj/ehx095 [ Links ]
34. Rieker O, Düber C, Schmiedt W, von Zitzewitz H, Schweden F, Thelen M. Prospective comparison of CT angiography of the legs with intraarterial digital subtraction angiography. AJR Am J Roentgenol 1996;166(2):269-276. https://doi.org/10.2214/ajr.166.2.8553929 [ Links ]
35. Ligush J, Reavis SW, Preisser JS, Kimberley J, Hansen KJ. Duplex ultrasound scanning defines operative strategies for patients with limb-threatening ischemia. J Vasc Surg 1998;28(3):482-491. https://doi.org/10.1016/s0741-5214(98)70134-x [ Links ]
36. Hou X, Chu G, Yu Y. Prospects of contrast-enhanced ultrasonography for the diagnosis of peripheral arterial disease: A meta-analysis. J Ultrasound Med 2018;37(5):1081-1090. https://doi.org/10.1002/jum.14451 [ Links ]
37. Nicolaides AN, Kakkos SK, Kyriacou E, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52(6):1486-1496. https://doi.org/10.1016/j.jvs.2010.07.021 [ Links ]
38. Madani A, Beletsky V, Tamayo C, Spence JD. High risk asymptomatic carotid stenosis: Ulceration on 3D ultrasound v. TCD microemboli. Neurology 2011;77(8):744-750. https://doi.org/10.1212/wnl.0b013e31822b0090 [ Links ]
39. Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJW. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA 2009;301(4):415-424. https://doi.org/10.1001/jama.30L4.415 [ Links ]
40. Martin ML, Tay KH, Flak B, et al. Multidetector CT angiography of the aortoiliac system and lower extremities: A prospective comparison with digital subtraction angiography. AJR Am J Roentgenol 2003;180(4):1085-1091. https://doi.org/10.2214/ajr.180.4.1801085 [ Links ]
41. Andras A, Ferker B. Screening for peripheral arterial disease. Cochrane Database Syst Rev 2014;7(4):CD010835. https://doi.org/10.1002/14651858.CD010835.pub2 [ Links ]
42. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA 2001;286(13):1599-1606. https://doi.org/10.1001/jama.286.13.1599 [ Links ]
43. Heald CL, Fowkes FG, Murray GD, Price JF, Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis 2006;189(1):61-69. https://doi.org/10.1016/j.atherosclerosis.2006.03.011 [ Links ]
44. Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, van Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7-year follow-up study. J Clin Epidemiology 2004;57(3):294-300. https://doi.org/10.1016/j.jclinepi.2003.09.003 [ Links ]
45. Alahdab F, Wang AT, Elraiyah TA, et al. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg 2015;61(3):42S-53S. https://doi.org/10.1016/j.jvs.2014.12.008 [ Links ]
46. Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: The Rotterdam study. Arch Intern Med 2000;160(19):2934-2938. https://doi.org/10.1001/archinte.160.19.2934 [ Links ]
47. Morcos R, Louka B, Tseng A, et al. The evolving treatment of peripheral arterial disease through guideline-directed recommendations. J Clin Med 2018;7(1):9. https://doi.org/10.3390/jcm7010009 [ Links ]
48. Erb W. Klinische Beitrage ze Pathologie des intermitterenden Hinkens. Munch Wed Wschr 1911;2:2487. [ Links ]
49. Kannel WB, Shurtleff D. The Framingham study. Cigarettes and the development of intermittent claudication. Geriatics1973;28:61-68. [ Links ]
50. Cole CW, Hill GB, Farzad E, et al. Cigarette smoking and peripheral arterial occlusive disease. Surgery 1993;114(4):753-756. [ Links ]
51. Murabito JM, D'Agostino RB, Silvershatz H, Wilson WF. Intermittent claudication. A risk profile from the Framingham heart study. Circulation 1997;96(1):44-49. https://doi.org/10.1161/01.cir.96.1.44 [ Links ]
52. Fowkes FG, Housley E, Riemersma RA, et al. Smoking, lipids, glucose intolerance and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh artery study. Am J Epidemiol 1992;135(4):331-340. https://doi.org/10.1093/oxfordjournals.aje.a116294 [ Links ]
53. Powell JT, Edwards RJ, Worrell PC, Franks PJ, Greenhalgh RM, Poulter NR. Risk factors associated with the development of peripheral arterial disease in smokers: A case control study. Atherosclerosis 1997;129(1):41-48. https://doi.org/10.1016/s0021-9150(96)06012-1 [ Links ]
54. Kannel WB, McGee DL. Update on some epidemiological features of intermittent claudication. J Am Geriar Soc 1985;33:13-18. https://doi.org/10.1111/j.1532-5415.1985.tb02853.x [ Links ]
55. Hirsch AT, Treat-Jacobson D, Lando HA, Hatsukami DK. The role of tobacco cessation, antiplatelet and lipid lowering therapies in the treatment of peripheral arterial disease. Vasc Med 1999;2(3):243-251. https://doi.org/10.1177/1358863x9700200314 [ Links ]
56. Willigendeal EM, Tejink JA, Bartelink MK, Peters RJ, Buller HR, Prins MH. Smoking and patency of lower extremity bypass grafts: A meta-analysis. J Vasc Surg 2005;42(1):67-74. https://doi.org/10.1016/j.jvs.2005.03.024 [ Links ]
57. Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh artery study. Eur Heart J 1999;20(5):344-353. https://doi.org/10.1053/euhj.1998.1194 [ Links ]
58. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J AM Coll Cardiol 1997;29(7):1422-1432. https://doi.org/10.1016/s0735-1097(97)00079-x [ Links ]
59. Krupski WC. The peripheral vascular consequence of smoking. Ann Vasc Surg 1991;5(3):291-304. https://doi.org/10.1007/bf02329389 [ Links ]
60. Adams MR, Jesup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: Reversibility with oral L-arginine but not vitamin C. J AM Coll Cardiol 1997;29(3):491-497. https://doi.org/10.1016/s0735-1097(96)00537-2 [ Links ]
61. Pittilo RM, Clarke JM, Harris D, et al. Cigarette smoking and platelet adhesion. Br J Haematol 1984;58(4):627-632. https://doi:10.1111/j.1365-2141.1984.tb06109.x [ Links ]
62. Fitzgerald GA, Oates JA, Nowak J. Cigarette smoking and homoeostatic function. Am Heart J 1988;115:267-271. https://doi:10.1016/0002-8703(88)90648-5 [ Links ]
63. Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic medication of smoking-associated hemodynamic and metabolic events. N Engl J Med 1976;295(11):573-577. https://doi.org/10.1056/nejm197609092951101 [ Links ]
64. Turner JA, McNicol MW, Sillett RW. Distribution of carboxyhaemoglobin concentrations in smokers and non-smokers. Thorax 1986;41(1):25-27. https://doi.org/10.1136/thx.41.L25 [ Links ]
65. Terry ML, Berkowitz HD, Kerstein MD. Tobacco - its impact on vascular disease. Surg Clin North Am 1998;78(3):409-429. https://doi.org/10.1016/s0039-6109(05)70323-6 [ Links ]
66. Rigotti NA. Strategies to help a smoker who is struggling to quit. JAMA 2012;308(15):1573-1580. https://doi.org/10.1001%2Fjama.2012.13043 [ Links ]
67. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioral interventions for smoking cessation. Cochrane Database Syst Rev 2016;10:CD008286. https://doi.org/10.1002/14651858.cd008286.pub2 [ Links ]
68. Rosen LJ, Galili T, Kott J, Goodman M, Freedman LS. Diminishing benefit of smoking cessation medications during the first year: A meta-analysis of randomised controlled trails. Addiction 2018;113(5):805-816. https://doi.org/10.1111/add.14134 [ Links ]
69. Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy v. control for smoking cessation. Cochrane Database Syst Rev 2018;5:CD000146. https://doi.org/10.1002/14651858.CD000146.pub5 [ Links ]
70. Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial antagonists for smoking cessation. Cochrane Database Syst Rev 2016;5:CD006103. https://doi/10.1002/14651858.CD006103.pub7 [ Links ]
71. Camenga DR, Tindle HA. Weighing the risks and benefits of electronic cigarette use in high-risk population. Med Clin North Am 2018;102(4):765-779. https://doi.org/10.1016/j.mcna.2018.03.002 [ Links ]
72. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116(9):1509-1526. https://doi.org/10.1161/circresaha.116.303849 [ Links ]
73. Heart Protection Study Collaborative Group. Randomised trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20 536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg 2007;45(4):645-654. https://doi.org/10.1016/j.jvs.2006.12.054 [ Links ]
74. Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database of Syst Rev 2007;4:CD000123. https://doi.org/10.1002%2F14651858.CD000123.pub2 [ Links ]
75. Youssef F, Seifalian AM, Jagroop IA, et al The early effect of lipid lowering treatment on carotid and femoral intima media thickness (IMT). Eur J Vasc Endovasc Surg 2002;23(4):358-364. https://doi.org/10.1053/ejvs.2002.1611 [ Links ]
76. Schillinger M, Exner M, Mlekusch W, et al. Statin therapy improves cardiovascular outcome of patients with peripheral arterial disease. Eur Heart J 2004;25(9):742-748. https://doi.org/10.1016/j. ehj.2004.02.012 [ Links ]
77. Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER). Am J Cardiol 2010;106(2):204-209. https://doi.org/10.1016/j.amjcard.2010.03.018 [ Links ]
78. Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dL with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011;57916):1666-1675. https://doi.org/10.1016/j.jacc.2010.09.082 [ Links ]
79. Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: Randomised controlled trial. BMJ 2002;325(7373):1139. http://doi.org/10.1136/bmj.325.7373.1139 [ Links ]
80. Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med 2009;361(22):2113-2122. https://doi.org/10.1056/nejmoa0907569 [ Links ]
81. Navarese EP, Kolodziejczak M, Schulze V, et al Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia. A systematic review and meta-analysis. Ann Intern Med 2015;163(1):40-51. https://doi.org/10.7326/m14-2957 [ Links ]
82. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;362(6):381-386. https://doi.org/10.1056/nejm199202063260605 [ Links ]
83. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: Factorial randomised placebo-controlled trial of asprin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840. https://doi.org/10.1136/bmj.a1840 [ Links ]
84. Fowkes FG, Price JF, Stewart MC, et al. Asprin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: A randomised controlled trial. JAMA 2010;303(9):841-848. https://doi.org/10.1001/jama.2010.221 [ Links ]
85. Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. BMJ 2002;324(7329):71-86. https://doi.org/10.1136/bmj.324.7329.71 [ Links ]
86. Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Asprin for the prevention of cardiovascular events in patients with peripheral arterial disease. JAMA 2009;301(18):1909-1919. https://doi.org/10.1001/jama.2009.623 [ Links ]
87. Caprie Steering Committee. A randomised, blinded, trial of clopidogrel v. asprin in patients at risk of ischemic events (CAPRIE). Lancet 1996;348(9038):1329-1339. https://doi.org/10.1016/s0140-6736(96)09457-3 [ Links ]
88. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel plus asprin v. asprin alone for the prevention of atherothrombosis events (CHARISMA). N Engl J Med 2006;354(16):1706-1717. https://doi.org/10.1056/nejmoa060989 [ Links ]
89. Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor v. clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376(1):32-40. https://doi.org/10.1056/NEJMoa1611688 [ Links ]
90. Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral arterial disease: Results from TRA20 P- TIMI 50. Circulation 2013;127(14):1522-1529. https://doi.org/10.1161/circulationaha.112.000679 [ Links ]
91. Katsanos K, Spiliopoulos S, Saha P, et al. Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: A systemic review and network meta-analysis. PLoS ONE 2015;10(8):e0135692. https://doi.org/10.1371/journal.pone.0135692 [ Links ]
92. Schmit K, Dolor RJ, Jones WS, et al. Comparative effectiveness review of antiplatelet agents in peripheral artery disease. J Am Heart Assoc 2014;3(6):e001330. https://doi.org/10.1161/JAHA.113.001330 [ Links ]
93. Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377(14):1319-1330. https://doi.org/10.1056/NEJMoa1709118 [ Links ]
94. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without asprin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391(10117):219-229. https://doi.org/10.1016/s0140-6736(17)32409-1 [ Links ]
95. Warfarin Antiplatelet Vascular Evaluation Trial Investigators, Anand S, Yusuf S, et al. Oral anticoagulation and antiplatelet therapy and peripheral arterial disease. N Engl J Med 2007;357(3):217-227. https://doi.org/10.1056/nejmoa065959 [ Links ]
96. Lamberts M, Lip GY, Ruwald MH, et al. Antithrombotic treatment in patients with heart failure and associated atrial fibrillation and vascular disease: A nationwide cohort study. J Am Coll Cardiol 2014;63(24):2689-2698. https://doi.org/10.1016/j.jacc.2014.03.039 [ Links ]
97. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39(33):3021-3104. https://doi.org/10.1093/eurheartj/ehy339 [ Links ]
98. Thomopoulos C, Parati G, Zanchetti A. Effects in individuals with high-normal and normal blood pressure: Overview and meta-analyses of randomised trials. J Hypertens 2017;35(11):2150-2160. https://doi.org/10.1097/hjh.0000000000001547 [ Links ]
99. Brunstrom M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: A systematic review and meta-analysis. JAMA Intern Med 2018;178(1):28-36. https://doi.org/10.1001/jamainternmed.2017.6015 [ Links ]
100. Shapiro BP, Ambrosius WT, Blackshear JL, et al. Impact of intensive v. standard blood pressure management by tertiles of blood pressure in the systolic blood pressure intervention trial. Hypertension 2018;71(6):1064-1074. https://doi.org/10.1161/HYPERTENSIONAHA.117.10646 [ Links ]
101. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016;387(10022):957-967. https://doi.org/10.1016/s0140-6736(15)01225-8 [ Links ]
102. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens 2015;33(7):1321-1341. https://doi.org/10.1097/hjh.0000000000000614 [ Links ]
103. Ojji DB, Mayosi B, Francis V, et al. Comparison of dual therapies for lowering blood pressure in black Africans. N Engl J Med 2019;380(25):2429-2439. https://doi.org/10.1056/nejmoa1901113 [ Links ]
104. McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med 1999;159(4):387-392. https://doi.org/10.1001/archinte.159.4.387 [ Links ]
105. Sigvant B, Lundin F, Wahlberg E. The risk of disease progression in peripheral arterial disease is higher than expected: A meta-analysis of mortality and disease progression in peripheral arterial disease. Eur J Vasc Endovasc Surg 2016;51(3):395-403. https://doi.org/10.1016/j.ejvs.2015.10.022 [ Links ]
106. Laing SP, Greenhalgh RM. Standard exercise test to assess peripheral arterial disease. Br Med J 1980;280:13-16. https://doi.org/10.1136%2Fbmj.280.6206.13 [ Links ]
107. Nicolai SP, Viechtbauer W, Kruidenier LM, Candel MJ, Prins MH, Teijink JA. Reliability of treadmill testing in peripheral arterial disease: A meta-regression analysis. J Vasc Surg 2009;50(2):322-329. https://doi.org/10.1016/j.jvs.2009.01.042 [ Links ]
108. Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomised, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol 2002;90(12):1314-1319. https://doi.org/10.1016/s0002-9149(02)02869-2 [ Links ]
109. Salhiyyah K, Forster R, Senanayake E, Abdel-Hadi M, Booth A, Michaels JA. Pentoxifylline for intermittent claudication. Cochrane Database of Syst Rev 2015;9:CD005262. https://doi.org/10.1002/14651858.CD005262.pub3 [ Links ]
110. Robertson L, Andras A. Prostanoids for intermittent claudication. Cochrane Database Syst Rev 2013;4:CD000986. https://doi.org/10.1002/14651858.CD000986.pub3 [ Links ]
111. Pedersen TR, Kjekshus J, Pyörälä K, et al Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). Am J Cardiol 1998;81(3):333-335. https://doi.org/10.1016/s0002-9149(97)00904-1 [ Links ]
112. Mondillo S, Ballo P, Barbati R, et al Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med 2003;114(5):359-364. https://doi.org/10.1016/s0002-9343(03)00010-x [ Links ]
113. De Backer TL, Vander Stichele R, Lehert P, Van Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database Syst Rev 2012;12:CD001368. https://doi.org/10.1002%2F14651858.CD001368.pub4 [ Links ]
114. Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD000990. https://doi.org/10.1002/14651858.CD000990.pub3 [ Links ]
115. European Association of Cardiovascular Prevention and Rehabilitation Committee for Science Guidelines; EACPR, Corrà U, et al Secondary prevention through cardiac rehabilitation: Physical activity counselling and exercise training. Eur Heart J 2010;31(16):1967-1974. https://doi.org/10.1093/eurheartj/ehq236 [ Links ]
116. Fokkenrood HJ, Bendermacher BL, Lauret GJ, Willigendael EM, Prins MH, Teijink JA. Supervised exercise therapy v. non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev 2013;8:CD005263. https://doi.org/10.1002/14651858.CD005263.pub3 [ Links ]
117. Gommans LN, Fokkenrood HJ, van Dalen HC, Scheltinga MR, Teijink JA, Peters RJ. Safety of supervised exercise therapy in patients with intermittent claudication. J Vasc Surg 2015;61(2):512-518. https://doi.org/10.1016/j.jvs.2014.08.070 [ Links ]
118. Bermingham SL, Sparrow K, Mullis R, et al. The cost effectiveness of supervised exercise for the treatment of intermittent claudication. Eur J Vasc Endovasc Surg 2013;46(6):707-714.https://doi.org/10.1016/j.ejvs.2013.09.005 [ Links ]
119. Lauret GJ, Fakhry F, Fokkenrood HJ, Hunink MG, Teijink JA, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database Syst Rev 2014;7:CD09638. https://doi.org/10.1002/14651858.CD009638.pub 2 [ Links ]
120. Galaria II, Davies MG. Percutaneous transluminal revascularisation for iliac occlusive disease: Long-term outcomes in Trans-Atlantic Inter-Society Consensus A and B lesions. Ann Vasc Surg 2005;19(3):352-360. https://doi.org/10.1007/s10016-005-0010-8 [ Links ]
121. Greenhalgh RM, Belch JJ, Brown LC, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: Results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovasc Surg 2008;36(6):680-688. https://doi.org/10.1016/j.ejvs.2008.10.007 [ Links ]
122. Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise v. primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the claudication: Exercise v. endoluminal revascularisation (CLEVER) study. Circulation 2012;125(1):130-139. https://doi.org/10.1161/circulationaha.111.075770 [ Links ]
123. Kim TH, Ko YG, Kim U, et al Outcomes of endovascular treatment of chronic total occlusion of the infrarenal aorta. J Vasc Surg 2011;53(6):1542-1549. https://doi.org/10.1016/j.jvs.2011.02.015 [ Links ]
124. Moise MA, Alvarez-Tostado JA, Clair DG, et al. Endovascular management of chronic infrarenal aortic occlusion. J Endovasc Ther 2009;16(1):84-92. https://doi.org/10.1583/08-2526. [ Links ]!
125. Bosch JL, Hunink MG. Meta-analysis of the results of percutaneous transluminal angioplasty and stent placement for aortoiliac occlusive disease. Radiology 1997;204(1):87-96. https://doi.org/10.1148/radiology.204.1.9205227 [ Links ]
126. Mwipatayi BP, Thomas S, Wong J, et al. A comparison of covered v. bare expandable stents for the treatment of aortoiliac occlusive disease. J Vasc Surg 2011;54(6):1561-1570. https://doi.org/10.1016/j.jvs.2011.06.097 [ Links ]
127. Pereira CE, Albers M, Romiti M, Brochado-Neto FC, Pereira CA. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg 2006;44(3):510-517. https://doi.org/10.1016/j.jvs.2006.04.054 [ Links ]
128. Klinkert P, Schepers A, Burger DH, van Bockel JH, Breslau PJ. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomised controlled trial. J Vasc Surg 2003;37(1):149-155. https://doi.org/10.1067/mva.2002.86 [ Links ]
129. Hunink MG, Wong JB, Donaldson MC, Meyerovitz MF, Harrington DP. Patency results of percutaneous and surgical revascularisation for femoropopliteal arterial disease. Med Decis Making 1994;14(1):71-81. https://doi.org/10.1177/0272989x9401400109 [ Links ]
130. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation v. balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery. Circ Cardiovasc Interv 2010;3(3):267-276. https://doi.org/10.1583/11-3627.1 [ Links ]
131. Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomised and single-arm clinical studies. J Am Coll Cardiol 2013;61(24):2417-2427. https://doi.org/10.1016/j.jacc.2013.03.034 [ Links ]
132. Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results ofthe Zilver PTX randomised trial. Circulation 2016;133(15):1472-1483. https://doi.org/10.1161/circulationaha.115.016900 [ Links ]
133. Tepe G, Laird J, Schneider P, Brodmann M, et al. Drug-coated balloon v. standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomised trial. Circulation 2015 Feb 3;131(5):495-502. https://doi.org/10.1161/circulationaha.114.011004 [ Links ]
134. Rosenfield K, Jaff MR, White CJ, et al Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med 2015;373(2):145-153. https://doi.org/10.1056/nejmoa1406235 [ Links ]
135. Lammer J, Zeller T, Hausegger KA, et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomised VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). J Am Coll Cardiol 2013;62(15):1320-1327. https://doi.org/10.1016/j.jacc.2013.05.079 [ Links ]
136. Indes JE, Pfaff MJ, Farrokhyar F, et al Clinical outcomes of 5 358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: A systematic review and meta-analysis. J Endovasc Ther 2013;20(4):443-455. https://doi.org/10.1583/13-4242.1 [ Links ]
137. Grimme FA, Goverde PC, Verbruggen PJ, Zeebregts CJ, Reijnen MM. Editors choice: First results of the covered endovascular reconstruction of the aortic bifurcation (CERAB) technique for aortoiliac occlusive disease. Eur J Vasc Endovasc Surg 2015;50(5):638-647. https://doi.org/10.1016/j.ejvs.2015.06.112 [ Links ]
138. Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein v. PTFE for above-knee femoropopliteal bypass. A review ofthe literature. Eur J Vasc Endovasc Surg 2004;27(4):357-362. https://dolorg/10.1016/j.ejvs.2003.12.027 [ Links ]
139. Almasri J, Adusumalli J, Asi N, et al. A systematic review and meta-analysis of revascularisation outcomes of infrainguinal chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2018;68(2):624-633. https://doi.org/10.1016/j.jvs.2018.01.066 [ Links ]
140. Mills JL Sr, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: Risk stratification on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59(1):220-234. https://doi.org/10.1016/j.jvs.2013.08.003 [ Links ]
141. Wylie EJ, Kerr E, Davies O. Experimental and clinical experiences with the use of fascia lata applied as a graft about major arteries after thromboendarterectomy and aneurysmorrhaphy. Surg Gynecol Obstet 1951;93(3):257-272. [ Links ]
142. Rutherford RB. Aortobifemoral bypass, the gold standard: Technical considerations. Semin Vasc Surg 1994;7:11-16. [ Links ]
143. Back MR, Johnson BL, Shames ML, Bandyk DF. Evolving complexity of open aortofemoral reconstruction done for occlusive disease in the endovascular era. Ann Vasc Surg 2003;17(6):596-603. https://doi.org/10.1007/s10016-003-0063-5 [ Links ]
144. Bredahl K, Jensen LP, Schroeder TV, Sillesen H, Nielsen H, Eiberg JP. Mortality and complications after aortic procedures for chronic aortoiliac occlusive disease. J Vasc Surg 2015;62(1):75-82. https://doi.org/10.1016/j.jvs.2015.02.025 [ Links ]
145. Reed AB, Conte MS, Donaldson MC, Mannick JA, Whittemore AD, Belkin M. The impact of patient age and aortic size on the results of aortobifemoral bypass grafting. J Vasc Surg 2003;37(6):1219-1225. https://doi.org/10.1016/s0741-5214(02)75179-3 [ Links ]
146. Ricco JB, Probst H, French University Surgeons Association. Longterm results of a multicentre randomised study on direct v. crossover bypass for unilateral iliac artery occlusive disease. J Vasc Surg 2008;47(1):45-53. https://doi.org/10.1016/j.jvs.2007.08.050 [ Links ]
147. Rutherford RB, Patt A, Pearce WH. Extra-anatomic bypass: A closer view. J Vasc Surg 1987;6(5):437-446. [ Links ]
148. Kashyap VS, Pavkov ML, Bena JF, et al The management of severe aortoiliac occlusive disease: Endovascular therapy rivals open reconstruction. J Vasc Surg 2008;48(6):1451-1457. https://doi.org/10.1016/j.jvs.2008.07.004 [ Links ]
149. Chang RW, Godey PP, Baek JH, et al. Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease. J Vasc Surg 2008;48(2):362-367. https://doiorg/10.1016/j.jvs.2008.03.042 [ Links ]
150. Chiu KW, Davies RS, Nightingale PG, Bradbury AW, Adam DJ. Review of direct anatomical open surgical management of atherosclerotic aorto-iliac occlusive disease. Eur J Vasc Endovasc Surg 2010;39(4):460-471. https://doi.org/10.1016/j.ejvs.2009.12.014 [ Links ]
151. Passman MA, Taylor LM, Moneta GL, et al. Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Vasc Surg 1996;23(2):263-269. https://doi.org/10.1016/s0741-5214(96)70270-7 [ Links ]
152. Valentine RJ, Hansen ME, Myers SI, et al The influence of sex and aortic size on late patency after aortofemoral revascularisation in young adults. J Vasc Surg 1995;21(2):296-305. https://doi.org/10.1016/s0741-5214(95)70270-9 [ Links ]
153. Adam DJ, Beard JD, Cleveland T, et al. Bypass v. angioplasty in severe ischemia of the leg (BASIL) trial. Lancet 2005;366(9501):1925-1934. https://doi.org/10.1016/s0140-6736(05)67704-5 [ Links ]
154. Abu Dabrh AM, Steffen MW, Asi N, et al Bypass surgery v. endovascular interventions in severe or critical limb ischemia. J Vasc Surg 2016;63(1):244-253. https://doi.org/10.1016/j.jvs.2015.07.068 [ Links ]
155. Ramanan B, Ahmed A, Wu B, et al. Determinants of midterm functional outcomes, wound healing, and resources used in a hospital-based limb preservation program. J Vasc Surg 2017;66(6):1765-1774. https://doi.org/10.1016/j.jvs.2017.05.102 [ Links ]
156. Simons JP, Goodney PP, Flahive J, et al. A comparative evaluation of risk-adjustment models for benchmarking amputation-free survival after lower extremity bypass. J Vasc Surg 2016;63(4):990-997. https://doi.org/10.1016/j.jvs.2015.09.051 [ Links ]
157. Cull DL, Manos G, Hartley MC, et al. An early validation of the Society for Vascular Surgery lower extremity threatened limb classification system. J Vasc Surg 2014;60(6):1535-1541. https://doi.org/10.1016/j.jvs.2014.08.107 [ Links ]
158. Dorigo W, Pulli R, Castelli P, et al. A multicentre comparison between autologous saphenous vein and heparin-bonded expanded polytetrafluoroethylene (ePTFE) graft in the treatment of critical limb ischemia in diabetics. J Vasc Surg 2011;54(5):1332-1338. https://doi.org/10.1016/j.jvs.2011.05.046 [ Links ]
159. Neville RF, Capone A, Amdur R, Lidsky M, Babrowicz J, Sidawy AN. A comparison of tibial artery bypass performed with heparin-bonded expanded polytetrafluoroethylene and great saphenous vein to treat critical limb ischemia. J Vasc Surg 2012;56(4):1008-1014. https://doi.org/10.1016/j.jvs.2012.03.020 [ Links ]
160. Conte MS. Challenges of distal bypass surgery in patients with diabetes: Patient selection, techniques, and outcomes. J Vasc Surg 2010;52(3):96S-103S. https://doi.org/10.1016/j.jvs.2010.06.015 [ Links ]
161. Monahan TS, Owens CD. Risk factors for lower-extremity vein graft failure. Semin Vasc Surg 2009;22(4):216-226. https://doi.org/10.1053/j.semvascsurg.2009.10.003 [ Links ]
162. Conte MS, Belkin M, Upchurch GR, Mannick JA, Whittemore AD, Donaldson MC. Impact of increasing comorbidity on infrainguinal reconstruction: A 20-year perspective. Ann Surg 2001;233(3):445-452. https://doi.org/10.1097%2F00000658-200103000-00021 [ Links ]
163. Pomposelli FB, Kansal N, Hamdan AD, et al. A decade of experience with dorsalis pedis artery bypass: Analysis of outcome in more than 1 000 cases. J Vasc Surg 2003;37(2):307-315. https://doi.org/10.1067/mva.2003.125 [ Links ]
164. Neville RF, Attinger CE, Bulan EJ, Ducic I, Thomassen M, Sidawy AN. Revascularisation of a specific angiosome for limb salvage: Does the target artery matter? Ann Vasc Surg 2009;23(3):367-373. https://doi.org/10.1016/j.avsg.2008.08.022 [ Links ]
165. Conte MS, Pomposelli FB, Clair DG, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication. J Vasc Surg 2015;61(3):2S-41S. https://doi.org/10.1016/j.jvs.2014.12.009 [ Links ]
166. Ye W, Liu CW, Ricco JB, Mani K, Zeng R, Jiang J. Early and late outcomes of percutaneous treatment of Trans-Atlantic Inter-Society Consensus Class C and D aorto-iliac lesions. J Vasc Surg 2011;53(6):1728-1737. https://doi.org/10.1016/j.jvs.2011.02.005 [ Links ]
167. Sharafuddin MJ, Hoballah JJ, Kresowik TF, Sharp WJ. Kissing stent reconstruction of the aorto-iliac bifurcation. Perspect Vasc Surg Endovasc Ther 2008;20(1):50-60. https://doi.org/10.1177/1531003507313224 [ Links ]
168. Dorigo W, Piffaretti G, Benedetto F, et al A comparison between aorto-bifemoral bypass and aorto-iliac kissing stents in patients with complex aorto-iliac obstructive disease. J Vasc Surg 2016;65(1):99-107. https://doi.org/10.1016/j.jvs.2016.06.107 [ Links ]
169. Taeymans K, Groot Jebbink E, Holewijn S, et al Three-year outcome of the covered endovascular reconstruction of the aortic bifurcation technique for aortoiliac occlusive disease. J Vasc Surg 2018;67(5):1438-1447. https://doi.org/10.1016/j.jvs.2017.09.015 [ Links ]
170. Soga Y, Iida O, Kawasaki D, et al. Contemporary outcomes after endovascular treatment for aorto-iliac artery disease. Circ J 2012;76(11):2697-2704. https://doi.org/10.1253/circj.cj-12-0492 [ Links ]
171. Antoniou GA, Sfyroeras GS, Karathanos C, et al. Hybrid endovascular and open treatment of severe multi-level lower extremity arterial disease. Eur J Vasc Endovasc Surg 2009;38(5):616-622. https://doi.org/10.1016/j.ejvs.2009.06.016 [ Links ]
172. Dosluoglu HH, Lall P, Cherr GS, Harris LM, Dryjski ML. Role of simple and complex hybrid revascularisation procedures for symptomatic lower extremity occlusive disease. J Vasc Surg 2010;51(6):1425-1435. https://doi.org/10.1016/j.jvs.2010.01.092 [ Links ]
173. Krankenberg H, Zeller T, Ingwersen M, et al. Self-expanding v. balloon expandable stents for iliac artery occlusive disease: The randomised ICE trial. J Ann Coll Cardiac Interv 2017;10(16):1694-1704. https://doi.org/10.1016/j.jcin.2017.05.015 [ Links ]
174. Mwipatayi BP, Sharma S, Daneshmand A, et al. Durability of the balloon-expandable covered v. bare-metal stents in the Covered v. Balloon Expandable stent Trial (COBEST) for the treatment of aortoiliac occlusive disease. J Vasc Surg 2016;64(1):83-94. https://doi.org/10.1016/j.jvs.2016.02.064 [ Links ]
175. Teraa M, Conte MS, Moll FL, Verhaar MC. Critical limb ischaemia: Current trends and future directions. J Am Heart Assoc 2016;5(2):e002938. https://doi.org/10.1161/JAHA.115.002938 [ Links ]
176. Katsanos K, Tepe G, Tsetis D, Fanelli F. Standards of practice for superficial femoral and popliteal artery angioplasty and stenting. Cardiovasc Intervent Radiol 2014;37(3):592-603. https://doi.org/10.1007/s00270-014-0876-3 [ Links ]
177. Nguyen BN, Conrad MF, Guest JM, et al. Late outcomes of balloon angioplasty and angioplasty with selective stenting for superficial femoral-popliteal disease are equivalent. J Vasc Surg 2011;54(4): 1051-1057. https://doi.org/10.1016/j.jvs.2011.03.283 [ Links ]
178. Jens, S, Conijn, AP, Koelemay MJW, Bipat S, Reekers JA. Randomised trials for endovascular treatment of infrainguinal arterial disease: Systematic review and meta-analysis (Part 1: Above the Knee). J Vasc Endovasc Surg 2014;47(5):524-535. https://doi.org/10.1016/j.ejvs.2014.02.011 [ Links ]
179. Anantha-Narayanan M, Shah SM, Jelani Q, et al. Drug-coated balloon v. plain old balloon angioplasty in femoropopliteal disease: An updated meta-analysis of randomised controlled trials. Catheter Cardiovasc Interv 2019;94(1):139-148. https://doi.org/10.1002/ccd.28176 [ Links ]
180. Meecham L, Bate G, Smitaa Patel S, Bradbury AW. A Comparison of clinical outcomes following femoropopliteal bypass or plain balloon angioplasty with selective bare metal stenting in the bypass v. angioplasty in severe ischaemia of the limb (BASIL) trial. Eur J Vasc Endovasc Surg 2019;58(1):52-59. https://doi.org/10.1016/j.ejvs.2019.01.006 [ Links ]
181. Poncyljusz W, Falkowski A, Safranow K, Rae M, Zawierucha D. Cutting-balloon angioplasty v. balloon angioplasty as treatment for short atherosclerotic lesions in the superficial femoral artery: Randomised controlled trial. Cardiovasc Intervent Radiol 2013;36(6):1500-1507. https://doi.org/10.1007/s00270-013-0603-5 [ Links ]
182. Canaud L, Alric P, Berthet JP, Marty-Ané C, Mercier G, Branchereau P. Infrainguinal cutting balloon angioplasty in de novo arterial lesions. J Vasc Surg 2008;48(5):1182-1188. https://doi.org/10.1016/j.jvs.2008.06.053 [ Links ]
183. Dick P, Sabeti S, Mlekusch W, et al. Conventional balloon angioplasty v. peripheral cutting balloon angioplasty for treatment of femoropopliteal artery in-stent restenosis: Initial experience. Radiology 2008;248(1):297-302. https://doi.org/10.1148/radiol.2481071159 [ Links ]
184. Lugenbiel I, Grebner M, Zhou Q, et al. Treatment of femoropopliteal lesions with the AngioSculpt scoring balloon - results from the Heidelberg PANTHER registry. Vasa 2018;47(1):49-55. https://doi.org/10.1024/0301-1526/a000671 [ Links ]
185. Mustapha JA, Lansky A, Shishehbor M, et al. A prospective, multicentre study of the chocolate balloon in femoropopliteal peripheral arterial disease: The Chocolate BAR registry. Catheter Cardiovasc Interv 2018;91(6):1144-1148. https://doi.org/10.1002/ccd.27565 [ Links ]
186. Klein AJ, Chen SJ, Messenger JC, et al. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter Cardiovasc Interv 2009;74(5):787-798. https://doi.org/10.1002/ccd.22124 [ Links ]
187. Duda SH, Pusich B, Richter G, et al. Sirolimus-eluting stents for the treatment of obstructive superficial femoral artery disease: Six-month results. Circulation 2002;106(12):1505-1509. https://doi.org/10.1161/01.cir.0000029746.10018.36 [ Links ]
188. Duda SH, Bosiers M, Lammer J, et al., Sirolimus-eluting v. bare nitinol stent for obstructive superficial femoral artery disease; the SIROCCO II trial. J Vasc Interv Radiol 2005;16(3):331-338. https://doi.org/10.1097/01.rvi.0000151260.74519.ca [ Links ]
189. Speck U, Cremers B, Kelsch B, et al. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ Cardiovasc Interv 2012;5(3):392-400. https://doi.org/10.1161/circinterventions.111.967794 [ Links ]
190. Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358(7):689-699. https://doi.org/10.1056/nejmoa0706356 [ Links ]
191. Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries: Paclitaxel-coated v. uncoated balloon: Femoral paclitaxel randomised pilot trial. Circulation 2008;118(13):1358-1365. https://doi.org/10.1161/circulationaha.107.735985 [ Links ]
192. Scheinert D, Duda S, Zeller T, et al. The LEVANT I trial for femoropopliteal revascularisation: Firstin-human randomised trial of low-dose drug-coated balloon v. uncoated balloon angioplasty. JACC Cardiovasc Interv 2014;7(1):10-19. https://doi.org/10.1016/j.jcin.2013.05.022 [ Links ]
193. Werk M, Albrecht T, Meyer DR, et al. Paclitaxel coated balloons reduce restenosis after femoropopliteal angioplasty: Evidence from the randomised PACIFIER trial. Circ Cardiovasc Interv 2012;5(6):831-840. https://doi.org/10.1161/circinterventions.112.971630 [ Links ]
194. Tepe G, Laird J, Schneider P, et al. Drug-coated balloon v. standard percutaneous transluminal angioplasty for the treatment of superficial femoral and/or popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomised trial. Circulation 2015;131(5):495-502. https://doi.org/10.1161/circulationaha.114.011004 [ Links ]
195. Krankenberg H, Tübler T, Ingwersen M, et al. Drug-coated balloon v. standard balloon for superficial femoral artery in-stent restenosis: The randomised femoral artery in-stent restenosis (FAIR) trial. Circulation 2015;132(23):2230-2236. https://doi.org/10.1161/circulationaha.115.017364 [ Links ]
196. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel coated balloons and stents in the femoropopliteal artery of the leg: A systematic review and meta-analysis of randomised controlled trials. J Am Heart Assoc 2018;7(24):e011245. https://doi.org/10.1161/JAHA.118.011245 [ Links ]
197. Ramaiah V, Gammon R, Kiesz S, et al. Mid-term outcomes from the Talon registry: Treating peripherals with SilverHawk: Outcomes collection. J Endovasc Ther 2006;13(5):592-602. https://doi.org/10.1583/05-1780mr.1 [ Links ]
198. McKinsey JF, Zeller T, Rocha-Singh KJ, Jaff MR, Garcia LA, DEFINITIVE LE Investigators. Lower extremity revascularisation using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv 2014;7(8):923-933. https://doi.org/10.1016/j.jcin.2014.05.006 [ Links ]
199. Zeller T, Rastan A, Sixt S, et al. Long-term results after directional atherectomy of femoral-popliteal lesions. J Am Coll Cardiol 2006;48(8):1573-1578. https://doi.org/10.1016/j.jacc.2006.07.031 [ Links ]
200. Shammas NW, Coiner D, Shammas GA, Dippel EJ, Christensen L, Jerin M. Percutaneous lower extremity arterial interventions with primary balloon angioplasty v. Silverhawk atherectomy and adjunctive balloon angioplasty: Randomised trial. J Vasc Interv Radiol 2011;22(9):1223-1228. https://doi.org/10.1016/j.jvir.2011.05.013 [ Links ]
201. Zeller T, Krankenberg H, Steinkamp H, et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: The multicentre pathway PVD trial. J Endovasc Ther 2009;16(6):653-662. https://doi.org/10.1583/09-2826.1 [ Links ]
202. Mehta M, Zhou Y, Patty PS, et al. Percutaneous common femoral artery interventions using angioplasty, atherectomy, and stenting. J Vasc Surg 2016;64(2):369-379. https://doi.org/10.1016/j.jvs.2016.03.418 [ Links ]
203. Davis T, Ramaiah V, Niazi K, Martin Gissler H, Crabtree T. Safety, and effectiveness of the Phoenix atherectomy system in lower extremity arteries: Early and mid-term outcomes from the prospective multicentre EASE study. Vascular 2017;25(6):563-575. https://doi.org/10.1177/1708538117712383 [ Links ]
204. Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° Trial: A randomised, prospective, multicentre, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol 2014;26(8):355-360. [ Links ]
205. Dippel EJ, Makam P, Kovach R, et al. Randomised controlled study of excimer laser atherectomy for treatment of femoropopliteal in-stent restenosis: Initial results from the EXCITE ISR trial (EXCImer Laser Randomised Controlled Study for Treatment of FemoropopliTEal In-Stent Restenosis). JACC Cardiovasc Interv 2015;8(1):92-101. https://doi.org/10.1016/j.jcin.2014.09.009 [ Links ]
206. Diamantopoulos A, Katsanos K. Atherectomy of the femoral-popliteal artery: A systematic review and meta-analysis of randomised controlled trials. J Cardiovasc Surg (Torino) 2014;55(5):655-665. [ Links ]
207. Ramkumar N, Martinez-Camblor P, Columbo JA, Osborne NH, Goodney PP, O'Malley AJ. Adverse events after atherectomy: Analysing long-term outcomes of endovascular lower extremity revascularisation techniques. J Am Heart Assoc 2019;8(12):e012081. https://doi.org/10.1161/JAHA.119.012081 [ Links ]
208. Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: Twelve-month results of the DEFINITIVE AR study. Circ Cardiovasc Interv 2017;10(9):e004848. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004848 [ Links ]
209. Bhatt H, Janzer S, George JC. Crossing techniques and devices in femoropopliteal chronic total occlusion intervention. Cardiovasc Revasc Med 2017;18(8):623-631. https://doi.org/10.1016/j.carrev.2017.06.002 [ Links ]
210. Schneider PA, Caps MT, Nelken N. Re-entry into the true lumen from the subintimal space. J Vasc Surg 2013;58(2):529-534. https://doi.org/10.1016/j.jvs.2013.03.002 [ Links ]
211. Liang GZ, Zhang FX. Novel devices and specialised techniques in recanalisation of peripheral artery chronic total occlusions (CTOs) - a literature review. Int J Cardiol 2013;165(3):423-429. https://doi.org/10.1016/j.ijcard.2012.03.033 [ Links ]
212. Taleb M, Burket MW, Cooper CJ. A role for embolic protection in the management of acute limb ischemia. Tech Vasc Interv Radiol 2011;14(2):75-79. https://doi.org/10.1053/j.tvir.2011.01.004 [ Links ]
213. Lam RC, Shah S, Faries PL, McKinsey JF, Kent KC, Morrissey NJ. Incidence and clinical significance of distal embolisation during percutaneous interventions involving the superficial femoral artery J Vasc Surg 2007;46(6):1155-1159. https://doi.org/10.1016/j.jvs.2007.07.058 [ Links ]
214. Shammas NW, Dippel EJ, Coiner D, Shammas GA, Jerin M, Kumar A. Preventing lower extremity distal embolisation using embolic filter protection: Results of the PROTECT registry. J Endovasc Ther 2008;15(3):270-276. https://doi.org/10.1583/08-2397.1 [ Links ]
215. Karnabatidis D, Katsanos K, Kagadis GC, et al. Distal embolism during percutaneous revascularisation of infra-aortic arterial occlusive disease: An underestimated phenomenon. J Endovasc Ther 2006;13(3):269-280. https://doi.org/10.1583/05-1771.1 [ Links ]
216. Mendes BC, Oderich GS, Fleming MD, et al. Clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without embolic protection devices. J Vasc Surg 2014;59(2):359-367. https://doi.org/10.1016%2Fj.jvs.2013.07.119 [ Links ]
217. Lookstein RA, Lewis S. Distal embolic protection for infrainguinal interventions: How to and when? Tech Vasc Interv Radiol 2010;13(1):54-58. https://doi.org/10.1053/j.tvir.2009.10.007 [ Links ]
218. Bornak A, Milner R. Current debate on the role of embolic protection devices. Vasc Endovascular Surg 2012;46(6):441-446. https://doi.org/10.1177/1538574412452160 [ Links ]
219. Ward TJ, Piechowiak RL, Patel RS, et al. Revascularisation for critical limb ischemia using the SpiderFX embolic protection device in the below-the-knee circulation: Initial results. J Vasc Interv Radiol 2014;25(10):1533-1538. https://doi.org/10.1016/j.jvir.2014.07.016 [ Links ]
220. Müller-Hülsbeck S1, Hümme TH, Philipp Schäfer J, et al. Final results of the protected superficial femoral artery trial using the FilterWire EZ system. Cardiovasc Intervent Radiol 2010;33(6):1120-1127. https://doi.org/10.1007/s00270-010-9936-5 [ Links ]
221. Bates MC, Campbell JE. Pitfalls of embolic protection. Tech Vasc Interv Radiol 2011;14(2):101-107. https://doi.org/10.1053/j.tvir.2011.01.008 [ Links ]
222. Allie DE. To PROTECT or not to PROTECT? In lower extremity angioplasty procedures, "Why Not?' is the question! J Endovasc Ther 2008;15(3):277-282. https://doi.org/10.1583/08-2397c1 [ Links ]
223. Wholey M. The role of embolic protection in peripheral arterial atherectomy. Tech Vasc Interv Radiol 2011;14(2):65-74. https://doi.org/10.1053/j.tvir.2011.01.003 [ Links ]
224. Hitchner E, Zayed M, Varu V, Lee G, Aalami O, Zhou W. A prospective evaluation of using IVUS during percutaneous superficial femoral artery interventions. Ann Vasc Surg 2015;29(1):28-33. https://doi.org/10.1016/j.avsg.2014.07.026 [ Links ]
225. Iida O, Takahara M, Soga Y, et al Efficacy of intravascular ultrasound in femoropopliteal stenting for peripheral artery disease with TASC II class A to C lesions. J Endovasc Ther 2014;21(4):485-492. https://doi.org/10.1583/14-4721r.1 [ Links ]
226. Scheinert D, Scheinert S, Sax J, et al Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol 2005;45(2):312-315. https://doi.org/10.1016/j.jacc.2004.11.026 [ Links ]
227. Chowdhury MM, McLain AD, Twine CP. Angioplasty v. bare metal stenting for superficial femoral artery lesions. Cochrane Database Syst Rev 2014;24(6):CD006767. https://doi.org/10.1002/14651858.CD006767.pub3 [ Links ]
228. Werner M, Piorkowski M, Thieme M, et al SUMMIT registry: One-year outcomes after implantation of the EPIC self-expanding nitinol stent in the femoropopliteal segment. J Endovasc Ther 2013;20(6):759-766. https://doi.org/10.1583/13-4430r.1 [ Links ]
229. Laird JR, Jain A, Zeller T, et al Nitinol stent implantation in the superficial femoral artery and proximal popliteal artery: Twelve-month results from the complete SE multicentre trial. J Endovasc Therapy 2014;21(2):202-212. https://doi.org/10.1583/13-4548r.1 [ Links ]
230. Mwipatayi BP, Perera K, Daneshmand A, et al. First-in-man experience of self-expanding nitinol stents combined with drug-coated balloon in treatment of femoropopliteal occlusive disease. Vascular 2018;6(1):3-11. https://doi.org/10.1177/1708538117705805 [ Links ]
231. Garcia LA, Rosenfield KR, Metzger CD, et al. SUPERB final 3-year outcomes using interwoven nitinol biomimetic supera stent. Catheter Cardiovasc Interv 2017;89(7):1259-1267. https://doi.org/10.1002/ccd.27058 [ Links ]
232. Sibé M, Kaladji A, Boirat C, et al. French multicentre experience with the GORE TIGRIS vascular stent in superficial femoral and popliteal arteries. J Vasc Surg 2017;65(5):1329-1335. https://doi.org/10.1016/j.jvs.2016.11.056 [ Links ]
233. Morice MC, Serruys PW, Sousa JE, et al. A randomised comparison of a sirolimus-eluting stent with a standard stent for coronary revascularisation. N Engl J Med 2002;346(23):1773-1780. https://doi.org/10.1056/nejmoa012843 [ Links ]
234. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents v. standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349(14):1315-1323. https://doi.org/10.1056/nejmoa035071 [ Links ]
235. Duda SH, Bosiers M, Lammer J, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: Long-term results from the SIROCCO trial. J Endovasc Ther 2006;13(6):701-710. https://doi.org/10.1583/05-1704.1 [ Links ]
236. Lammer J, Bosiers M, Zeller T, et al. First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease. J Vasc Surg 2011;54(2):394-401. https://doi.org/10.1016/j.jvs.2011.01.047 [ Links ]
237. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty v. implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354(18):1879-1888. https://doi.org/10.1056/nejmoa051303 [ Links ]
238. Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: The TAXUS-IV trial. Circulation 2004;109(16):1942-1947. https://doi.org/10.1161/01.cir.0000127110.49192.72 [ Links ]
239. Van der Giessen WJ, Lincoff AM, Schwartz RS, et al Marked inflammatory sequelae to implantation of biodegradable and non-biodegradable polymers in porcine coronary arteries. Circulation 1996;94(7):1690-1697. https://doi.org/10.1161/01.cir.94.7.1690 [ Links ]
240. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: Twelve-month Zilver PTX randomised study results. Circ Cardiovasc Interv 2011;4(5):495-504. https://doi.org/10.1161/circinterventions.111.962324 [ Links ]
241. Fischer M, Schwabe C, Schulte KL. Value of the hemobahn/viabahn endo-prosthesis in the treatment of long chronic lesions of the superficial femoral artery: 6 years of experience. J Endovasc Ther 2006;13(3):281-290. https://doi.org/10.1583/05-1799. [ Links ]!
242. Saxon RR, Chervu A, Jones PA, et al. Heparin-bonded expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol 2013;24(2):165-173. https://doi.org/10.1016/j. jvir.2012.10.004 [ Links ]
243. Ohki T, Kichikawa K, Yokoi H, et al. Outcomes of the Japanese multicentre Viabahn trial of endovascular stent grafting for superficial femoral artery lesions. J Vasc Surg 2017;66(1):130-142. https://doi.org/10.1016/j.jvs.2017.01.065 [ Links ]
244. Lammer J, Zeller T, Hausegger KA, et al. Sustained benefit at 2-years for covered stents v. bare-metal stents in Llng SFA lesions: The VIASTAR trial. Cardiovasc Intervent Radiol 2015;38:25-32. https://doi.org/10.1007/s00270-014-1024-9 [ Links ]
245. Bosiers M, Deloose K, Callaert J, et al Superiority of stent-grafts for in-stent restenosis in the superficial femoral artery: Twelve-month results from a multicentre randomised trial. J Endovasc Ther 2015;22(1):1-10. https://doi.org/10.1177/1526602814564385 [ Links ]
246. Reijnen MMPJ, van Walraven LA, Fritschy WM, et al. 1-Year results of a multicentre randomised controlled trial comparing heparin-bonded endoluminal to femoropopliteal bypass. JACC: Cardiovascular Interventions 2017;10(22):2320-2331. https://doi.org/10.1016/j.jcin.2017.09.013 [ Links ]
247. Khalili H, Jeon-Slaughter H, Armstrong EJ, et al. Atherectomy in below-the-knee endovascular interventions: One-year outcomes from the XLPAD registry. Catheter Cardiovasc Interv 2019;93(3):488-493. https://doi.org/10.1002/ccd.27897 [ Links ]
248. Prasad A, Saab F. Endovascular treatment of below-the-knee chronic total occlusions. In: Banerjee S, ed. Practical approach to peripheral arterial chronic total Ooclusion. Singapore: Springer, 2017:45-74. [ Links ]
249. Mustapha JA, Finton SM, Diaz-Sandoval LJ, Saab FA, Miller LE. Percutaneous transluminal angioplasty in patients with infrapopliteal arterial disease - systematic review and meta-analysis. Circ Cardiovasc Interv 2016;9(5):e003468. https://doi.org/10.1161/circinterventions.115.003468. [ Links ]
250. Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg 2008;47(5):975-981. https://doi.org/10.1016/j.jvs.2008.01.005 [ Links ]
251. Das TS, McNamara T, Gray B, et al Primary cryoplasty therapy provides durable support for limb salvage in critical limb ischemia patients with infrapopliteal lesions: 12-month follow-up results from the BTK Chill trial. J Endovasc Ther 2009;16(2):19-30. https://doi.org/10.1583/08-2652.1 [ Links ]
252. Haghighat L, Altin SE, Attaran RR, Mena-Hurtado C, Regan CJ. Review of the latest percutaneous devices in critical limb ischemia. J Clin Med 2018;7(4):82. https://doi.org/10.3390/jcm7040082 [ Links ]
253. Rundback JH, Herman KC. Optimal use of atherectomy in critical limb ischemia. Tech Vasc Interventional Rad 2014;17(3):211-218. https://doi.org/10.1053/j.tvir.2014.08.010 [ Links ]
254. Abdullah O, Omran J, Enezate T, et al. Percutaneous angioplasty v. atherectomy for treatment of symptomatic infra-popliteal arterial disease. Cardiovasc Revasc Med 2018;19(4):423-428. https://doi.org/10.1016/j.carrev.2017.09.014 [ Links ]
255. Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev 2014;17(3):CD006680. https://doi.org/10.1002/14651858.CD006680.pub2 [ Links ]
256. Safian RD, Niazi K, Runyon JP, et al. Orbital atherectomy for infrapopliteal disease: Device concept and outcome data for the OASIS trial. Catheter Cardiovasc Interv 2009;73(3):406-412. https://doi.org/10.1002/ccd.21898 [ Links ]
257. Brodmann M, Holden A, Zeller T. Safety and feasibility of intravascular lithotripsy for treatment of below-the-knee arterial stenoses. J Endovasc Ther 2018;25(4):499-503. https://doi.org/10.1177/1526602818783989 [ Links ]
258. Blevins WA, Schneider PA. Endovascular management of critical limb ischemia. Eur J Vasc Endovasc Surg 2010;39(6):756-761. https://doi.org/10.1016/j.ejvs.2010.02.008 [ Links ]
259. Fanelli F, Cannavale A, Corona M, Lucatelli P, Wlderk A, Salvatori FM. The DEBELLUM' lower limb multilevel treatment with drug eluting balloon randomised trial: 1-year results. J Cardiovasc Surg (Torino) 2014;55(2):207-216. [ Links ]
260. Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below thekKnee angioplasty evaluation (DEBATE-BTK): A randomised trial in diabetic patients with critical limb ischemia. Circulation 2013;128(6):615-621. https://doi.org/10.1161/circulationaha.113.001811 [ Links ]
261. Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon v. standard balloon angioplasty for infrapopliteal arterial revascularisation in critical limb ischemia: 12-month results from the IN.PACT DEEP randomised trial. J Am Coll Cardiol 2014;64(15):1568-1576. https://doi.org/10.1016/j.jacc.2014.06.1198 [ Links ]
262. Thieme M, Lichtenberg M, Brodmann M, Cioppa A, Scheinert D. Lutonix 014 DCB global below the knee registry study: Interim 6-month outcomes. J Cardiovasc Surg (Torino) 2018;59(2):232-236. https://doi.org/10.23736/s0021-9509.18.10269-2 [ Links ]
263. Zeller T, Micari A, Scheinert D, et al. The IN.PACT DEEP clinical drug-coated balloon trial: 5-year outcomes. JACC Cardiovasc Interv 2020;13(4):431-443. https://doi.org/10.1016/j.jcin.2019.10.059 [ Links ]
264. Randon C, Jacobs B, De Ryck F, Vermassen F. Angioplasty or primary stenting for infrapopliteal lesions: Results of a prospective randomised trial. Cardiovasc Intervent Radiol 2010;33(2):260-269. https://doi.org/10.1007/s00270-009-9765-6 [ Links ]
265. Brodmann M, Froehlich H, Dorr A, et al. Percutaneous transluminal angioplasty v. primary stenting in infrapopliteal arteries in critical limb ischemia. Vasa 2011;40(6):482-490. https://doi.org/10.1024/0301-1526/a000152 [ Links ]
266. Bosiers M, Scheinert D, Peeters P, et al. Randomised comparison of everolimus-eluting v. bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg 2012;55(2):390-398. https://doi.org/10.1016/j.jvs.2011.07.099(DESTINY Trial) [ Links ]
267. Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents v. bare-metal stents for treatment of focal lesions in infra-popliteal arteries: A double-blind, multicentre, randomised clinical trial. Eur Heart J 2011;32(18):2274-2281. https://doi.org/10.1093/eurheartj/ehr144(YUKON-BTX Trial) [ Links ]
268. Scheinert D1, Katsanos K, Zeller T, et al. A prospective randomised multicentre comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol 2012;60(22):2290-2295. https://doi.org/10.1016/j.jacc.2012.08.989 [ Links ]
269. Spreen MI, Martens JM, Knippenberg B, et al. Long-term follow-up of the PADI trial: Percutaneous transluminal angioplasty v. drug-eluting stents for infrapopliteal lesions in critical limb ischemia. J Am Heart Assoc 2017;6(4):e004877. https://doi.org/10.1161/JAHA.116.004877 [ Links ]
270. Zhou Y, Lin S, Zhang Z, et al. A network meta-analysis of randomised controlled trials comparing treatment modalities for infrapopliteal lesions in critical limb ischemia. Ann Vasc Surg 2019;60:424-434. https://doi.org/10.1016/j.avsg.2019.02.021 [ Links ]
271. Hsu CCT, Kwan GNC, Singh D, Rophael JA, Anthony C, van Driel ML. Angioplasty v. stenting for infrapopliteal arterial lesions in chronic limb-threatening ischaemia. Cochrane Database Syst Rev 2018;1212:CD009195. https://doi.org/10.1002/14651858.CD009195.pub2 [ Links ]
272. Liu X, Zheng G, Wen S. Drug-eluting stents v. control therapy in the infrapopliteal disease: A meta-analysis of eight randomised controlled trials and two cohort studies. Int J Surg 2017;44:166-175. https://doi.org/10.1016/j.ijsu.2017.06.075 [ Links ]
273. Zhang J, Xu X, Kong J, et al. Systematic review and meta-analysis of drug-eluting balloon and stent for infrapopliteal artery revascularisation. Vasc Endovascular Surg 2017;51(2):72-83. https://doi.org/10.1177/1538574416689426 [ Links ]
274. Varcoe RL, Paravastu SC, Thomas SD, Bennett MH. The use of drug-eluting stents in infrapopliteal arteries: An updated systematic review and meta-analysis of randomised trials. Int Angiol 2019;38(2):121-135. https://doi.org/10.23736/s0392-9590.19.04049-5 [ Links ]
275. Singh P, Harper Y, Oliphant CS, et al. Peripheral interventions and antiplatelet therapy : Role in current practice. World J Cardiol 2017;9(7):583-593. https://doi.org/10.4330%2Fwjc.v9.i7.583 [ Links ]
276. Yang JK, Jimenez JC, Jabori S. Antiplatelet therapy before, during, and after extremity revascularisation. J Vasc Surg 2014;60(4):1085-1091. https://doi.org/10.1016/j.jvs.2014.07.006 [ Links ]
277. Robertson L, Ghouri M, Kovacs F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev 2012;8:CD002071. https://doi.org/10.1002/14651858.CD002071.pub3 [ Links ]
278. Cassar K, Ford I, Greaves M, Bachoo P, Brittenden J. Randomised clinical trial of the antiplatelet effects of aspirin-clopidogrel combination v. aspirin alone after lower limb angioplasty. Br J Surg 2005;92(2):159-165. https://doi.org/10.1002/bjs.4810 [ Links ]
279. Tepe G, Bantleon R, Brechtel K, et al. Management of peripheral arterial interventions with mono or dual antiplatelet therapy - the MIRROR study: A randomised and double-blinded clinical trial. Eur Radiol 2012;22(9):1998-2006. https://doi.org/10.1007/s00330-012-2441-2 [ Links ]
280. Strobl FF, Brechtel K, Schmehl J, et al. Twelve-month results of a randomised trial comparing mono with dual antiplatelet therapy in endovascularly treated patients with peripheral artery disease. J Endovasc Ther 2013;20(5):699-706. https://doi.org/10.1583/13-4275mr.1 [ Links ]
281. Thott O, Granath F, Malmstedt J, Wahlgren CM. Dual antiplatelet therapy improves outcome in diabetic patients undergoing endovascular femoropopliteal stenting for critical limb ischaemia. Eur J Vasc Endovasc Surg 2017;53(3):403-410. https://doi.org/10.1016/j.ejvs.2016.12.014 [ Links ]
282. Arya S, Zettervall SL, Ultee KHJ, Schermerhorn ML. Dual antiplatelet therapy is associated with prolonged survival after lower extremity revascularisation. Eur J Vasc Endovasc Surg 2017;53(3):403-410. [ Links ]
283. Weem SMOP, van Haelst STW, den Ruijter HM, Moll FL, de Borst GJ. Lack of evidence for dual antiplatelet therapy after endovascular arterial procedures: A meta-analysis. Eur J Vasc Endovasc Surg 2016;52(2):253-262. https://doi.org/10.1016/j.ejvs.2016.04.023 [ Links ]
284. Spiliopoulos S, Fragkos G, Katsanos K, Diamantopoulos A, Karnabatidis D, Siablis D. Long-term outcomes following primary drug-eluting stenting of infrapopliteal bifurcations. J Endovasc Ther 2012;19(6):788-796. https://doi.org/10.1583/jevt-12-3993r.1 [ Links ]
285. Bedenis R, Lethaby A, Maxwell H, Acosta S, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst Rev 2015;2:CD000535. https://doi.org/10.1002/14651858.CD000535.pub3 [ Links ]
286. Dutch Bypass Oral Anticoagulants or Aspirin (BOA) Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin study): A randomised trial. Lancet 2000;355(9201):346-351. [ Links ]
287. Sarac TP, Huber TS, Back MR, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg 1998;28(3):446-457. https://doi.org/10.1016/s0741-5214(98)70130-2 [ Links ]
288. Brumberg RS, Back MR, Armstrong PA, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg 2007;46(6):1160-1166. https://doi.org/10.1016/j.jvs.2007.07.046 [ Links ]
289. Belch JJ, Dormandy J, CASPAR Writing Committee, et al. Results of the randomised, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg 2010;52(4):825-833. https://doi.org/10.1016/j.jvs.2010.04.027 [ Links ]
290. Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularisation. N Engl J Med 2020;382(21):1994-2004. https://doi.org/10.1056/nejmoa2000052 [ Links ]
291. Iftikhar O, Oliveros K, Tafur AJ, Casanegra AI. Prevention of femoropopliteal in-stent restenosis with cilostazol: A meta-analysis. Angiology 2016;67(6):549-555. https://doi.org/10.1177/0003319715604768 [ Links ]
292. Soga Y, Takahara M, Iida O, et al. Efficacy of cilostAzol for below-the-knee artery disease after balloon angioplasty in patients with severe limb ischemia (CABBAGE trial). Ann Vasc Surg 2017;45:22-28. https://doi.org/10.1016/j.avsg.2017.05.029 [ Links ]
293. Zierler RE, Jordan WD, Lal BK, et al The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J Vasc Surg 2018;68(1):256-284. https://doi.org/10.1016/j.jvs.2018.04.018 [ Links ]
294. Davies AH, Hawdon AJ, Sydes MR, Thompson SG; VGST participants. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the Vein Graft Surveillance randomised Trial (VGST). Circulation 2005;112(13):1985-1991. https://doi.org/10.1161/circulationaha.104.518738 [ Links ]
295. Bui TD, Mills JL Sr, Ihnat DM, Gruessner AC, Goshima KR, Hughes JD. The natural history of duplex-detected stenosis after femoropopliteal endovascular therapy suggests questionable clinical utility of routine duplex surveillance. J Vasc Surg 2012;55(2):346-352. https://doi.org/10.1016/j.jvs.2011.08.010 [ Links ]
296. Abu Dabrh AM, Mohammed K, Farah W, et al. Systematic review and meta-analysis of duplex ultrasound surveillance for infrainguinal vein bypass grafts. J Vasc Surg 2017;66(6):1885-1891. https://doi.org/10.1016/j.jvs.2017.06.113 [ Links ]
297. Ihlberg L, Luther M, Tierala E, Lepäntalo M. The utility of duplex scanning in infrainguinal vein graft surveillance: Results from a randomised controlled study. Eur J Vasc Endovasc Surg 1998;16(1):19-27. https://doi.org/10.1016/s1078-5884(98)80087-7 [ Links ]
298. Ihlberg L, Luther M, Albäck A, Kantonen I, Lepäntalo M. Does a completely accomplished duplex-based surveillance prevent vein-graft failure? Eur J Vasc Endovasc Surg 1999;18(5):395-400. https://doi.org/10.1053/ejvs.1999.0935 [ Links ]
299. Lundell A, Lindblad B, Bergqvist D, Hansen F. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: A prospective randomised study. J Vasc Surg 1995;21(1):26-34. https://doi.org/10.1016/s0741-5214(95)70241-5 [ Links ]
300. Golledge J, Beattle DK, Greenhalgh RM, Davies AH. Have the results of infrainguinal bypass improved with the widespread utilisation of postoperative surveillance? Eur J Vasc Endovasc Surg 1996;11(4):388-392. https://doi.org/10.1016/s1078-5884(96)80168-7 [ Links ]
301. Dunlop P, Sayers RD, Naylor AR, Bell PR, London NJ. The effect of a surveillance programme on the patency of synthetic infrainguinal bypass grafts. Eur J Vasc Endovasc Surg 1996;11(4):441-445. https://doi.org/10.1016/s1078-5884(96)80179-1 [ Links ]
302. Aune S, Pedersen OM, Trippestad A. Surveillance of above-knee prosthetic femoropopliteal bypass. Eur J Vasc Endovasc Surg 1998;16(6):509-512. https://doi.org/10.1016/s1078-5884(98)80242-6 [ Links ]
303. Mills JL. Mechanisms of vein graft failure: The location, distribution, and characteristics of lesions that predispose to graft failure. Semin Vasc Surg 1993;6(2):78-91. [ Links ]
304. Patel SD, Zymvragoudakis V, Sheehan L, et al. The efficacy of salvage interventions of distal bypass grafts. J Vasc Surg 2016;63(1):126-133. https://doi.org/10.1016/j.jvs.2015.07.093 [ Links ]
305. Avino AJ, Bandyk DF, Gonsalves AJ, et al. Surgical and endovascular interventions of infrainguinal vein graft stenosis. J Vasc Surg 1999;29(1):60-70. https://doi.org/10.1016/s0741-5214(99)70361-7 [ Links ]
306. Schneider PA, Caps MT, Nelken N. Infrainguinal vein graft stenosis: Cutting balloon angioplasty as the first-line treatment of choice. J Vasc Surg 2008;47(5):960-966. https://doi.org/10.1016/j.jvs.2007.12.035 [ Links ]
307. Kitrou P, Parthipun A, Diamantopoulos A, et al. Paclitaxel-coated balloons for failing peripheral bypass grafts: The BYPACS study. J Cardiovasc Surg (Torino) 2014;55(2):217-224. [ Links ]
308. Löfberg AM, Karacagil S, Ljungman C, et al. Distal percutaneous transluminal angioplasty through infrainguinal bypass grafts. Eur J Vasc Endovasc Surg 2002;23(3):212-219. https://doi.org/10.1053/ejvs.2001.1584 [ Links ]
309. Hagino RT, Sheehan MK, Jung I, Canby ED, Suri R, Toursarkissian B. Target lesion characteristics in failing vein graft predicts the success of endovascular or open surgical revision. J Vasc Surg 2007;46(6):1167-1172. https://doi.org/10.1016/j.jvs.2007.08.017 [ Links ]
310. Kinstner CM, Lammer J, Willfort-Ehringer A, et al. Paclitaxel-eluting balloon v. standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery: 1-year results of the PACUBA trial. JACC Cardiovasc Interv 2016;9(13):1386-1392. https://doi.org/10.1016/j.jcin.2016.04.012 [ Links ]
311. Ott I, Cassese S, Groha P, et al. ISAR-PEBIS (Paclitaxel-eluting balloon v. conventional balloon angioplasty for in-stent restenosis of superficial femoral artery): A randomised trial. J Am Heart Assoc 2017;6(7):e006321. https://doi.org/10.1161/JAHA.117.006321 [ Links ]
312. Kayssi A, Al-Jundi W, Papia G, et al. Drug-eluting balloon angioplasty v. uncoated balloon angioplasty for the treatment of in-stent restenosis of the femoropopliteal arteries. Cochrane Database Syst Rev 2019;1:CD012510. https://doi.org/10.1002/14651858.CD012510.pub2 [ Links ]
313. Maillet A, Desormais I, Rivière AB, et al Peripheral atheromatous arterial disease in the young: risk factors, clinical features, and prognosis from the COPART cohort. Angiology 2017;68(10):893-898. https://doi.org/10.1177/0003319717699501 [ Links ]
314. Sise MJ, Shackford SR, Rowley WR, Pistone FJ. Claudication in young adults: A frequently delayed diagnosis. J Vasc Surg 1989;10(1):68-74. https://doi.org/10.1016/0741-5214(89)90287-5 [ Links ]
315. Morbi A, Gohel MS, Hamady M, Cheshire NJ, Bicknell CD. Lower-limb ischemia in the young patient: Management strategies in an endovascular era. Ann Vasc Surg 2012;26(4):591-599. https://doi.org/10.1016/j.avsg.2011.06.008 [ Links ]
316. White JV, Ryjewski C. Progress in the endovascular treatment of intermittent claudication: Rationale for changes in the TASC classification. Semin Vasc Surg 2007;20(1):54-61. https://doi.org/10.1053/j.semvascsurg.2007.02.010 [ Links ]
317. Van Marle, Mistry PP, Botes K. HIV-occlusive vascular disease. S Afr J Surg 2009;47(2):36-42. [ Links ]
318. Lavingia KS, Dua A, Rothenberg KA, et al. Surgical management of functional popliteal entrapment syndrome in athletes. J Vasc Surg 2019;70(5):1555-1562. https://doi.org/10.1016/j.jvs.2019.01.068 [ Links ]
319. Menon D, Onida S, Davies AH. Overview of arterial pathology related to repetitive trauma in athletes. J Vasc Surg 2019;70(2):641-650. https://doi.org/10.1016/j.jvs.2019.02.002 [ Links ]
320. Lavie G, Merdler I, Barenboim E, Nitecki SS. A young adult male with peripheral artery disease. Acta Clin Belg 2015;70(1):65-68. https://doi.org/10.1179/2295333714y.0000000081 [ Links ]
321. Torrealba JI, Osman M, Kelso R. Hypercoagulability predicts worse outcomes in young patients undergoing lower extremity revascularisation. J Vasc Surg 2019;70(1):175-180. https://doi.org/10.1016/j.jvs.2018.09.062 [ Links ]
322. Berridge DC, Kessel DO, Robertson I. Surgery v. thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev 2013;6:CD002784. https://doi.org/10.1002/14651858.CD002784 [ Links ]
323. Wang JC, Kim AH, Kashyap VS. Open surgical or endovascular revascularisation for acute limb ischaemia. J Vasc Surg 2016;63(1):270-278. https://doi.org/10.1016/j.jvs.2015.09.055 [ Links ]
324. Taha AG, Byrne RM, Avgerinos ED, et al. Comparative effectiveness of endovascular v. surgical revascularisation for acute lower extremity ischaemia. J Vasc Surg 2015;61(1):147-154. https://doi.org/10.1016/j.jvs.2014.06.109 [ Links ]
325. Muli Jogi RK, Damodharan K, Leong HL, et al. Catheter-directed thrombolysis v. percutaneous mechanical thrombectomy in the management of acute limb ischaemia: A single centre review. CVIR Endovasc 2018;1(1):35. https://doi.org/10.1186/s42155-018-0041-1 [ Links ]
326. Byrne RM, Taha AG, Avgerinos E, et al. Contemporary outcomes of endovascular interventions for acute limb ischaemia. J Vasc Surg 2014;59(4):988-995. https://doi.org/10.1016/j.jvs.2013.10.054 [ Links ]
327. Leung DA, Blitz LR, Nelson T, et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischaemia: Results from the PEARL registry. J Endovasc Ther 2015;22(4):546-557. https://doi.org/10.1177/1526602815592849 [ Links ]
328. Kwok CHR, Fleming S, Chan KKC, et al. Aspiration thrombectomy v. conventional catheter directed thrombolysis as first line treatment for non-iatrogenic acute lower limb ischaemia. J Vasc Interv Radiol 2018;29(5):607-613. https://doi.org/10.1016/j.jvir.2017.11.030 [ Links ]
329. Vorwerk D, Triebe S, Ziegler S, Ruppert V. Percutaneous mechanical thrombo-embolectomy in acute lower limb ischaemia. Cardiovasc Intervent Radiol 2019;42(2):178-185. https://doi.org/10.1007/s00270-018-2129-3 [ Links ]
330. Nicoloff AD, Taylor LM Jr, McLafferty RB, Moneta GL, Porter JM. Patient recoveries after infrainguinal bypass grafting for limb salvage. J Vasc Surg 1998;27(2):256-263. https://doi.org/10.1016/s0741-5214(98)70356-8 [ Links ]
331. Goshima KR, Mills Sr. JL, Hughes JD. A new look at outcomes after infrainguinal bypass surgery: Traditional reporting standards systematically underestimate the expenditure of effort required to attain limb salvage. J Vasc Surg 2004;39(2):330-335. https://doi.org/10.1016/j.jvs.2003.10.020 [ Links ]
332. Taylor SM, York JW, Cull DL, Kalbaugh CA, Cass AL, Langan EM 3rd. Clinical success using patient-oriented outcome measures after lower extremity bypass and endovascular intervention for ischemic tissue loss. J Vasc Surg 2009;50(3):534-541. https://doi.org/10.1016/j.jvs.2009.03.030 [ Links ]
333. Taylor SM, Cull DL, Kalbaugh CA, et al. Comparison of interventional outcomes according to preoperative indication: A single centre analysis of 2 240 limb revascularisations. J Am Coll Surg 2009;208(5):770-778. https://doi.org/10.1016/j.jamcollsurg.2009.01.025 [ Links ]
334. Owens CD, Ho KJ, Conte MS. Risk factors for failure of lower-extremity revascularisation procedures: Are they different for bypass and percutaneous procedures? Semin Vasc Surg 2008;21(3):143-153. https://doi.org/10.1053/j.semvascsurg.2008.05.007 [ Links ]
335. Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: A multicentre, randomised trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006;43(4):742-751. https://doi.org/10.1016/j.jvs.2005.12.058 [ Links ]
336. Taylor SM, Kalbaugh CA, Gray BH, et al. The LEGS score: A proposed grading system to direct treatment of chronic lower extremity ischemia. Ann Surg 2003;237(6):812-818. https://doi.org/10.1097%2F01.SLA.0000071563.19208.50 [ Links ]
337. Androes MP, Kalbaugh CA, Taylor SM, et al. Does a standardisation tool to direct invasive therapy for symptomatic lower extremity peripheral arterial disease improve outcomes? J Vasc Surg 2004;40(5):907-915. https://doi.org/10.1016/j.jvs.2004.08.046 [ Links ]
338. Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: Derivation and validation of a model to predict amputation-free survival using multicentre surgical outcomes data. J Vasc Surg 2008;48(6):1464-1471. https://doi.org/10.1016/j.jvs.2008.07.062 [ Links ]
339. Schanzer A, Goodney PP, Li Y, et al; Vascular study group of Northern New England. Validation of the PIII CLI risk score for the prediction of amputation-free survival in patients undergoing infrainguinal autogenous vein bypass for critical limb ischemia. J Vasc Surg 2009;50(4):769-775. https://doi.org/10.1016/j.jvs.2009.05.055 [ Links ]
340. Biancari F, Salenius JP, Heikkinen M, Luther M, Ylönen K, Lepäntalo M. Risk-scoring method for prediction of 30-day postoperative outcome after infrainguinal surgical revascularisation for critical lower-limb ischemia: A Finnvasc registry study. World J Surg 2007;31(1):217-225. https://doi.org/10.1007/s00268-006-0242-y [ Links ]
341. Kechagias A, Perälä J, Ylönen K, Mahar MA, Biancari F. Validation of the Finnvasc score in infrainguinal percutaneous transluminal angioplasty for critical lower limb ischemia. Ann Vasc Surg 2008;22(4):547-551. https://doi.org/10.1016/j.avsg.2008.01.007 [ Links ]
342. Bradbury AW, Adam DJ, Bell J, et al. Bypass v. angioplasty in severe ischaemia ofthe leg (BASIL) trial: A survival prediction model to facilitate clinical decision making. J Vasc Surg 2010;51(5 Suppl): 52S-68S. https://doi.org/10.1016/j.jvs.2010.01.077 [ Links ]
343. Benson RA, Meecham LA, Hewitt CA, Bradbury AW. Comparison of immediate and long-term outcomes in men and women undergoing revascularisation for chronic limb threatening ischaemia in the bypass v. angioplasty in severe ischaemia of the leg (BASIL-1) trial. Eur J Vasc Endovasc Surg 2019;58(2):224-228. https://doi.org/10.1016/j.ejvs.2019.03.001 [ Links ]
344. Meltzer AJ, Graham A, Connolly PH, et al. The comprehensive risk assessment for bypass (CRAB) facilitates efficient perioperative risk assessment for patients with critical limb ischemia. J Vasc Surg 2013;57(5):1186-1195. https://doi.org/10.1016/j.jvs.2012.09.083 [ Links ]
345. Brizuela Sanz JA, Gonzalez Fajardo JA, Taylor JH, Río Solá L, Munoz Moreno MF, Vaquero Puerta C. Design of a new risk score in critical limb ischaemia: The ERICVA model. Eur J Vasc Endovasc Surg 2016;51(1):90-99. https://doi.org/10.1016/j.ejvs.2015.09.025 [ Links ]
346. Bradbury AW, Adam DJ, Bell J, et al. Bypass v. angioplasty in severe ischaemia of the leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomised to a bypass surgery-first or a balloon angioplasty-first revascularisation strategy. J Vasc Surg.2010;51(5 Suppl):5S-17S. https://doi.org/10.1016/j.jvs.2010.01.073 [ Links ]
347. Bradbury AW, Adam DJ, Bell J, et al. Bypass v. angioplasty in severe ischaemia of the leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010;51(5 Suppl):18S-31S. https://doi.org/10.1016/j.jvs.2010.01.074 [ Links ]
348. Forbes JF, Adam DJ, Bell J, et al. Bypass v. angioplasty in severe ischaemia of the leg (BASIL) trial: Health-related quality of life outcomes, resource utilisation, and cost effectiveness analysis. J Vasc Surg 2010;51(5 Suppl):43S-51S. https://doi.org/10.1016/j.jvs.2010.01.076 [ Links ]
349. Bradbury AW, DJ Adam, J Bell, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first v. a balloon angioplasty- first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The bypass v. angioplasty in severe ischaemia of the leg (BASIL) trial. Health Technol Assess 2010;14(14):71-88. https://doi.org/10.3310/hta14140 [ Links ]
350. Menard MT, Farber A, Assmann SF, et al. Design and rationale of the best endovascular v. best surgical therapy for patients with critical limb ischemia (BEST-CLI) trial. J Am Heart Assoc 2016;5:e003219. https://doi.org/10.1161/JAHA.116.003219 [ Links ]
351. Popplewell MA, Davies H, Jarrett H, et al. Bypass v. angioplasty in severe ischaemia of the leg-2 (BASIL-2) trial: Study protocol for a randomised controlled trial. Trials 2016;17:11. https://doi.org/10.1186/s13063-015-1114-2 [ Links ]
352. Hunt BD, Popplewell MA, Davies H, et al Balloon v. stenting in severe ischaemia ofthe leg-3 (BASIL-3): Study protocol for a randomised controlled trial. Trials 2017;18:224. https://doi.org/10.1186/s13063-017-1968-6 [ Links ]
353. Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev 2013;2:CD004001. https://doi.org/10.1002/14651858.CD004001.pub3 [ Links ]
354. Setacci C, de Donato G, Teraa M, et al. Chapter IV: Treatment of critical limb ischaemia. Eur J Vasc Endovasc Surg 2011;42(Suppl 2):S43-S59. https://doi.org/10.1016/s1078-5884(11)60014-2 [ Links ]
355. Zaki M, Elsherif M, Tawfick W, El Sharkawy M, Hynes N, Sultan S. The role of sequential pneumatic compression in limb salvage in non-reconstructable critical limb ischemia. Eur J Vasc Endovasc Surg 2016;51(4):565-571. https://doi.org/10.1016/j.ejvs.2015.12.025 [ Links ]
356. Schreve MA, Vos CG, Vahl AC, et al. Venous arterialisation for salvage of critically ischaemic limbs: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2017;53(3):387-402. https://doi.org/10.1016/j.ejvs.2016.11.007 [ Links ]
357. Vietto V, Franco JV, Saenz V, Cytryn D, Chas J, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev 2018;1:CD006544. https://doi.org/10.1002/14651858.CD006544.pub3 [ Links ]
358. Anderson JL, Halperin JL, Albert NM, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127(13):1425-1443. https://doi.org/10.1161/cir.0b013e31828b82aa [ Links ]
359. Smith FB, Bradbury A, Fowkes G. Intravenous naftidrofuryl for critical limb ischaemia. Cochrane Database Syst Rev 2012;7:CD002070. https://doi.org/10.1002/14651858.CD002070.pub2 [ Links ]
360. Lambert MA, Belch JJ. Medical management of critical limb ischaemia: Where do we stand today? J Intern Med 2013;274(4):295-307. https://doi.org/10.1111/joim.12102 [ Links ]
361. Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2015;24(6):CD004123. https://doi.org/10.1002/14651858.CD004123.pub4 [ Links ]
362. Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower-extremity ulcers in patients with diabetes: Results of the DAMO2CLES multicentre randomised clinical trial. Diabetes Care 2018;41(1):112-119. https://doi.org/10.2337/dc17-0654 [ Links ]
363. Ní Laoire Á, Murtagh FEM. Systematic review of pharmacological therapies for the management of ischaemic pain in patients with non-reconstructable critical limb ischaemia. BMJ Support Palliat Care 2018;8(4):400-410. https://doi.org/10.1136/bmjspcare-2017-001359 [ Links ]
364. Wilson WR, Bower TC, Creager MA, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: A scientific statement from the American Heart Association. Circulation 2016;134(20):412-460. https://doi.org/10.1161/cir.0000000000000457 [ Links ]
365. Gharamti A, Kanafani ZA. Vascular graft infections: An update. Infect Dis Clin North Am 2018;32(4):789-809. https://doi.org/10.1016/j.idc.2018.06.003 [ Links ]
366. Seeger JM, Pretus HA, Welborn MB, Ozaki CK, Flynn TC, Huber TS. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass grafting and aortic graft removal. J Vasc Surg 2000;32(3):451-459. https://doi.org/10.1067/mva.2000.109471 [ Links ]
367. Batt M, Feugier P, Camou F, et al A meta-analysis of outcomes after in situ reconstructions for aortic graft infection. Angiology 2018;69(5):370-379. https://doi.org/10.1177/0003319717710114 [ Links ]
368. Back MR. Graft Infection. Rutherford's vascular surgery and endovascular therapy. In: Sidawy AN, Perler BA, eds. Philadelphia: Elsevier, 2019:2088-2136. [ Links ]
369. Samson RH, Veith FJ, Janko GS, Gupta SK, Scher LA. A modified classification and approach to the management of infections involving peripheral arterial prosthetic grafts. J Vasc Surg 1988;8(2):147-153. [ Links ]
370. Sah BR, Husmann L, Mayer D, et al. Diagnostic performance of 18F-FDG-PET/CT in vascular graft infections. Eur J Vasc Endovasc Surg 2015;49(4):455-464. https://doi.org/10.1016/j.ejvs.2014.12.024 [ Links ]
371. Hodgkiss-Harlow KD, Bandyk DF. Antibiotic therapy of aortic graft infection: Treatment and prevention recommendations. Semin Vasc Surg 2011;24(4):191-198. https://doi.org/10.1053/j.semvascsurg.2011.10.013 [ Links ]
372. Calligaro KD, Veith FJ, Schwartz ML, Dougherty MJ, DeLaurentis DA. Differences in early v. late extracavitary arterial graft infections. J Vasc Surg 1995;22(6):680-685. https://doi.org/10.1016/s0741-5214(95)70058-7 [ Links ]
373. Aavik A, Lieberg J, Kals J, Pulges A, Kals M, Lepner U. Ten years' experience of treating aorto-femoral bypass graft infection with venous allografts. Eur J Vasc Endovasc Surg 2008;36(4):432-437. https://doi.org/10.1016/j.ejvs.2008.06.034 [ Links ]
374. O'Connor S, Andrew P, Batt M, Becquemin JP. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 2006;44(1):35-38. https://doi.org/10.1016/j.jvs.2006.02.053 [ Links ]
375. Causey MW, Ahmed A, Wu B, et al. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg 2016;63(6):1563-1573. https://doi.org/10.1016/j.jvs.2016.01.011 [ Links ]
376. Chakfé N, Diener H, Lejay A, et al. European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of vascular graft and endograft infections. Eur J Vasc EndovascSurg 2020;59:339-384. [ Links ]
 Correspondence:
Correspondence:
N G Naidoo
Nadraj.Naidoo@uct.ac.za
Accepted 25 October 2021
Contributing authors
A T O Abdool Carrim,1 MB ChB, FRCS ;
A J Arian,2 MB BS, FRCS, FCS, Cert Vasc Surg;
N J Cloete,3 MB ChB, FCS, Cert Vasc Surg;
B Dube,4 MB ChB, FCS, MPhil;
T P Fernandes,5 MB ChB, FCS, Cert Vasc Surg;
M V Forlee,6 MB ChB, FCS, Cert Vasc Surg;
R Goga,7 MB ChB, FCS, Cert Vasc Surg;
H Louwrens,8 MB ChB, MMed (Surg), FCS, Cert Vasc Surg;
A F Malan,9 MB ChB, FCS, MMed (Surg), Cert Vasc Surg;
K Michalowski,10 MB ChB, FCS, FRCS;
T Monareng,1 MB BCh, BDS, FCS, Cert Vasc Surg;
P Moodley,11 MB ChB, FCS, Cert Vasc Surg;
J de Villiers Odendaal,8 MB ChB, FCS, MMed (Surg), Cert Vasc Surg;
J Pillai,12 MB BCh, BSc, FCS, Cert Vasc Surg;
D Shone,13 MB ChB, MMed (Surg), FCS, Cert Vasc Surg;
K Sibartie,14 MB BCh, BAO, MRCS, FCS, Cert Vasc Surg;
M P Tarkowski,15 MB ChB, FCS, Cert Vasc Surg;
S C Tsotetsi,16 MB ChB, FCS, Cert Vasc Surg;
J van Marle,5 MB ChB, MMed (Surg), FCS;
I Cassimjee,1 MB BCh, MMed (Surg), DPhil, FCS, Cert Vasc Surg;
R G Botha,13 MB ChB, MMed (Surg), FCS, DA
1 Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
2 Department of Surgery, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
3 Department of Surgery, Sefako Makgatho Health Sciences University, Johannesburg, South Africa
4 Department of Surgery, Livingstone Hospital and Greenacres Private Hospital, Port Elizabeth, South Africa
5 Unitas Hospital, Lyttelton, Pretoria, South Africa
6 Kingsbury Hospital, Claremont, Cape Town, South Africa
7 Department of Vascular Surgery, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
8 Department of Surgery, Faculty of Health Sciences, University of Stellenbosch and Tygerberg Hospital, Cape Town, South Africa
9 Department of Surgery, Universitas Hospital, Bloemfontein, South Africa
10 Milnerton MediClinic and Netcare Blaauwberg Hospital, Milnerton, Cape Town, South Africa
11 Life Westville Hospital, Durban, South Africa
12 Flora Clinic, Floracliffe, Johannesburg, South Africa
13 St George's Medical Centre, Port Elizabeth, South Africa
14 Department of Surgery, Faculty of Health Sciences, University of Cape Town, South Africa
15 Hillcrest Private Hospital, Durban, South Africa
16 Department of Surgery, Steve Biko Academic Hospital, Pretoria, South Africa














