Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.6 Pretoria Jun. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i6.16247
RESEARCH
Pharmacist-led medication therapy management of diabetes club patients at a primary healthcare clinic in Cape Town, South Africa: A retrospective and prospective audit
F Sonday; A Bheekie; M van Huyssteen
MPharm ; School of Pharmacy, Faculty of Natural Sciences, University of the Western Cape, Cape Town, South Africa
ABSTRACT
BACKGROUND. Diabetes mellitus (DM) is a complex chronic condition and remains a public health concern worldwide. In South Africa (SA), many patients with DM access public sector primary healthcare clinics, and those who are considered to be stable are referred to the club system, which is managed by a multidisciplinary team. Patients who have DM are often diagnosed with concurrent medical conditions, resulting in multiple medication therapies that lead to medication therapy problems (MTPs). Prescriber adherence to standard treatment guidelines (STGs) is aimed at improving glycaemic control to minimise complications and decrease healthcare costs. The pharmacist's role in medication therapy management (MTM) for DM is underutilised in public sector healthcare facilities.
OBJECTIVES. To evaluate the implementation of a pharmacist-led MTM intervention to optimise the management of stable patients with type 2 DM attending a diabetes club at a Cape Town community day centre.
METHOD. An evaluation study design using a case study approach was conducted over 8 months from November 2016 to June 2017. A retrospective and prospective audit was conducted from patient folders of stable patients who attended the club. Quantitative data were extracted from the folders. A trained pharmacist audited baseline (pre-intervention) data. Prescribing staff were notified of therapeutic discrepancies through written pharmacist's pharmacotherapeutic recommendations (intervention). Pharmacist-led interventions audited prescriber adherence to SA STGs and the Essential Medicines List, and prescriber responses to the pharmacist's recommendations (post-intervention) were recorded as accepted, partially accepted or rejected. Estimated costs were calculated for rational and irrational prescribing of aspirin during the MTM process.
RESULTS. Of 104 patient folders audited, most were for females (n=70; 67.3%). A total of 453 MTPs were identified, averaging four interventions per folder reviewed. The most common MTPs identified were the absence of basic clinical data: body mass index not documented (22.5%) in the folder, no medical indication noted (19.2%), and laboratory tests not requested (18.3%) by clinicians. Prescriber acceptance of the pharmacist's recommendations was found to be low (26.8%), suggestive of clinical inertia. Aspirin was found to be irrationally prescribed to patients with DM (15.4%).
CONCLUSION. Pharmacists can identify, resolve and prevent MTPs and rationalise appropriate medication therapy in patients with DM. Prescriber uptake of pharmacists' pharmacotherapeutic recommendations seems overlooked. Pharmacist-led workshops to advocate for rational prescribing are needed to mitigate MTPs among stable patients with type 2 DM at public sector healthcare facilities.
Diabetes mellitus (DM) is identified as one of the most challenging public health concerns.[1] In 2019, there were an estimated 463 million adults with DM worldwide and 19.4 million in Africa.[2] In South Africa (SA), the age-adjusted comparative prevalence of DM was 12.7% in 2019, and 23% of the health budget was spent on diabetes care,[2] imposing an economic burden on the country's fragmented health system.[3, 4] A Cape Town study (2008) further reported a high prevalence (28.2%) of type 2 DM.[5]
DM forms part of SAs quadruple burden of disease[6] that requires comprehensive management at a primary care level. Despite evidence demonstrating the benefits of attaining glycaemic control, management of this disease is still largely lacking. Glycaemic targets are not being met, which can lead to diabetic complications.[7] Suboptimal management of patients with DM at primary healthcare (PHC) level can be attributed to lack of prescriber adherence to guidelines and failure to draw blood samples for laboratory testing, while recording of the body mass index (BMI) is often overlooked.'8 In Tshwane district, findings from a review of patient folders noted limited recordings of glycated haemoglobin (HbAlc), a lipogram or total cholesterol test result, and kidney function with a serum creatinine level.[9]
In addition, patients with type 2 DM often present with comorbidities[10, 11] and receive multiple medications which could lead to medication therapy problems (MTPs) that interfere with desired therapeutic outcomes.[12] MTPs may include wrong choice of medicine, incorrect dosage, medicine-disease interactions, adherence problems,[13] polypharmacy, and dosage adjustment in renal failure.[10] Through their extensive training in medication therapy,[14] pharmacists are ideally suited to interpret the patient's physical, clinical and laboratory data relative to therapeutic guidelines to assess the effectiveness and safety of each individual patient's medicine regimen and offer recommendations to preservers[12] to optimise diabetes management at the PHC level.
An Ethiopian pharmacist-led intervention study for patients with type 2 DM further identified MTPs such as additional therapy, ineffective therapy, very low dosages and limited prescribing of a statin.[15] Elsewhere, studies have also demonstrated that pharmacists have identified and prevented such problems and have made recommendations to clinicians, who either acknowledged and acted on the recommendation or rejected the pharmacist's recommendation to resolve the MTP.[16, 18] Such medication therapy interventions in adult patients with type 2 DM led to an improvement in HbAlc, lipid profile and BMI and a decrease in fasting plasma glucose and blood pressure,[19] and were found to save costs for the health system.[20]
While numerous pharmacist intervention studies are conducted in hospital settings, pharmacist-led medication therapy management (MTM) interventions among stable diabetes club patients at a community day centre (CDC) in Cape Town have not yet been documented. A multidisciplinary team can offer regular and comprehensive therapeutic assessments of clinical, biochemical and physical parameters to minimise the risk of developing long-term complications of DM.[21] Rational prescribing is achieved when prescribers adhere to the standard treatment guidelines (STGs), Essential Medicines List (EML)[22] and updated pharmacotherapeutic approaches[8,23] to optimise medication therapy and health outcomes among patients with DM.[21]
Objectives
This study aimed to audit the implementation of a pharmacist-led MTM intervention to optimise the management of stable patients with type 2 DM.
Methods
Study design
An evaluation design using a case study approach was undertaken at a single CDC. A non-invasive technique was designed to follow the CDC's routine operational procedures, including those involving prescribers.
Setting
The study was conducted in a subdistrict of Cape Town. The CDC has a functional club system[24] whereby patients with DM who adhere to their medicine regimen and have minimal changes in their clinical status (classified as stable)[25] are referred to the club, to which they return every 6 months for their follow-up appointment. The first issue of medication is dispensed at the facility's pharmacy. Thereafter, patients collect their repeat medication through the chronic dispensing unit (CDU) at a decentralised pick-up point (off site) located closest to the community[26]
The CDC offers chronic care for diseases such as diabetes, hypertension, epilepsy and asthma on specific club days. Forty appointments for stable patients are reserved for the Thursday diabetes club per week (-160 per month). 'Stable' diabetes club patients are seen by either the club doctor or a clinical nurse practitioner (CNP) and their appointment dates are recorded in a club register. The CNP refers patients to the doctors when assessment of their clinical data shows them to be poorly controlled. Such patients are identified as 'unstable'.
The club system was introduced to improve patient flow and reduce the workload and waiting times at the CDC. The organisational flow of this facility's club system is outlined in Fig. 1. A reception clerk is assigned to have the club patient folders pre-drawn a day before the club appointment date, to fast-track folder access for the club patients for the next day (1). On the club day, a staff nurse records physical (weight and height) and clinical (blood pressure, fasting plasma glucose and dipstick urinalysis) measurements in the folder (2). At this facility, the staff nurse is expected to measure and record the patient's height and weight, and the prescribers to calculate and record the BMI. A health promoter offers health talks to educate the club patients about lifestyle changes (A). The patient's blood sample is taken for laboratory tests (blood lipid panel, HbAlc, serum creatinine) 2 weeks prior to their club appointment (B). Patient laboratory test results are obtained within 3 days, depending on resource availability (network points, computers, printers) at the CDC. The club doctor or CNP examines the patient, reviews laboratory test results, and prescribes the patient's chronic medication during the consultation (3). Prescriptions are written up for a period of 6 months and club patients only receive their initial 1 -month supply of chronic medication from the pharmacy (4). For the next 5 months, until the 6-month follow-up club appointment, stable patients are required to collect their chronic medication at the CDU off site (C).
Study participants
The target population included stable patients with type 2 DM who attended the diabetes club and the facility staff who managed those patients. The research pharmacist (FS) collected data via a folder review and did not have face-to-face contact with patients. Inclusion criteria for folder review included adult patients (> 18 years) diagnosed with type 2 DM, categorised as 'stable' and attending the diabetes club, and with a valid 6-month prescription. Patients attending other chronic disease clubs or diagnosed with type 1 DM were excluded for folder review, as the focus of the study was adult type 2 DM owing to the high prevalence of the condition in Cape Town.[5]
Prescribing staff who managed the club patients and consented to participate in the study were recruited. They consisted of 2 doctors and 2 CNPs. The doctors had a medical bachelor's degree with <10 years of experience, whereas the CNPs had a diploma in clinical nursing science, health assessment, treatment and care, with >10 years of experience.
Sample method and size
Non-probability convenience sampling was used. The minimum number of stable diabetes club patient folders required for review was calculated using the formula:1271 η= z2p(l-p)/d2 (n=100); ζ = 1.96 for 95% confidence level, d = 0.05, and the prevalence of DM in the population was assumed to be 7%. To accommodate for missing or incomplete data, the sample size was increased by 10%, so a minimum of 110 folders were required for review
Data collection tools and process
The pharmacist's MTM data tools were a patient data extraction sheet, an intervention log sheet, an assessment worksheet, an intervention label, and a reminder prompt cover page that was mounted on the patient folder (Appendix 1, https://www.samedical.org/file/1828).
The researcher, a pharmacist trained in pharmacotherapeutics, attended a 1-year course, 'Integrated applied therapeutics: Fundamentals of rational prescribing' (2015), offered by Pharmacy Education International, an approved South African Pharmacy Council provider.![28] One of the key competencies was MTM for non-communicable diseases using the PHC STGs and EML.
The researcher used the pharmacotherapy work-up notes to categorise the MTPs (n=8) and their types (n=35),[12] using the PHC STGs and EML (2014) and government circular Η141/2017[29·30] to audit each folder. A list of MTP categories and types is provided in Appendix 1. Guidelines used in practice generally consist of the Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) guidelines.[31] The STGs for the management of DM are synthesised from the SEMDSA guidelines in the public sector. In this study, STGs therefore serve as a key reference to audit diabetes patient folders.[29] The Practical Approach to Care Kit (PACK) Primary Care Guide for the Adult,[32] formerly known as Primary Care 101,[33] is a clinical decision support tool designed for use by a clinician, tailored for low-income, extremely resource-constrained primary care settings with a high patient burden. The PACK uses a symptom-based approach and a standardised approach to the routine care of patients with chronic condition(s).[32] Clinicians are advised to use the PACK in conjunction with the latest edition of the PHC STGs and EML.[32] Even though PACK guidelines are used in clinics, the latest edition of the EML takes precedence when differences in treatment guideline recommendations exist.[34]
The data collection process was phased (Appendix 2, https://www.samedical.org/file/1828) to accommodate the clinic's routine diabetes club workflow (described earlier). The pharmacist's pharmacotherapeutic intervention was performed 1 week prior to the patient's club appointment date to enable prescribers to respond to the written pharmacist recommendations.
The research pharmacist obtained permission from the CNP to acquire the patient list from the club register to access diabetes patient folders from the reception department on a weekly basis. At phase 1, a 2-week pilot study was conducted prospectively. Patient folders ()i=7) that met the criteria were audited to test the pharmacist's MTM data tools and make amendments, and those folders were excluded from the main study, which was conducted over 8 months (November 2016 - June 2017).
The folders of stable patients with type 2 DM were randomly selected for audit, retrospectively. At phase 2, baseline information (demographics, comorbidities, and physical, clinical and biochemical parameters) was recorded on a predesigned patient data sheet. Owing to workload constraints, folder audits were performed by the research pharmacist before the patient's Thursday club appointment date. The pharmacist-led MTM intervention was dependent on the availability of the blood results that were meant to be taken 2 weeks prior to the club appointment. During phase 3, the intervention phase, the pharmacist logged MTM interventions and pharmacotherapeutic recommendation( s) for the prescribers' attention, on a log sheet, based on the information in the file and the latest blood results recorded for the patient. The research pharmacist only undertook one round of pharmacist-led interventions. A cover sheet was mounted on the front of the patient folder, intended to alert prescribers to an intervention label containing the pharmacist's recommendation, which was attached to the existing prescription inside the folder. The pharmacist's recommendations were offered before the patient returned for their follow-up appointment at the diabetes club within 6 months after their previous appointment. After the club visit, the pharmacist retrieved the folders from the reception department and continued to audit the folders retrospectively for the prescribers' response. Prescriber responses to the pharmacist's recommendations were noted at phase 4, post-intervention, as accepted' (had been agreed to and noted accordingly onto the prescription), 'partially accepted' (not completely rejected) or 'rejected' (not accepted) on the pharmacist's assessment worksheet. At phase 5, the patient's 6-month follow-up, physical and laboratory data (HbAlc, total cholesterol, serum creatinine) were re-assessed and audited. Data were compared with baseline and post-intervention data. A comparison of cost estimates between rational and irrational prescribing of aspirin was calculated for 28 days over a 6-month expenditure period: tender price ZAR5.38 for a single (14s) pack unit (Western Cape Master Procurement Catalogue, November 2016).
Ethical considerations
Ethics approval was obtained from the University of the Western Cape Biomedical Research Ethics Committee (ref. no. BM/16/4/11) and the Western Cape Department of Health (ref. no. WC_2016RP43_75). Facility staff was informed of the study before it began. Written informed consent was obtained from the prescribing staff members who agreed to participate in the study.
Statistical analysis
Data collected were captured into an Excel 2007 spreadsheet (Microsoft, USA), and SPSS statistics software, version 24 (IBM, USA), was used to analyse the data, which consisted of descriptive and inferential statistics. The means, standard deviations (SDs), and minimum and maximum figures for the patients' baseline characteristics were calculated. In an inferential approach, data were analysed using means, standard errors and 95% confidence intervals. Statistical significance was considered at p<0.05. Prescriber responses to the pharmacist's intervention were included in the data analysis. A paired-sample f-test was applied to compare data obtained from folders of stable patients at baseline (pre-intervention), at prescriber response to pharmacist-led intervention (post-intervention), and at 6-month club follow-up. Pre-prescriber practice and post-prescriber response were compared following the pharmacist's recommendations. No statistically significant correlations between the pharmacist's intervention and any of the clinical parameters and laboratory tests were found when using the paired-sample f-test, consequently resulting in no conclusive findings.
Results
Study sample demographic data and clinical characteristics
A total of 104 patient folders were included in the study. The mean (SD) age of the patients was 57.7 (9.2) years, and 67.3% of them were female. There was a mean of 3.1 (0.9) chronic illnesses per patient and the mean BMI was 31.6 (7.2) kg/m2, indicating overweight or moderately obese patients. Blood pressure was recorded for all patients (100%) and fasting plasma glucose in 99.0%. Table 1 presents the demographic and clinical indicators for the study sample.
At baseline, only 43 patients (2015) and 54 patients (2016) had their HbAlc levels checked. The mean (SD) HbAlc in 2015 was 8.6% (1.8%) and in 2016 it was 8.5% (1.9%). Optimal glycaemic control (HbAlc <7%) was only achieved in 8.6% (2015) and 11.5% (2016) of patients. Laboratory tests performed by healthcare staff are shown in Table 2.
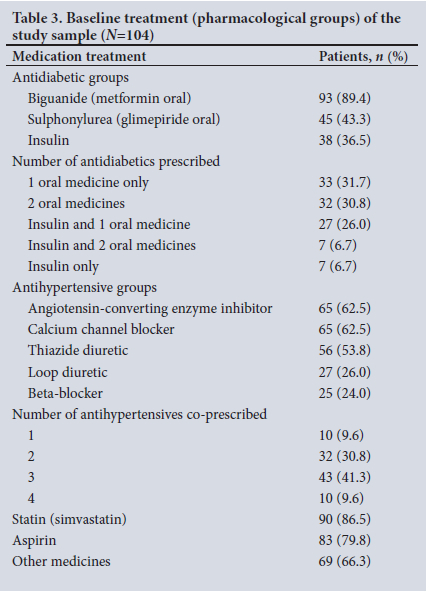
Table 3 documents the pharmacological treatment prescribed for stable patients with type 2 diabetes at baseline. Almost all patients were prescribed metformin (89.4%), either as monotherapy or in combination, a statin (86.5%) and aspirin (79.8%).The most commonly prescribed antihypertensive medicine groups were angiotensin-converting enzyme inhibitors (62.5%) and calcium channel blockers (62.5%), followed by thiazide diuretics, loop diuretics and beta-blockers.
MTPs identified and prescribers' response to the pharmacist's recommendations
MTP types that were identified and prescriber responses to the pharmacist's recommendations are outlined in Table 4. There was a total of 453 interventions, an average of 4 per patient. At post-intervention, the highest number of MTPs identified were BMI not documented (n=102; 22.5%), no medical indication noted (n=87: 19.2%) for a prescribed medicine, and laboratory tests not requested (n=83; 18.3%). Laboratory tests that were absent at baseline (2015 and 2016) were HbAlc, total cholesterol and serum creatinine (Table 2).
Overall, prescribers rejected more than two-thirds (n=314; 69.7%) and accepted a quarter (n=123; 26.8%) of the pharmacist-led interventions (Table 4).
Of the 102 interventions for BMI not documented, doctors rejected 47 and CNPs 36 pharmacist's recommendations. Rejection of the pharmacist's recommendations for the MTP laboratory tests not requested was two-fold higher among the doctors (n=38; 8.4%) compared with the CNPs (n=19; 4.2%). Doctors and CNPs showed similar rejection trends for the pharmacist's recommendation relating to the MTPs no medical indication noted (n=31; 6.9% and n=28: 6.2%. respectively), synergistic/potentiating effects of medicines (n=23: 5.1% and n=26; 5.7%, respectively), and inappropriate (low and high) dosage (n=25; 5.6% and n=22; 4.8%, respectively).
The label seemed to have had negligible influence on prescriber behaviour change.
Estimated expenditure associated with irrational prescribing of aspirin
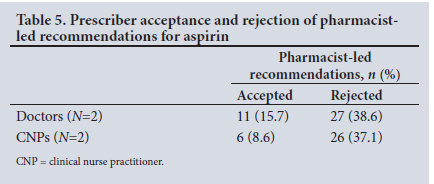
Although the majority of MTPs were identified as BMI not documented. the MTP type no medical indication noted (n=87) was the second most common MTP that attracted attention during the pharmacist intervention study. The prescribed medicine that emerged as a particular concern for the research pharmacist during the study was aspirin. Of the 87 pharmacist-led interventions for the MTP no medical indication noted, 70 (15.4%) were directly related to irrational prescribing of aspirin (Table 5). Prescriber rejection of pharmacist-led recommendations for aspirin led to over three-fold expenditure estimates for 28-day (ZAR196.37) and 6-month (ZAR1 178.22) supplies, when compared with prescriber acceptance of the pharmacist's recommendation (ZAR53.80 and ZAR322.80, respectively) (Western Cape Master Procurement Catalogue, November 2016).
Discussion
Overall findings from the pharmacist-led interventions illustrate that diabetes management at the primary care facility is suboptimal. The mean HbAlc (2015) in this study was 8.6%. Figures were similar in studies in the Tshwane district[9] and Cape Town,[35] both reporting a mean HbAlc of 8.8%, which exceeds the 7% target[36] and indicates that diabetes is suboptimally managed and that current practice interventions are not effective.
Our exploratory study attempted to implement 'pharmacist-led' MTM interventions to determine the effectiveness of prescribed medication therapy in the management of stable patients with type 2 diabetes attending a diabetes club for routine care at the CDC. The pharmacist recommendations were made according to evidence-based STGs. In this study, there was an average of 4 interventions per patient, which is similar to the number in a study in Denmark'[37] but two times higher than that recorded in a Malaysian study.[11] The MTP categories and types listed in our study were framed on the Pharmacotherapy Workup Notes,[12] while other researchers have used the Problem Intervention Documentation coding system[37] and the Pharmaceutical Network Care Europe tool version 5.01.[38] Variation in MTP instrument framework and the country-specific reference guidelines being used would offer different outcomes on prescription reviews.
The most common MTP types documented by the pharmacist were BMI not documented (n=102; 22.5%), no medical indication noted (n=87; 19.2%) and laboratory tests not requested (n=83; 18.3%).
The BMI in a patient with DM is a cornerstone of therapeutic efficacy monitoring and subsequent decision-making, as it assesses obesity status and cardiovascular risk factors.[39] A deteriorating BMI impedes treatment efficacy because lifestyle interventions are not adequate.[40] Since the staff nurse is expected to measure and record the patient's height and weight, it is the prescribers responsibility to calculate and record the patient's BMI in the folder at each clinical visit.[39]
The most prominent medicine that was prescribed without an indication was aspirin, which underscores poor prescriber adherence to evidence-based guidelines. As per guidelines, low-dose aspirin is only indicated in the secondary prophylaxis for cardiovascular disease, requiring a diagnosis of myocardial infarction, cerebrovascular accident, ischaemic heart disease or peripheral vascular disease to be noted in the folder to make its use rational. Although the pharmacist-led recommendations had alerted prescribers to consider removing aspirin from the patients' regimen, almost three-quarters (n=53: 75.7%) were rejected (Table 5), indicating poor prescriber adherence to the government circular on aspirin.[30] Inappropriate prescribing of aspirin was also addressed in an Italian study that reported lower findings of only 2.6% of cases where there was no reason for aspirin use,[41] whereas our study reported 15.4% of cases with no medical indication noted for aspirin use.
Other SA studies have also noted the absence of laboratory testing, [42,43]which particularly impedes the pharmacist's ability to determine whether the prescribed treatment is effective and safe for the patient. In addition, the absence of a co-ordinated system to track, review and file laboratory test data underpins a poor medical recordkeeping system.[44]
Prescriber acceptance in this study was low (about a third of interventions were accepted). A possible reason for low prescriber acceptance of pharmacist-led interventions in this study may be clinical inertia.[45] Clinical inertia is the failure to set glycaemic targets, and implement and escalate treatment to achieve these therapeutic goals.[46] The cause of clinical inertia is multifactorial, involving patient, physician and health system factors, which explains the poor glycaemic control in SA. Physicians tend to work in isolation, especially at PHC facilities.[45] A 1-year retrospective audit review in KwaZulu-Natal Province found that the poor control and management of patients with type 2 DM at public sector facilities could be attributed to clinical inertia.[46] In the present study, it was thought that clinical inertia could be due to a high workload in the PHC setting, limited prescribing staff, and time constraints. A Cape Town study conducted an appreciative inquiry at 15 community health centres and reported that staff had to deal with a high patient workload, reducing the consultation time for individual patients and resulting in poor quality of care.[47] The high patient load and time constraints at public sector healthcare facilities therefore requires a multidisciplinary approach to converge the scope of practice among facility staff to optimise diabetes management.
Pharmacists in the public sector traditionally operate as mechanical dispensers in outpatient public health facilities, with minimal focus on MTM and clinical interaction with prescribing staff.[48] To optimally manage diabetes at PHC club level, staff roles and responsibilities in multidisciplinary teams should therefore be clearly delineated to ensure that patient data (weight, BMI, blood pressure, laboratory investigations) are noted in the file.[4]
It was noted that costs could potentially be saved by acceptance of a pharmacist-led aspirin intervention, and the fact that an estimated massive three-fold loss was incurred as a result of irrational prescribing is a concern, especially for a constrained health system. Findings from another Cape Town district-based study found that increasing costs were attributed to the number of comorbidities, and that prescribing patterns for DM and hypertension, in relation to the PHC STGs and EML, should be assessed.[3]
MTM provides trained pharmacists with an opportunity to manage patients with chronic diseases, and evaluate and address MTPs through pharmacist-led interventions that have demonstrated both positive clinical and positive economic outcomes.[12]
With most patients with type 2 DM attending public sector primary care facilities rather than hospitals,[49] the findings of the present study indicate that pharmacist-led interventions using evidence-based guidelines can assist a multidisciplinary team in identifying, intervening and preventing MTPs and thus delaying the onset of complications. Furthermore, the pharmacist's responsibility in MTM is key when evaluating physical, clinical and biochemical data to help make timeous adjustments to the patient's medication therapy
While pharmacists are trained in medication therapy,[14] contextual constraints such as a high patient load, inadequate staff, patients not utilising the clinic system as intended, and an increasing administrative load in public sector facilities precludes them from offering such a practice.[50] In essence, effective task-shifting of the pharmacist's administrative load is required to redefine the role of the pharmacist as an integral member of the PHC team,[50] that depends on patient acceptance, professional dedication, interprofessional collaboration, and funding an appropriate legislative framework.[51]
This study demonstrates the potential role of pharmacists to intervene and promote rational prescribing of medicine at a PHC level in Cape Town.
Study limitations
Data were collected at only one PHC facility, and the study findings therefore lack generalisability. The absence of a control group and the lack of an independent review of the pharmacist's intervention to make comparative assessments do not enable conclusive findings. Owing to the small sample size of the prescribers (n=4) who participated in the pharmacist-led intervention, conclusions regarding prescriber uptake of the intervention cannot be based on the responses of the prescribers, despite the fact that the patient folder sample and number of responses were adequate. The study can therefore only be regarded as an audit of pharmacist-identified prescriber practices in the population of 104 stable patients with type 2 DM (compared with evidence-based STGs for the management of these patients).
Conclusions and recommendations
The study findings demonstrate continued poor management of type 2 DM in primary care and the potential role of a trained pharmacist to evaluate the MTM of chronic stable patients. It also indicates irrational prescribing of aspirin, which begs the question: how effectively are government circulars and guideline updates being disseminated among healthcare personnel at facility level? Regular facility-based pharmacist-led workshops could promote rational prescribing by advocating for the provision of pharmaceutical care in primary care and policy through task-shifting. The next step is to replicate the pharmacist-led MTM study in other CDCs located in the same subdistrict.
Declaration. The research for this study was done to fulfil the requirements for FS's master's degree in pharmacy at the University of the Western Cape.
Acknowledgements. The authors thank the healthcare facility staff for their assistance with and support of the study. The National Research Foundation of South Africa provided funding. Staff at the School of Pharmacy at the University of the Western Cape offered academic input.
Author contributions. All authors conceptualised and implemented the study. FS was responsible for data collection. AB and MvH worked with FS in drafting and write-up of the manuscript.
Funding. Funding for the study was provided by the National Research Foundation of South Africa (ref. no. CEC14061870029).
Conflicts of interest. None.
References
1. World Health Organization. Global report on diabetes. Executive summary. 21 April 2016. https://www.who.int/publications/i/item/9789241565257 (accessed 18 February 2020). [ Links ]
2. International Diabetes Federation. Diabetes Atlas. 9th ed. Brussels and Belgium. IDF, 2019. http://www.idf.org/ (accessed 21 July 2021). [ Links ]
3. Isaacs A Manga N, le Grange C, Hellenberg DA Titus V, Sayed R. Quality of care and cost of prescriptions for diabetes and hypertension at primary healthcare facilities in the Cape Town metrópole. S Afr Fam Pract 2015,57(3).187-193. https://doi.org/10.1080/20786190.2014.976988 [ Links ]
4. Kalain A, Omole OB. Lifestyle advice, processes of care and glycaemic control amongst patients with type 2 diabetes in a South African primary care facility. Afr J Prim Health Care Fam Med 2020,12( 1).2163. https://doi.org/10.4102/phcfm.vl2il.2163 [ Links ]
5. Erasmus RT, Soita DJ, Hassan MS, et al. High prevalence of DM andmetabolic syndrome in a South African coloured population. Baseline data of a study in Bellville, Cape Town. S Afr Med J 2012,102(11):841-844. http://doi.org/10.7196/SAMI.5670 [ Links ]
6. Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet 2009,374(9693):934-947. http://doi.org/10.1016/S0140-6736(09)61087-4 [ Links ]
7. Pillay S, Aldous C, Mahomed F. Diabetic patients served at a regional level hospital. What is their clinical picture? J Endocrinol Metab Diabetes S Afr 2015:20(1)-.60-66. https://doi.org/10.1080/16089677.2015.1030856 [ Links ]
8. Igbojiaku OJ, Harbor OC, Ross A. Compliance with diabetes guidelines at a regional hospital in KwaZulu-Natal, South Africa. Afr J Prim Health Care Fam Med 2013,5(1).447. https://doi.org/10.4102/phcfm.v5i1.447 [ Links ]
9. Webb EM, Rheeder P, vanZyl DG. Diabetes care and complications in primary care in the Tshwane district of South Africa. Prim Care Diabetes 2015,9(2).147-154. https://doi.org/10.1016/j.pcd.2014.05.002 [ Links ]
10. Munger MA. Polypharmacy and combination therapy in the management of hypertension in elderh patients with co-morbid DM. Drugs Aging 2010,27(11).871-883. https://doi.org/10.2165/11538650-000000000-00000 [ Links ]
11. Huri HZ, Wee HE Drug related problems in type 2 diabetes patients with hypertension. A cross-sectional retrospective study. BMC Endocr Disord 2013U3.2. https://doi.org/10.1186/1472-6823-13-2 [ Links ]
12. Cipolle RJ, Strand LM, Morley PC. Pharmaceutical Care Practice. The Patient-Centered Approach to Medication Management 3rded New York. McGrawHill Professional, 2012. [ Links ]
13. Shareef J, Fernandes J, Samaga L. Assessment of clinical pharmacist interventions in drug therapy in patients with DM in a tertiary care teaching hospital. Diab Metab Syndr 201610(2).82-87. https://doi.org/10.1016/j.dsx.2015.09.017 [ Links ]
14. Ells AW, Sherman JJ. Community and clinical pharmacy services. A step-by-step approach. In. Westberg SM, Reidt SL, Sorensen TD, eds. Medication Therapy Management 1st ed New York. McGraw-Hill Education, 2013:49-61. [ Links ]
15. Yimama M, Jarso Η Desse TA. Determinants of drug-related problems among ambulatory type 2 diabetes patients with hypertension comorbidity in southwest Ethiopia. A prospective cross sectional study. BMC Res Notes 2018;11(1):679..https://doi.org/10.1186/sl3104-018-3785-8 [ Links ]
16. Chung AY, Anand S, Wong IC, et al. Improving medication safety and diabetes management in Hong Kong: A multidisciplinary approach. Hong Kong Med J 2017;23(2):158-167. https://doi.org/10.12809/hkmj165014 [ Links ]
17. Kheir N, Adam A, Hooper R. Pharmacist-documented interventions during the dispensing process in a primary health care facility in Qatar. Drug Healthc Patient Saf 2009:1.73-80. https://doi.org/10.2147/dhps.s5534 [ Links ]
18. Chua SS, Kok LC, Yusof FA, et al Pharmaceutical care issues identified by pharmacists in patients with diabetes, hypertension or hyperlipidaemia in primary care settings. BMC Health ServRes 201212(1).388. https://doi.org/10.1186/1472-6963-12-388 [ Links ]
19. Pousinho S, Morgado M, Falcão A Alves G. Pharmacist interventions in the management of type 2 diabetes mellitus. A systematic review of randomised controlled trials. J Manag Care Spec Pharm 2016;22(5):493-515. https://doi.org/10.18553/jmcp.2016.22.5.493 [ Links ]
20. Pontefract B, KingBS, King C A GothardDM. Impact of pharmacist-led diabetes management in primary care clinics. Innov Pharm 2018-9(2).1-8. https://doi.org/10.24926/iip.v9i2.985 [ Links ]
21. American Diabetes Association. Standards of medical care in diabetes - 2013. Diabetes Care 2013,36(Suppl 1)S11-S66. https://doi.org/10.2337/dcl3-S011 [ Links ]
22. National Department of Health, South Africa. National Drug Policy for South Africa. Pretoria. NDoH, 1996. https://www.gov.za/sites/default/files/gcis_document/201409/drugpolO.pdf (accessed 15May 2017). [ Links ]
23. Kapadia-Kundu N, Sullivan TM, Safi B, Trivedi G, Velu S. Understanding health information needs and gaps in the health care system inUttar Pradesh, India. J Health Comm2012,17(Suppl2).30-45. https://doi.org/10.1080/10810730.2012.666625 [ Links ]
24. Slingers N, de Villiers PJ. Evaluation of the effect of the introduction of a hypertension club on the management of hypertension at a community health centre in the Cape Town Metrópole. S Afr Fam Pract 2009,51(2).143-147. https://doi.org/10.1080/20786204.2009.10873830 [ Links ]
25. Meinties G, Maartens G. Guidelines for antiretroviral therapy in adults. South Afr J HIV Med 2012,13(3).114-133. https://doi.org/10.4102/sajhivmed.vl3i3.125 [ Links ]
26. Magadzire BP, Marchai Β, Ward Κ. Improving access to medicines through centralised dispensing in the public sector. A case study of the Chronic Dispensing Unit in the Western Cape Province, South Africa. BMC Health ServRes 2015,15(1). 1-8. https://doi.org/10.1186/sl2913-015-1164-x [ Links ]
27. Larsen RJ, Marx ML. An Introduction to Mathematical Statistics. 2nd ed. New York Prentice Hall, 1986. [ Links ]
28. Perkin Ε Integrated Applied Therapeutics. Fundamentals of Rational Prescribing Training Manual. Cape Town. Pharmacy Education International, 2014. [ Links ]
29. National Department of Health, South Africa. Essential Drugs Programme. Standard Treatment Guidelines and Essential Medicines List for South Africa. Primary Healthcare Level, 2014 edition. Pretoria. NDoH 2014. http://www.kznhealth.gov.za/pharmacy/edlphc2014a.pdf (accessed 20 August 2016). [ Links ]
30. Western Cape Department of Health, South Africa. Aspirin medicine use evaluation feedback, October 2016. Circular No.: H141/2017. [ Links ]
31. Amod A, Ascott-Evans BH Berg GI, et al. The 2012 SEMDSA guideline for the management of type 2 diabetes. J Endocrinol Metab Diabetes S Afr 2012,17(2).S1-S95. https://doi.org/10.1080/22201009.2012.10872287 [ Links ]
32. Western Cape Department of Health, South Africa. PACK primary care guide for the adult 2020, Western Cape edition. 2020. https://knowledgetranslation.co.za/pack/wc-south-áfrica/ (accessed 7 February 2022). [ Links ]
33. Folb N, Timmerman V, Levitt NS, et aL Multimorbidity, control and treatment of non-communicable diseases among primary healthcare attenders in the Western Cape, South Africa. S Afr Med J2015;105(8):642-647. https://doi.org/10.7196/SAMJnew.7882 [ Links ]
34. Western Cape Department of Health, South Africa. Antimicrobial Stewardship Training Manual. November 2018. Reference No.. 18/2/10/2. [ Links ]
35. Steyn K, Lombard C, Gwebushe N, et aL Implementation of national guidelines, incorporated within structured diabetes and hypertension records at primary level care in Cape Town, South Africa. A randomised controlled trial. Glob Health Action 2013;6:20796. https://doi.org/10.3402/gha.v6i0.20796 [ Links ]
36. Soetedjo NN, McAllister SM, Ugarte-Gii C, et al Disease characteristics and treatment of patients with DM attending government health services in Indonesia, Peru, Romania and South Africa. Trop Med Int Health 2018,23(10)-.1118-1128. https://doi.org/10.1111/tmi.l3137 [ Links ]
37. Haugbolie LS, Sorensen EW Drug-related problems in patients with angina pectoris, type 2 diabetes and asthma - interviewing patients at home. Pharm World Sci 2006,28(4).239-247. https://doi.org/10.1007/si1096-006-9023-9 [ Links ]
38. Mechessa DF, Kebede B. Drug-related problems and their predictors among patients with diabetes attending the ambulatory clinic of Gebre Tsadik Shawo General HospitaL Southwest Ethiopia. Diabetes Metab Syndr Obes 2020-13.3349-3357. https://doi.org/10.2147/DMSO.S267790 [ Links ]
39. Rose SA, Turchin A, Grant RW Meigs JB. Documentation of body mass index and control of associated risk factors in a large primary care network. BMC Health Serv Res 2009,9(1).236. https://doi.org/10.1186/1472-6963-9-236 [ Links ]
40. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 DM. Int J Mol Sci 2020;21(17):6275. https://doi.org/10.3390/ijms21176275 [ Links ]
41. Filippi A, Bianchi C, Parazzini F, Cricelii Q Sessa E, Mazzaglia G. A national survey on aspirin patterns of use and persistence in community outpatients in Italy. Eur J Cardiovasc PrevRehabii 2011,18(5).695-703. https://doi.org/10.1177/1741826710397850 [ Links ]
42. Pinchevsky Y, Butkow W Raai FJ, Chirwa T. The implementation of guidelines in a South African population with type 2 diabetes. J Endocrinol Metab Diabetes S Afr 2013;18(3):154-158. https://doi.org/10.1080/22201009.2013.10844554 [ Links ]
43. Rampersad K, Rangiah S, Kendon M. Compliance with local diabetic guidelines at a district hospital in KwaZulu-Natal, South Africa. S Afr Fam Pract 2019-,61(2):60-64. https://doi.org/10.1080/20786190.2018.1507565 [ Links ]
44. Pirkle CM, Dumont A, Zunzunegui MV. Medical recordkeeping, essential but overlooked aspect of quality of care in resource-limited settings. Int J Qual Health Care 2012,24(6).564-567. https://doi.org/10.1093/intqhc/mzs034 [ Links ]
45. Strain WD, Blüher M, Paidánius P. Clinical inertia in individualising care for diabetes. Is there time to do more in type 2 diabetes? Diabetes Ther 2014,5(2).347-354. https://doi.org/10.1007/s13300-014-0077-8 [ Links ]
46. Govender RD, Gathiram P, Panajatovic M. Poor control and management of type 2 DM at an under-resourced South African hospital. Is it a case of clinical inertia? S Afr Fam Pract 2017,59(5).154-159. https://doi.org/10.1080/20786190.2017.1307909 [ Links ]
47. Mash R, Levitt NS, van Vuuren U, Martell R. Improving the annual review of diabetic patients in primary care. An appreciative inquiry in the Cape Town District Health Services. S Afr Fam Pract 2008,50(5).50-50d. https://doi.org/10.1080/20786204.2008.10873764 [ Links ]
48. Coomber P, Clavarino A, Ballard E, Luetsch K. Doctor-pharmacist communication in hospitals. Strategies, perceptions, limitations and opportunities. Int) Clin Pharm 2018,40(2).464-473. https://doi.org/10.1007/ S11096-018-0592-1 [ Links ]
49. Pillay S, Lutge E, Aldous C. The burden of DM in KwaZulu-Natal's public sector: A 5-year perspective. S Afr Med J 2016;106(4):384-388. https://doi.org/10.7196/SAMJ.2016.v106i4.9920 [ Links ]
50. Bobbins AC, Burton S, Fogarty TL. Different models of pharmaceutical services and care in primary healthcare clinics in the Eastern Cape, South Africa. Challenges and opportunities for pharmacy practice. Afr J Prim Health Care Fam Med 2020,12(1):2323. https://doi.org/10.4102/phcfm.vl2il.2323 [ Links ]
51. Nyamazana TN, Manyama TM, Tshitake RT. A review on the prevention and management of DM complications and the role of the pharmacist. S Afr Pharm J 2020,87(4).22-25. https://hdl.handle.net/10520/ejc-mp_sapj-v87-n4-a7 [ Links ]
 Correspondence:
Correspondence:
F Sonday
fsonday786@hotmail.com
Accepted 9 March 2022














