Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.6 Pretoria Jun. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i6.16079
RESEARCH
Diagnostic performance of dobutamine stress echocardiography: A South African experience
L SchermanI; C CilliersI; D OdendaalII; J A SaaimanIII; M J HeradienIV, V; A P DippenaarVI; Y SwartVII; J LouwVII; P A BrinkVIII, IX; P van der BijllX
IBTech; SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
IIMSc; SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
IIIMB ChB, MMed (Int Med); SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
IVMMed (Int Med), PhD; SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
VMMed (Int Med), PhD; Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VIMB ChB, MMed (Int Med); SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
VIIRN; SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
VIIIMMed (Int Med), PhD; SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
IXMMed (Int Med), PhD; Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
XMMed (Int Med), PhD; SA Endovascular, Kuils River Netcare Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND. Dobutamine stress echocardiography (DSE) is a well-established modality for the diagnosis of coronary artery disease, but there are no reported diagnostic data in southern Africa.
OBJECTIVES. To compare the safety, sensitivity and specificity of a South African (SA) DSE programme with larger, international series.
METHODS. All patients undergoing DSE from 2019 to 2021 at a single SA centre were included. A new wall motion abnormality (>2 segments) signified inducible ischaemia.
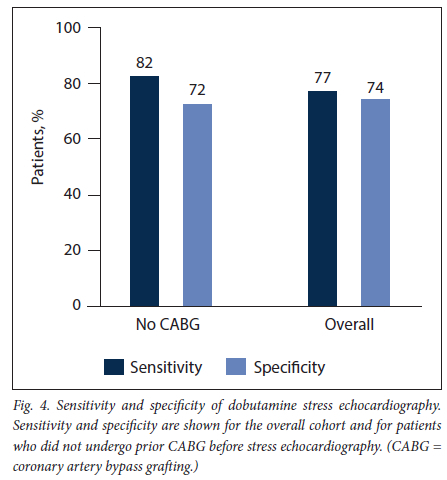
RESULTS. A total of 106 patients (mean (standard deviation) age 61 (11) years, 68% male) were analysed. Six patients (6%) experienced chest pain during DSE and 4 (4%) developed an atrial arrhythmia. The sensitivity and specificity for epicardial coronary stenosis were 77% and 74%, respectively, changing to 82% and 72% when excluding those who had previous coronary artery bypass surgery.
CONCLUSION. The sensitivity, specificity and safety of an SA DSE programme were comparable to international series. A DSE programme is feasible in a resource-constrained environment.
Dobutamine stress echocardiography (DSE) is a well-established modality for the diagnosis and risk stratification of coronary artery disease, and has demonstrated high sensitivity and specificity, as well as an excellent safety record, in large meta-analyses.[1]
The use of DSE is not yet widespread in southern Africa, and there are no published data on the diagnostic performance of DSE in this geographical region. Since DSE is more cost-effective than other non-invasive diagnostic modalities for the identification of inducible ischaemia, e.g. adenosine stress cardiac magnetic resonance imaging and single-photon emission computed tomography, demonstrating its appropriate use in a resource-constrained environment is highly relevant.[2]
Objectives
The objective of our study was therefore to compare the safety sensitivity and specificity of a South African (SA) DSE programme for the diagnosis of epicardial coronary artery disease with larger, international series.
Methods
Study population and data collection
All adult patients aged >18 years who underwent routine DSE and invasive coronary angiography from 2019 to 2021 at a single centre (SAEndovascular, Kuils River Netcare Hospital, Cape Town, SA) were included from an ongoing clinical registry. Patients with non-diagnostic tests (e.g. suboptimal echocardiographic windows), implantable cardiac devices (including where pacing stress echocardiography was performed) and DSE for any indication (e.g. distinguishing severe from pseudosevere aortic stenosis or viability testing) other than the diagnosis of inducible ischaemia were excluded (Fig. 1). Written informed consent was obtained pre-procedure for all patients undergoing DSE and invasive coronary angiography. The study protocol was approved by the Health Research Ethics Committee, Faculty of Medicine and Health Sciences, Stellenbosch University (ref. no. N20/10/106) and the Netcare Research Operations Committee (ref. no. UNIV-2021-0038).
DSE protocol and safety endpoints
Baseline transthoracic echocardiography was performed on all patients in the left lateral decubitus position with commercially available echocardiographic equipment (Vivid E95; General Electric Vingmed Ultrasound, USA), and imaging data were acquired with an M5S transducer (General Electric Vingmend Ultrasound). Depth and gain settings were optimised as required, and electrocardiogram (ECG)-triggered and two-dimensional data were digitally archived for off-line analysis (EchoPac 202; General Electric Vingmed Ultrasound). DSE was performed with continuous ECG and oximetry monitoring and dobutamine administered intravenously via a cannula into an antecubital or other suitable vein. Sphygmomanometric blood pressure recordings were made during each stage of the protocol. The DSE protocol comprised four stages, i.e. 'baseline', 'low dose' (typically acquired at a dobutamine infusion rate of 10 μg/kg/min), 'peak dose' (typically at a dobutamine infusion rate of 40 μg/kg/min), and 'recovery' (typically 3 minutes after the cessation of the dobutamine infusion).[3] Each stage consisted of acquisition of parasternal long- and short-axis views, as well as apical 4-, 2- and 3-chamber views. Three R-R intervals were saved digitally for every view in every stage of the protocol. Diagnostic endpoints were: (i) administration of the maximum dose of dobutamine; (ii) achievement of the target heart rate ((220 - age) χ 0.85 bpm); (iii) the presence of a new regional wall motion abnormality in at least two contiguous left ventricular segments; and (iv) chest pain typical of angina pectoris or ECG changes indicating myocardial ischaemia.[3] If the target heart rate could not be achieved with the maximal dose of dobutamine (40 μg/ kg/min), intravenous atropine sulphate was administered while maintaining the maximal dobutamine infusion rate, starting with a 0.25 mg atropine bolus, up to a maximum cumulative dose of 2 mg of atropine.'31 Nondiagnostic endpoints were: (;) intolerable symptoms; (ii) severe hypertension or hypotension; (iii) persistent supraventricular arrhythmias; and (iv) persistent ventricular arrhythmias. The test was concluded when: (t) the heart rate decreased to within 20 bpm of baseline; and (ii) all new regional wall motion abnormalities had resolved.[3] Segmental wall motion was analysed according to a 16-segment model, and was considered indicative of inducible ischaemia if >2 myocardial segments demonstrated a new wall motion abnormality (Fig. 2).[2,3] The inter-observer agreement was assessed on 20 randomly selected patients by means of Cohen's κ. There was substantial agreement, with κ=0.80 (95% confidence interval (CI) 0.54 - 1.06; p<0.001).
Invasive coronary angiography
Invasive coronary angiography was performed via radial or femoral arterial access, and the percentage vessel stenosis was assessed visually. An epicardial (native/saphenous vein graft/arterial graft) coronary artery lesion was deemed significant if it was >50% of the luminal diameter. Multivessel coronary artery disease was defined by a >50% luminal diameter lesion in more than one epicardial coronary artery or bypass graft. Successfully revascularised lesions were equated to a 0% luminal diameter stenosis. The inter-observer agreement was assessed on 20 randomly selected patients by means of Cohen's κ. Substantial agreement was found, with κ=0.79 (95% CI 0.52 - 1.06; p<0.001). Percutaneous treatment of epicardial coronary artery and bypass graft lesions was performed at the discretion of the operator.
Statistical analysis
Normality was assessed by visual comparison of data histograms with a normal probability curve, as well as Q-Q plots and detrended normal Q-Q plots. Continuous data are presented as means and standard deviations (SDs) when normally distributed and as medians and interquartile ranges (IQRs) when not normally distributed. Categorical data are expressed as frequencies and percentages. Sensitivity and specificity were calculated as proportions using cross-tabulation. Cohen's κ was employed to assess inter-observer agreement. All analyses were performed with SPSS for Windows version 25.0 (SPSS, USA).
Results
Baseline patient characteristics
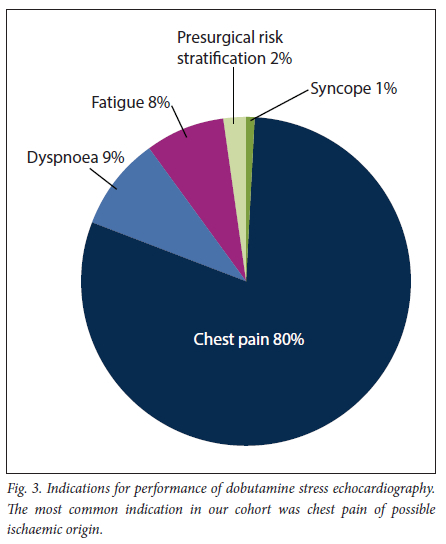
A total of 106 patients (mean (SD) age 61 (11) years, 68% male) were analysed. Of the patients, 78 (74%) had hypertension, 32 (30%) diabetes mellitus and 37 (35%) a first-degree family history of coronary artery disease, 9 (9%) were obese and 27 (25%) were smokers, 36 (34%) had received previous coronary artery bypass surgery, and 52 (49%) had undergone percutaneous revascularisation of a native or bypass vessel. The specific indications for performing DSE in our cohort are summarised in Fig. 3, with chest pain being the most frequent clinical question (80%).
DSE: Safety and diagnostic accuracy
The median (IQR) interval between DSE and invasive coronary angiography was 1 (0 - 13) month. Atropine was used in 69 patients (65%), and 6 individuals (6%) experienced chest pain during the test, while 4 (4%) developed an atrial arrhythmia. No persistent ventricular arrhythmias were recorded in the study population during DSE.
A total of 56 instances of inducible ischaemia were detected: none (0%) in the left mainstem territory, 13 (23%) in the left anterior descending coronary artery territory, 17 (30%) in the circumflex coronary artery territory and 26 (47%) in the right coronary artery territory. Fifty-five epicardial coronary artery lesions were visualised on invasive coronary angiography: none (0%) in the left mainstem territory 15 (27%) in the left anterior descending coronary artery territory 19 (35%) in the circumflex coronary artery territory and 21 (38%) in the right coronary artery territory. Eight patients (8%) had evidence of multivessel coronary artery disease. The sensitivity and specificity of DSE for detecting epicardial coronary stenosis were 77% and 74%, respectively (Fig. 4). Sensitivity improved to 82% and specificity decreased to 72% when patients who had undergone coronary artery bypass surgery were excluded.
Discussion
DSE for the diagnosis of epicardial coronary artery disease
Stress echocardiography may be used for both the diagnosis and the prognostication of coronary artery disease, and may be performed either with exercise or with a pharmacological stressor. While an exercise ECG remains the first-line investigation in most patients for the detection of inducible ischaemia, an alternative, non-invasive test is required if an exercise ECG is not feasible (e.g. patient deconditioning), uninterpretable (e.g. resting ST-segment abnormalities on ECG), incompletely performed (i.e. a submaximal effort by the patient) or inconclusive.[2] Dobutamine is commonly used as a pharmacological stressor, and causes myocardial ischaemia by increasing oxygen demand via stimulation of inotropy and chronotropy.[2] In order to detect ischaemia, dobutamine is administered as a continuous intravenous infusion, starting at a dose of 5 μg/kg/min and increasing every 3-5 minutes in increments, until a maximum dose rate of 40 μg/kg/min has been achieved. If a dobutamine infusion alone is insufficient to achieve the estimated target heart rate, intravenous atropine sulphate may be administered, which facilitates a higher heart rate owing to its parasympatholytic effect. The use of atropine increases the sensitivity of DSE without a loss of specificity.'1,41 DSE is a safe, reproducible test with a class I indication from the European Society of Cardiology.'2,51 Standardised guidelines on the indications and performance of DSE have been published.'2,3
Safety of DSE
A number of unwanted effects have been reported with DSE, including myocardial ischaemia, severe hypertension and hypotension, arrhythmias (supraventricular and ventricular), palpitations, urinary urgency, anxiety, nausea and death.[2] Serious adverse events occur at a rate of 3:1 000, which agrees closely with the incidence of safety endpoints reached in our study.[6]
Diagnostic accuracy of DSE
A wide range of sensitivities (54 - 96%) and specificities (62 -93%) have been reported for DSE.[1, 2] In a large (>35 000 patients) meta-analysis (where both sensitivity and specificity for inducible ischaemia were reported as 79%), mean age, gender and the use of beta-adrenoreceptor antagonists affected neither the sensitivity nor the specificity of DSE, and we therefore did not stratify our analysis according to age, gender or the use of beta-adrenoreceptor antagonists.[1] In addition, in the abovementioned meta-analysis, no difference was found in the sensitivity of DSE for the detection of epicardial coronary artery disease when a 50% diameter stenosis or a 70% diameter stenosis was used as a threshold on invasive coronary angiography.[1] Owing to the limited number of patients in our study we therefore opted for a 50% diameter stenosis, so as to increase the number of angiographic lesions available for analysis. Even though blinding of the results of coronary angiography does not appear to alter the sensitivity of DSE, we calculated the inter-observer variability, since it is the first report of DSE data from the current patient cohort.[1] Wall motion abnormalities on baseline echocardiography increase sensitivity and decrease specificity, and directly imply the presence of coronary artery disease in the appropriate context, and we excluded all patients with baseline wall motion abnormalities (in whom DSE was performed for myocardial viability assessment) for the calculation of sensitivity and specificity values.[3]
Implications for southern African cardiology
DSE is not yet widely used in sub-Saharan Africa, and as far as the authors are aware, there are no reported data on the diagnostic performance of DSE in southern Africa. Two series from West Africa have been published.[7, 8] Although DSE was found to be feasible in these West African reports, no data on test accuracy were reported.[7] Since DSE is more cost-effective than other non-invasive diagnostic modalities for the identification of inducible ischaemia, e.g. adenosine stress cardiac magnetic resonance imaging and single-photon emission tomography (which may also be limited in their availability), it is a highly relevant modality in the armamentarium of a clinician working in a resource-constrained environment. We have demonstrated that the establishment of a successful DSE programme is feasible in a southern African context, and we advocate for the proliferation of such programmes. Potential obstacles are the absence of a specific remuneration code in private practices, and the dedicated training that has to be undertaken before DSE can be performed safely and interpreted accurately[3]
Study limitations
This was a single-centre, retrospective analysis. Referral bias (retrospective identification of patients who underwent invasive coronary angiography) is inherent in the study design.'11 The accuracy of myocardial viability DSE could not be evaluated owing to the absence of systematic performance of a gold-standard test, e.g. late gadolinium-enhanced cardiac magnetic resonance imaging or positron emission tomography. Echocardiographic contrast media are not yet available in SA, and patients in whom endocardial border visualisation was inadequate underwent adenosine stress cardiac magnetic resonance imaging or single-photon emission computed tomography to evaluate inducible ischaemia. Although three-dimensional echocardiography data were acquired in some patients, this was not performed systematically, and we have not analysed or reported the results. Owing to the limited follow-up time, no outcome data are available yet.
Conclusions
The sensitivity, specificity and safety of an SA DSE programme were comparable to large, international series. A successful DSE programme is feasible in a local, resource-constrained environment.
Declaration. None.
Acknowledgements. None.
Author contributions. LS: conception and design of the study collection, analysis and interpretation of data, drafting of the manuscript, final approval of the manuscript; CC, DO, JAS, MJH, APD, YS, JL, PAB: conception and design of the study, revision of the manuscript, final approval of the manuscript; PvdB: conception and design of the study collection, analysis and interpretation of data, drafting of the manuscript, final approval of the manuscript.
Funding. None.
Conflicts of interest. MJH received financial support from Medtronic and is a Hamilton Naki scholar. The remaining authors have nothing to disclose.
References
1. Geleijnse ML, Krenning BJ, van Dalen BM, et al. Factors affecting sensitivity and specificity of diagnostic testing. Dobutamine stress echocardiography. J Am Soc Echocardiogr 2009,22(11). 11991208. https://doi.org/10.1016/j.echo.2009.07.006 [ Links ]
2. Sicari R, Nihoyannopoulos P, Evangelista A, et al Stress Echocardiography Expert Consensus Statement - Executive Summary. European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur Heart J 2009:30(3).278-289. https://doi.org/10.1093/eurheartj/ehn492 [ Links ]
3. Pellikka PA, Arruda-Olson A, Chaudhry FA, et al. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease. From the American Society of Echocardiography J Am Soc Echocardiogr 2020,33(1).1-41.e48. https://doi.org/10.1016/j.echo.2019.07.001 [ Links ]
4. Ling LH, Pellikka PA, Mahoney DW, et al. Atropine augmentation in dobutamine stress echocardiography. Role and incremental value in a clinical practice setting. J Am Coll Cardiol 1996,28(3).551-557. https://doi.org/10.1016/0735-1097(96)00195-7 [ Links ]
5. Task Force Members. Montalescot G, Sechtem U, Achenbach S, et al 2013 ESC guidelines on the management of stable coronary artery disease. The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013,34(38).2949-3003. https://doi.org/10.1093/eurheartj/eht296 [ Links ]
6. Secknus MA, Marwick TH. Evolution of dobutamine echocardiography protocols and indications. Safety and side effects in 3,011 studies over 5 years. J Am Coll Cardiol 1997,29(6).1234-1240. https://doi.org/10.1016/S0735-1097(97)00039-9 [ Links ]
7. Mbaye A, Yameogo NV, Ndiaye MB, et al [Screening of silent myocardial ischaemia by dobutamine stress echocardiography among type 2 diabetics at high cardiovascular risk in Senegal.J Ann Cardiol Angeiol (Paris) 2011,60(2).67-70. https://doi.org/10.1016/j.ancard.2010.07.001 [ Links ]
8. Sarr EM, Dieye O, Adama S, et al. Contribution of dobutamine stress echocardiography in the management of advanced ischemic and valvular heart diseases in Senegal. J Cardiovasc Thorac Surg 2017,2(4).1-5. https://doi.org/10.15226/2573-864X/2/4/00124 [ Links ]
 Correspondence:
Correspondence:
L Scherman
ariskascherman@yahoo.com
Accepted 2 February 2022














