Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.5b Pretoria Mai. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i5b.16098
RESEARCH
VACCINE STRATEGY
Sisonke phase 3B open-label study: Lessons learnt for national and global vaccination scale-up during epidemics
A E GogaI, II; L-G BekkerIII; N GarrettIV, V; S TakuvaVI, VII, VIII; I SanneIX; J OdhiamboX; F MayatXI; L FairallXII, XIII; Z BreyXIV; L BamfordXV; G TannaXVI; G GrayXVII
IMB ChB, FCPaed (SA), PhD; South African Medical Research Council, South Africa
IIMB ChB, FCPaed (SA), PhD; Department of Paediatrics and Child Health, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
IIIMB ChB, PhD; Desmond Tutu HIV Centre, Faculty of Health Sciences, University of Cape Town, South Africa
IVMBBS, PhD; Centre for the AIDS Programme of Research in South Africa (CAPRISA), Durban, South Africa
VMBBS, PhD; School of Nursing and Public Health, Discipline of Public Health Medicine, University of KwaZulu-Natal, Durban, South Africa
VIMSc; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
VIIMSc; Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIIMSc; School of Health Systems and Public Health, Faculty of Health Sciences, University of Pretoria, South Africa
IXMB ChB; Right to Care, Johannesburg, South Africa
XMSc; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA
XIMSc; Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XIIMB ChB, PhD; Desmond Tutu HIV Centre, Faculty of Health Sciences, University of Cape Town, South Africa
XIIIMB ChB, PhD; King's Global Health Institute, King's College London, UK
XIVMB ChB, PhD; Bill and Melinda Gates Foundation, Johannesburg, South Africa
XVMB ChB, PhD; National Department of Health, Pretoria, South Africa
XVIMPH; National Department of Health, Pretoria, South Africa
XVIIMB ChB, FCPaed (SA), DSc South African Medical Research Council, South Africa
ABSTRACT
Sisonke is a multicentre, open-label, single-arm phase 3B vaccine implementation study of healthcare workers (HCWs) in South Africa, with prospective surveillance for 2 years. The primary endpoint is the rate of severe COVID-19, including hospitalisations and deaths. The Sisonke study enrolled and vaccinated participants nationally at potential vaccination roll-out sites between 17 February and 26 May 2021. After May 2021, additional HCWs were vaccinated as part of a sub-study at selected clinical research sites. We discuss 10 lessons learnt to strengthen national and global vaccination strategies: (i) consistently advocate for vaccination to reduce public hesitancy; (ii) an electronic vaccination data system (EVDS) is critical; (iii) facilitate access to a choice of vaccination sites, such as religious and community centres, schools, shopping malls and drive-through centres; (iv) let digitally literate people help elderly and marginalised people to register for vaccination; (v) develop clear 'how to' guides for vaccine storage, pharmacy staff and vaccinators; (vi) leverage instant messaging platforms, such as WhatsApp, for quick communication among staff at vaccination centres; (vii) safety is paramount - rapid health assessments are needed at vaccination centres to identify people at high risk of serious adverse events, including anaphylaxis or thrombosis with thrombocytopenia syndrome. Be transparent about adverse events and contextualise vaccination benefits, while acknowledging the small risks; (viii) provide real-time, responsive support to vaccinees post vaccination and implement an accessible national vaccine adverse events surveillance system; (ix) develop efficient systems to monitor and investigate COVID-19 breakthrough infections; and (x) flexibility and teamwork are essential in vaccination centres across national, provincial and district levels and between public and private sectors.
The COVID-19 pandemic, caused by SARS-CoV-2, has had a devastating effect globally. By 30 August 2021, 216 million COVID-19 cases had been confirmed worldwide, resulting in >4 million deaths.[1] South Africa (SA), which houses 0.8% of the world's population,[2] accounted for 1% and 1.5% of reported global COVID-19 cases and deaths, respectively.[1-3] By the end of August 2021, SA was well into the third COVID-19 wave.[4] During the first and second waves, dramatic increases in hospitalisations and pressure on the healthcare system led to excess deaths estimated at 2 - 3 times higher than reported.[5] The second and third waves were fuelled by the beta and delta variants that were multiple times more transmissible than the ancestral strain of the virus.[6]
Vaccination, alongside non-pharmaceutical interventions, is a key pillar to control the COVID-19 pandemic. Almost 100 COVID-19 vaccines are at various stages of clinical development and 6 have received emergency use listing or prequalification.[7] These vaccines are based on the prototype Wuhan strain and primarily target the
SARS-CoV-2 spike protein.[8] Efficacy of 94 - 95% has been reported from phase 3 trials for the messenger RNA (mRNA) vaccines (BNT162b2 and mRNA-1273), commonly known as the Pfizer BioNTech and Moderna vaccines,[7,9,10] with 117 and 81 number needed to vaccinate to prevent 1 case of COVID-19, respectively. Efficacy of 22 - 92% has been reported for the viral vector vaccines (ChAdOx1, Gam-COVID-Vac and Ad26.COV2.S), commonly known as AstraZeneca, Sputnik V and Johnson & Johnson (J&J), against moderate-to-severe COVID-19 (Table 1).[11-13] For the inactivated COVID-19 vaccines, efficacy against symptomatic disease was 51% for CoronaVac and 79% for Sinopharm >14 days after the second dose. Data on the effectiveness of COVID-19 vaccines in real-life settings are emerging (Table 1).[14-16]
In January 2021, the SA government aimed to immunise 40 million individuals against COVID-19 by the end of 2021, starting with the ChAdOx1 nCoV-19 vaccine.[17] The national vaccination roll-out was paused in February 2021 after reports of low efficacy of the ChAdOx1 nCoV-19 against the beta variant in SA.[18] The Ad26.COV2.S J&J vaccine was tested during the ENSEMBLE phase 3 randomised, double-blind, placebo-controlled study, with almost 44 000 adults across 8 countries, including 7 000 participants enrolled and followed up at 32 sites in SA.[13] Data were gathered between August and December 2020. The analysis (cut-off date 22 January 2021) found that the vaccine was safe and the SA data demonstrated protection against the beta variant and severe disease and hospitalisation (Table 1). Given these findings, the vaccine was considered for the national roll-out programme.[13]
Ad26.COV2.S is a monovalent vaccine composed of a recombinant, replication-incompetent adenovirus type 26 (Ad26) vector, constructed to encode the SARS-CoV-2 spike (S) protein. This single-dose Ad26.COV2.S vaccine is estimated to remain stable for 2 years at -20°C, at least 3 months of which can be at temperatures of 2 - 8°C. This permits seamless distribution using the existing vaccine supply chain channels in low- and middle-income countries, such as SA.
Methods
Sisonke is a multicentre, open-label, single-arm phase 3B implementation study of healthcare workers (HCWs) in SA (ClinicalTrials.gov number, NCT04838795), with prospective surveillance for endpoints for 2 years. It was implemented while waiting for registration of the Ad26.COV2.S vaccine by the South African Health Products Regulatory Authority (SAHPRA). The study sought to vaccinate 500 000 HCWs ahead of the third COVID-19 wave in SA.
The primary endpoint was the rates of severe COVID-19 (hospitalisations and death) among vaccinated HCWs compared with the general unvaccinated SA population. This study is led by the South African Medical Research Council (SAMRC). The protocol was designed to be pragmatic and as near to real-world vaccination roll-out as possible.
The study began in 18 hospital-based vaccination sites overseen by 16 clinical research sites, before expanding to a total of 122 urban and rural vaccination sites located across all 9 SA provinces, overseen by 43 clinical research sites. The last vaccination was administered on 12 August 2021 through the Sisonke sub-study. HCWs were defined as 'all people engaged in actions whose primary intent is to enhance health.[19] For the first 2.5 months of the Sisonke study, patients-facing HCWs who worked on COVID-19 wards, intensive care units and operating theatres were prioritised for study enrolment. From 11 May 2021, the HCW definition expanded to non-patient-facing HCWs, support and administrative staff, staff at multilateral health agencies, laboratory staff, health research staff, community health workers, staff working in care homes, funeral workers and registered traditional health practitioners.
In order to participate in the Sisonke study, HCWs had to firstly register on the national electronic vaccination data system (EVDS). Secondly, they had to consent to study participation after reading an online consent form and answering 6 questions to test their understanding of the study. Thirdly, they had to consent to vaccination after a screening evaluation at the vaccination centre. The date of screening was typically the date of vaccination.
Eligible HCWs were >18 years of age, in the private or public service, who were willing and able to comply with the vaccination plan and other study procedures, and who were capable of providing electronic or paper-based signed informed consent. Participants who reported breastfeeding at the time of enrolment were included up until 13 April 2021, when SAHPRA requested their exclusion pending more safety data. SAHPRA granted permission to re-include breastfeeding women on 28 April 2021. Special vaccine advocates, including the president and deputy-president of SA, were also included.
Exclusion criteria were: (i) any significant acute or chronic medical condition that in the opinion of the principal investigator/ designee made the participant unsuitable for enrolment in the study, or jeopardised the safety or rights of the participant; and (ii) current participation in any other research studies that would interfere with the objectives of this study. Participants who reported being pregnant at time of enrolment or planning to conceive within 3 months were excluded from the study, but were later invited to participate in the Sisonke sub-study. For HCWs with a history of severe adverse reaction associated with a vaccine and/or severe allergic reaction (e.g. anaphylaxis) to any component of the vaccine, eligibility was determined after consultation with a protocol safety review team (PSRT). Following a pause called by the Food and Drug Administration (FDA) on 13 April 2021 to review unusual clotting events in vaccine recipients in the USA, participants with a history of major venous or arterial thrombosis with thrombocytopenia and those with a history of heparin-induced thrombocytopenia were excluded. Participants with a chronic history of severe clotting disorders were only included after approval by the PSRT. Vaccination within 14 - 90 days with other vaccines were not exclusionary, but were discussed with the PSRT or the principal investigators of the study. A 2-week gap had to be allowed between influenza vaccination and COVID-19 vaccination.
Research staff on the Sisonke study worked in collaboration with local designated vaccination sites. Sisonke study staff supported and trained vaccination site staff on standardised study procedures.
All vaccinated participants were entered into the national COVID-19 vaccination register through EVDS. The single-dose vaccine was administered to all participants as an intramuscular injection in the deltoid region of their non-dominant arm. All participants received a single dose of Ad26.COV2.S comprising 5 x 1010 viral particles/mL.
Results, lessons learnt and discussion
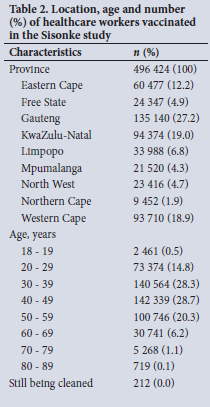
The Sisonke study enrolled and vaccinated 496 424 HCWs (Table 2). The majority of vaccinees were from Gauteng, Western Cape and KwaZulu-Natal provinces, in keeping with national population distributions. Approximately 28 200 vaccinations were administered in remote parts of the Eastern Cape and Northern Cape provinces.
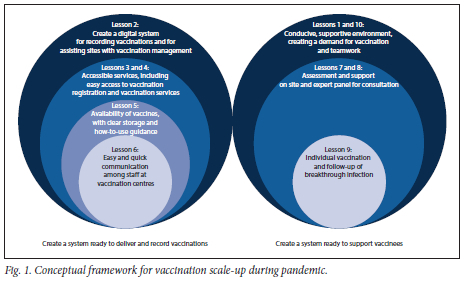
We highlight 10 challenges and lessons learnt (Table 3) using a framework that focuses on creating a system to deliver and report vaccinations and to support vaccinees (Fig. 1). We also highlight implications for any roll-out of vaccines during infectious disease epidemics or pandemics (Table 3).
Lesson 1: Consistently advocate for vaccination to reduce public hesitancy
During the study period there were reports of vaccine hesitancy in the mainstream and popular media relating to adverse events. We realised that a key advocacy message was that severe adverse reactions to vaccination are rare and can be managed, but severe COVID-19 is life threatening. In the Sisonke study, such vaccine-related questions were largely addressed through appropriate clear messaging and peer education using webinars, posters/leaflets, social media engagements and interviews on local, national and international news outlets. It was important for the Sisonke investigators and team to respond to queries arising from potential participants or stakeholders, and to dispel myths and misunderstandings with regard to COVID-19 vaccines.
Communicating risks became more complex when the rare blood-clotting condition was first reported. Sisonke messaging explained that headaches during the first 3 days could be managed with reassurance, but needed to be taken more seriously if severe with an onset between 4 and 20 days after vaccination or associated with blurred vision, weakness or difficulty speaking.
It is, however, important to communicate the risk of these events alongside the risks of COVID-19, so that people can make informed choices regarding vaccination.
In national vaccine roll-outs every vaccinee and HCW needs to be a vaccine advocate in their circles of influence.
Lesson 2: An electronic vaccination data system is critical
Paper forms were used to document vaccinations at some sites, often resulting in a delay in EVDS data capture. An EVDS is an important tool for real-time documentation of individual vaccinations and tracking of district, provincial and national progress. An EVDS also facilitates scheduling and real-time communication with vaccinees, recording vaccinee characteristics, ensuring standardisation of implementation and data quality. Critically, Sisonke enabled the National Department of Health (NDoH) to test the implementation of the EVDS. The electronic system should ensure that each person is linked with an occupation and place of work, which assists with monitoring the success of the vaccine roll-out.
Busy vaccination centres should use EVDS scheduling to avoid over-crowding, and queue marshals can be employed to monitor that vaccinees abide by their EVDS appointment time and to assist with social distancing.
Lesson 3: Facilitate access to a choice of vaccination sites, such as religious and community centres, schools, shopping malls and drive-through centres on weekdays and weekends
The limited number of vaccination sites meant that queues were long and HCWs had to wait, sometimes for ~3 hours, to be vaccinated. A key lesson was that vaccination sites should be easily accessible, using community centres, religious centres/halls, schools, shopping malls and drive-through centres, with parking space for the 15 minutes of observation. Partnering with local religious and community leaders is essential to achieve this.
Lesson 4: Let digitally literate people help elderly and marginalised people to register for vaccination
During the Sisonke study, registration for vaccination occurred mainly through a web-based portal. We learnt that registration should be allowed through various portals and systems, including WhatsApp and short message service (SMS), and that digitally literate people should help elderly and marginalised people to register for vaccination so that the digital divide does not exclude anyone. The opening of vaccination sites to walk-ins during the final week demonstrated that many HCWs had not refreshed their details or had missed SMS notifications. This situation emphasised the importance of allowing walk-ins during national vaccine roll-outs to maximise vaccine uptake.
Lesson 5: Develop clear 'how to' guides for vaccine storage, pharmacy staff and vaccinators
Nurses have prepared and administered vaccines for decades, but there has not been a recent vaccination campaign of this scale and complexity during a pandemic. COVID-19 vaccines are provided as small-volume injections. Ensuring that the volume is correctly drawn up is critical.
Cold-chain management also needs careful monitoring and accountability.
The Ad26.COV2.S and Pfizer-BioNTech vaccines have varying reconstitution and storage requirements. Pfizer must be reconstituted by injecting saline into the vial, neither can be shaken, needles cannot be changed between drawing up a dose and injecting it, and microdrops remaining in the needle nib increase wastage and risk sub-optimal dosing. The reconstituted vaccine must be stored between 2°C and 25°C and used within 6 hours of dilution. Consequently, close communication is needed between staff who reconstitute vaccine and staff who manage the vaccine queues, to prevent vaccine wastage.
The Sisonke protocol team realised the need for detailed resources on how to draw up each dose.[20] Study training therefore provided quality assurance, and a 3-step volume verification process was instituted to ensure that every dose counted. Furthermore, each dose was quality checked before leaving the pharmacy, and there was little wastage (<1%).
Allocating this process to dedicated trained teams and expanding the capacity of these teams optimised efficiency at vaccination centres and should be continued during any large-scale vaccination rollout. Many of the processes and tools developed for Sisonke have already been adapted and are being used in the national COVID-19 vaccination programme.
Lesson 6: Leverage instant messaging platforms, such as WhatsApp, for quick communication among staff at vaccination centres
Given the nature of COVID-19, information changed regularly. Providing factual and useful information to vaccination sites is key to enhance efficiency at such centres and allay concerns. We realised the need to distribute a wide range of tools from job aids, checklists, press statements and posters through WhatsApp groups to keep vaccination staff updated. These WhatsApp groups enabled principal investigators to rapidly implement changes on the ground and redistribute vaccine doses to avoid wastage, and allowed investigators and vaccination centre staff to support each other during long days and weeks.
Lesson 7: Safety - health assessments at vaccination sites and transparency regarding adverse events
Although severe allergic reactions to COVID-19 vaccines are rare, we realised that real-time rapid health assessments are needed at vaccination centres to identify people at risk of severe reactions. These assessments are important to identify those with a history of severe allergic reactions/anaphylaxis, who need to be administered medication before vaccination under medical supervision at specialised centres. Those with a history of allergy have to be identified and observed for 30 rather than 15 minutes.
In the Sisonke study, the rate of reported non-serious and serious adverse events with vaccination was low, with the majority of reported events being manifestations of mild-to-moderate reactogenicity (81%), while thromboembolic events occurred mainly in persons with risk factors for thromboembolism.[21]
Education and communication regarding these adverse events are needed early, frequently and honestly, and should juxtapose the benefits v. the risks of vaccination. All too often risks were communicated separately from the benefits of vaccination, generating fear and confusion, which was particularly true of the risk of thrombosis with thrombocytopenia syndrome related to vaccine administration. The study team realised that weighing risks against benefits is contextual. While the USA had the luxury of being at a far-advanced stage of their roll-out, with 37% of their population vaccinated by 13 April 2021 when the FDA recommended a pause, the proportion of the population vaccinated in SA was 0.5% (just <300 000), with a third wave rapidly approaching. Reciprocal licensure and safety arrangements must be considered against the contextual risk of suspending vaccination programmes because of rare events, despite limited access to vaccine options. For example, reports indicated that France and Poland did not suspend their use of Ad26.COV2.S while safety data were under review, providing an important precedent for determining policy based on vaccination coverage and community transmission.[22] As with movement restrictions, decisions informed by local data are advisable.
Lesson 8: Provide real-time, responsive support to vaccinees after vaccination
Adverse event reporting systems that are easily accessible, easy to use and data free are needed to maximise adverse event reporting and follow-up. In the Sisonke study, adverse event reporting included text message-based electronic reporting, 24/7 toll-free call centres, website links, health facility-based reporting, as well as encouragement of spontaneous case reporting.
The study team established an effective safety monitoring system based on both active (when the team follows up directly with vaccinees) and passive (when vaccinees are asked to report side-effects to the team) reporting.
A national roll-out should include an active national vaccine adverse event surveillance system and a safety desk that operates 24/7 and is responsive to vaccinees' concerns.
Lesson 9: Develop efficient systems to monitor and investigate COVID-19 breakthrough infections and deaths
During and after vaccination, monitoring and investigating breakthrough infections (BTIs) and deaths are critical to understand the emergence of new variants. We realised the need for a national BTI consortium that brings together teams from the National Institute for Communicable Diseases, the National Health Laboratory Service, the SAMRC Burden of Disease Research Unit, epidemiologists and private laboratories to ensure complete documentation of disease, hospitalisations and deaths, as well as viral genetic information. Furthermore, the Sisonke study showed that each severe BTI and death needed investigation and review by a team of experts to confirm the occurrence and establish temporality (in relation to vaccination or COVID-19). For any national rollout, similar systems are needed, and should be led by key national stakeholders and experts.
Lesson 10: Flexibility and teamwork are essential in vaccination centres, across national, provincial and district levels and between public and private sites
Sisonke's mandate was to reach as many HCWs as possible within 3 months with the research-allocated 500 000 doses of the Ad26. COV2.S vaccine imported for this purpose. This outreach was achieved through a public-private partnership in many sites, with the private sector either serving as vaccination centres or providing staff as vaccinators, pharmacists or syringe fillers.
Nothing was off limits for vaccination centre staff who engaged with health department teams, carried fridges, oversaw meticulous preparation of doses and consent processes and managed side-effects and reporting.
Conclusion
The Sisonke study team and collaborators made history by moving from the ENSEMBLE phase 3 trial results to the large-scale phase 3B study in <2 months. The Sisonke study is an example of what is possible when political will, science, hard work, partnership and a strong desire to act come together to serve public health.
Declaration. None.
Acknowledgements. South African Medical Research Council (study sponsor and oversight), Janssen Vaccines and Prevention (supply and transport of the study product to South Africa), National Department of Health, overseeing ethics committees, South African Health Products Regulatory Authority, Biocair, vaccine centres (hospitals), clinical research site principal investigators and teams, including the following: S Badal-Faesen (Themba Lethu HIV Research Unit and Clinical HIV Research Unit (CHRU)), S Barnabas (FAM-CRU), L G Bekker and S Mahoney (Desmond Tutu HIV Foundation-Emavundleni Research Centre), L Burgess (TREAD Research), W Brumskine (Aurum Institute Rustenburg Clinical Research Centre), R Dawson (University of Cape Town Lung Institute), A Diacon (TASK Central), T Dubula (Nelson Mandela Academic Clinical Research Unit), J Engelbrecht (Dr J M Engelbrecht Clinical Trial Site), K Gill (Desmond Tutu Health Foundation (DTHF) Masiphumelele Clinic), C Grobbelaar (Aurum Institute Clinical Research Centre Pretoria), L Hellstrom (Be Part-Yoluntu Centre), N Hussen (Worthwhile Clinical Trials), C Innes (Aurum Institute Klerksdorp Clinical Research Centre), N Joseph (Peermed CTC (Pty) Ltd T/A MERC), S Kassim (Desmond Tutu Health Foundation Clinical Trials Unit ), S Kotze (Synexus Stanza Research Centre), P Kotze (Qhakaza Mbokodo Research Clinic), E Lazarus (Perinatal HIV Research Unit), J Lombard (Josha Research), A Luabeya (South African Tuberculosis Vaccine Initiative (SATVI), Brewelskloof Hospital), D Makhaza (CAPRISA Vulindlela Clinic), R B Maboa (Ndlovu Research Centre), M Malahela (Setshaba Research Centre), D Malan (PHOENIX Pharma (Pty) Ltd), M Mamba (CRISMO Bertha Gxowa Research Centre), K Mngadi (Aurum Institute Tembisa Clinical Research Centre), L Naidoo (Chatsworth Clinical Research Site, SAMRC), N Naicker (CAPRISA eThekwini Clinic), V Naicker (Tongaat Clinical Research site, SAMRC), M Nchabaleng (Mecru Clinical Research Unit), T Nielsen (Aurum Institute), F Patel (Wits RHI-Shandukani Research), F Petrick (Mzansi Ethical Research Centre), E Spooner (Botha's Hill Clinical Research site, SAMRC), D Urbach (Synexus Helderberg Clinical Research Centre), E van Nieuwenhuizen (Synexus SA Watermeyer Clinical Research Centre ), A Ward (Ekhayavac TB Vaccine Trial Unit/Khayelitsha CRS (CIDRI UCT)).
Author contributions. GG, L-GB, AEG and NG conceptualised the Sisonke study. AEG wrote the first draft of the manuscript. All authors contributed to all sections of the article and reviewed the draft manuscript.
Funding. The Sisonke study is funded by the South African Medical Research Council, with funds received from National Treasury through the South African National Department of Health and by the Solidarity Fund, the ELMA Vaccines and Immunisation Foundation, the Michael and Susan Dell Foundation and the Bill and Melinda Gates Foundation.
Conflicts of interest. None.
References
1. World Health Organization. COVID-19. 2021. https://covid19.who.int/ (accessed 27 July 2021). [ Links ]
2. Worldometer. World population. 2021. https://www.worldometers.info/world-population/ (accessed 27 July 2021). [ Links ]
3. National Institute of Communicable Diseases. COVID-19 weekly epidemiological brief. 2021. https://www.nicd.ac.za/wp-content/uploads/2021/05/COVID-19-Weekly-Epidemiology-Brief-week-20-2021.pdf (accessed 27 July 2021). [ Links ]
4. Pitt R. Wits research professor warns of 'a very severe third wave in Gauteng'. The Maverick Citizen, 30 May 2021. https://www.dailymaverick.co.za/article/2021-05-30-wits-research-professor-warns-of-a-very-severe-third-wave-in-gauteng/ (accessed 27 July 2021). [ Links ]
5. Bradshaw D, Dorrington R, Laubscher R, et al. Tracking mortality in near to real time provides essential information about the impact of the COVID-19 pandemic in South Africa in 2020. S Afr Med J 2021;111(8):732-740. https://doi.org/10.7196/SAMJ.2021.v111i8.15809 [ Links ]
6. Abdool Karim S, de Oliveira T. New SARS-CoV-2 variants - clinical, public health, and vaccine implications. New Engl J Med 2021;384(19):1866-1868. https://doi.org/10.1056/nejmc2100362 [ Links ]
7. Olliaro P, Torreele E, Vaillant M. COVID-19 vaccine efficacy and effectiveness - the elephant (not) in the room. Lancet 2021;2(2):E279-E280. https://doi.org/10.1016/S2666-5247(21)00069-0 [ Links ]
8. Le TT, Cramer J, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov 2020;19(10):667-668. https://doi.org/10.1038/d41573-020-00151-8 [ Links ]
9. Baden L, El Sahly H, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384(5):403-416. https://doi.org/10.1056/NEJMoa2035389 [ Links ]
10. Polack F, Thomas S, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577 [ Links ]
11. Logunov D, Dolzhikova I, Shcheblyakov D, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397(10275):671-681. https://doi.org/10.1016/s0140-6736(21)00234-8 [ Links ]
12. Voysey M, Clemens S, Madhi S, et al. Safety and efficacy ofthe ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397(10269):99-111. https://doi.org/10.1016/s0140-6736(20)32661-1 [ Links ]
13. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. New Engl J Med 2021;384(23):2187-2201. https://doi.org/10.1056/NEJMoa2101544 [ Links ]
14. Nasreen S, Chung H, He S, et al Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. medRxiv 2021;06.28.21259420. https://doi.org/10.1101/2021.06.28.20 [ Links ]
15. Hemming K, Haines T, Chilton P, Girling A, Lilford R. The stepped wedge cluster randomised trial: Rationale, design, analysis and reporting. BMJ 2015;350:h391. https://doi.org/10.1136/bmj.h391 [ Links ]
16. Haas E, Angulo F, McLaughlin J, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021;97(10287):1819-1829. https://doi.org/10.1016/S0140-6736(21)00947-8 [ Links ]
17. National Department of Health. COVID-19 Coronavirus vaccine strategy. 2021. https://www.gov.za/covid-19/vaccine/strategy (accessed 11 June 2021). [ Links ]
18. Madhi S, Baillie V, Cutland C, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384(20):1885-1898. https://doi.org/10.1056/nejmoa2102214 [ Links ]
19. World Health Organization. Health workers. 2006. https://www.who.int/whr/2006/06_chap1_en.pdf (accessed 11 June 2021). [ Links ]
20. Knowledge Translation Unit University of Cape Town Lung Institute. COVID-19 vaccine resources (South Africa). 2021. https://knowledgetranslation.co.za/resources/covid-19-vaccine-resources-sa/ (accessed 11 June 2021). [ Links ]
21. Takuva S, Takalani A, Garrett N, et al Thromboembolic events in the South African Ad26.COV2.S vaccine study. N Engl J Med 2021;385(6):570-571. https://doi.org/10.1056/NEJMc2107920 [ Links ]
22. Wilson J. Europe scrambles as J&J vaccine delay deals another blow. AP News, 14 April 2021. https://apnews.com/article/europe-immunisations-portugal-poland-migrant-workers-5677f476109b7c279b9c5cb2ef57fc35 (accessed 17 November 2021). [ Links ]
 Correspondence:
Correspondence:
A E Goga
ameena.goga@mrc.ac.za
Accepted 29 November 2021














