Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 n.5 Pretoria May. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i5.16277
RESEARCH
Where have we come from and where are we going? The paediatric HIV programme in Johannesburg, South Africa, from 2004 to 2018: A retrospective analysis of programme trends
J L DunlopI, II; R R LilianIII; C L TaitIV; M MabitsiV; H StruthersVI, VII; J A MclntyreVIII, IX; K ReesX, XI
IMB BCh, MSc (Med) ; Anova Health Institute, Johannesburg, South Africa
IIMB BCh, MSc (Med) ; Department of Community Paediatrics, School of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMSc (Med); Anova Health Institute, Johannesburg, South Africa
IVMB ChB; Anova Health Institute, Johannesburg, South Africa
VMB BCh; Anova Health Institute, Johannesburg, South Africa
VIMSc, PhD; Anova Health Institute, Johannesburg, South Africa
VIIMSc, PhD; Division ofInfectious Diseases and HIV Medicine, Department ofMedicine, Faculty of Health Sciences, University of Cape Town, South Africa
VIIIMB ChB; Anova Health Institute, Johannesburg, South Africa
IXMB ChB; School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
XMB BCh Anova Health Institute, Johannesburg, South Africa
XIMB BChSchool of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. The paediatric HIV treatment programme in South Africa (SA) has grown since its inception in 2004. Despite this impressive scale-up of antiretroviral therapy (ART) in children, the proportion of children started on ART and retained in care remains unacceptably low, with only 47% of the 340 000 HIV-positive children in SA on ART in 2020. Johannesburg is one of the districts in SA with the largest number of children living with HIV who are not on ART, and is a priority district for paediatric case finding and retention.
OBJECTIVES. To describe the dynamics of the paediatric HIV programme in Johannesburg, SA.
METHODS. A secondary analysis was conducted on patient-level HIV treatment data from TIER.Net, the nationally mandated HIV/ART database. Children aged <15 years who received ART between January 2004 and June 2019 at public health facilities in Johannesburg were included. We reported the number of children on ART and the number who entered and exited the programme by age group over time, and analysed the trends of these indicators.
RESULTS. By December 2018, 7 630 children aged <15 years remained in Johannesburg's paediatric ART programme: 82.5% were aged 5 - <15 years, with 54.1% of these being 10 - <15 years old. During the study period, 19 850 children were newly initiated on ART. New initiations slowed from 2013, to range from 1 172 to 1 373 yearly. In 2018, 34.2% of initiators were aged <1 year, 24.2% 1 - <5 years and 41.6% 5 - <15 years. Despite these initiations, the number of children on ART only grew by 97 in 2018, owing to programme losses. In 2018, 924 children (12.1%) aged out, 35 (0.5%) died and 983 (12.9%) were lost to follow-up (LTFU), the latter having increased from 10.7% in 2017. Of children who aged out of the paediatric ART programme, 56.3% remained in care at the same facility.
CONCLUSION. Early in the SA ART roll-out, many children were found to be HIV infected and started on ART. This number started to slow in 2013, after which the growth rate of the paediatric HIV programme also began to slow. Scale-up of methods for identifying older children with HIV is needed. While ageing out of the paediatric programme is a consideration,the number of children LTFU remains unacceptably high. Further interrogation of barriers to paediatric retention is needed to help realise the Joint United Nations Programme on HIV and AIDS (UNAIDS) 90:90:90 goals for children in SA.
In 2004, South Africa (SA) began what has since become the world's largest antiretroviral therapy (ART) programme. Identifying and treating children with HIV is more complex than in adults, [1,2] and, ever since inception, children have lagged behind. By 2019, it was estimated that only 47% of the 340 000 HIV-positive children aged <15 years living in SA were receiving ART[3] and, despite universal access to ART, more than half of the HIV-positive children in SA were still not in care.
Around one-third of HIV-infected infants will die in the first 2 years without access to ART.[4] Much focus has therefore been placed on early infant diagnosis (EID) and linkage to ART. However, prevention of mother-to-child transmission (PMTCT) and EID strategies were historically not as robust as they have been more recently. Given the low HIV testing rates in children aged >5 years, many perinatally infected children remain untested[1,2,5]
Following diagnosis, HIV-positive children then need to be linked to ART. While initiation of ART on the same day is recommended[6] barriers such as lack of healthcare worker confidence in managing these children remain a challenge.[7] Lastly, retaining children in care and ensuring adherence to ART are more challenging than in adults. This is supported by an analysis of the long-term outcomes of children and adolescents in the IeDEA-Southern Africa Collaboration, which showed that adolescents with perinatally acquired HIV had suboptimal retention and adherence to ART during adolescence,[8] attributed to more frequent clinic visits as well as delaying disclosure to the child.[5,9,10] Lower retention and adherence affect virological suppression, which is the goal of ART.
The paediatric ART programme in SA includes all children from birth to <15 years of age who receive ART. The majority of children who receive ART do so from public health facilities, while a small number may access ART through private healthcare.
TIER.Net, an electronic patient database, is used to monitor and report on the programme in public health facilities.[11] Children living with HIV (CLHIV) enter a facility's paediatric ART programme by being initiated on ART following an HIV-positive diagnosis, by re-initiating ART after having interrupted treatment, or by transferring in from another facility or district where they were already receiving ART. Children leave the ART programme by moving into the adult programme when they reach the age of 15 (ageing out), transferring out to another facility, being lost to care, or dying. Ageing out is a factor specifically relevant to the paediatric HIV programme. Being lost to care, otherwise known as lost to follow-up (LTFU), means that a CLHIV leaves care and probably stops taking ART. This cohort may be over-estimated, with clients opting to move or 'self-transfer' to other facilities without notifying their previous facility.[12]
Objectives
As SA strives to achieve the Joint United Nations Programme on HIV and AIDS (UNAIDS) 90-90-90 targets, there is increasing acknowledgement of the need to focus on important subgroups such as CLHIV.[13] An understanding of the dynamics of the paediatric ART programme can tailor interventions to achieve these HIV care and treatment goals. This analysis aims to describe how a large public sector paediatric ART programme has changed over time, with shifting guidelines and dynamics. Using Johannesburg TIER. Net data, we aimed to describe the trends of the paediatric HIV programme in Johannesburg since its inception in 2004.
Methods
Study design
A secondary analysis was conducted on patient-level HIV treatment data from TIER.Net.
Setting
Johannesburg district, situated in Gauteng Province, is the most populous metropolitan area in SA,[14] with a population density of 3 200 people per km2. The district had an estimated ~14 800 CLHIV in September 2021, which is the second-highest estimated number of CLHIV in the country.1141 Johannesburg also ranks below average for ART coverage in CLHIV <15 years compared with the rest of the country, 44% compared with 51%.[15] In Johannesburg, this amounts to ~8 200 untreated CLHIV. Of all deaths of children aged 5 - 14 years in Johannesburg, ~10% are attributed to HIV-related causes.[14]
In order to monitor the HIV programme in SA, TIER.Net, the nationally mandated HIV programme database, is used by public health facilities to gather relevant visit information for each client in care.[11] This information is used by the Department of Health (DoH) for routine programme monitoring. Indicators that are prioritised include the overall size of the programme (number of children on ART) and new initiations in children aged <15 years.
Anova Health Institute is the President's Emergency Plan for AIDS Relief (PEPFAR)/United States Agency for International Development (USAID)-funded district support partner in Johannesburg and supports the DoH in providing care and treatment to HIV-positive children.
Record inclusion
TIER.Net records of all children aged <15 years who received ART between 1 January 2004 and 30 June 2019 at public health facilities in Johannesburg were included in the analysis.
Data processing
The Johannesburg district June 2019 TIER.Net export was used for the analysis. Records that had no ART start date available, as well as those for adults aged >15 years, were excluded from the analysis. Records that appeared to be incorrectly reported as children on the database were also excluded. These were identified where the date of birth was incorrectly recorded as being the same as the date of starting ART. Lastly, data quality of the remaining records was reviewed and records where there were crucial missing data or data quality concerns were removed. We then analysed our dataset of records of 30 733 children aged <15 years who were active in the paediatric ART programme (Fig. 1).
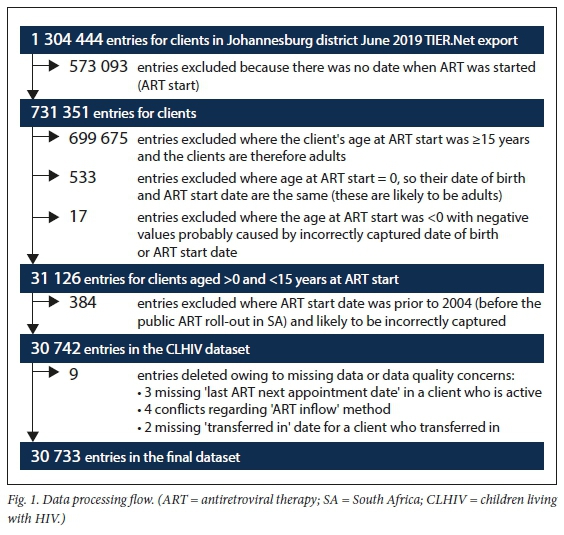
Measures
The following measures were used to establish how the paediatric ART programme has changed over time:
• the number of children who were active on ART at each time point (active on ART is defined as children who have not been transferred out, LTFU, aged out or died)
• mechanisms of how children entered the ART programme: newly initiated, re-initiated or transferred in (Fig. 2)
• mechanisms of how children left the paediatric ART programme: aged out, transferred out of the facility, LTFU or died (Fig. 2).
Analysis
The total number of children on ART at the end of each quarter, between January 2004 and December 2018, was reported. Children aged <15 years during the quarter were included in the paediatric ART programme, while those aged >15 years were considered to have aged out. Quarter 1 was the first 3 months (January - March) of the given year, with the other quarters following.
For each HIV programme entry and exit method, we calculated the frequency and percentage of records for each time period and compared these.
All data were disaggregated by age group (<1 year, 1 - <5 years, 5 -<10 years, 10 - <15 years).
The number of ART initiations and children on ART were aggregated to annual figures for reporting from 2004 to 2018.
The frequency and percentage of each entry and exit method was presented quarterly for 2017 and 2018.
For those who were started on ART at age <15 but aged out during the study period, we report the number of those clients still in care at the same facility but considered to be in adult care.
Ethical considerations
Permission was granted to conduct this analysis through the Human Sciences Research Council Ethics Committee (ref. no. REC 3/22/08/18).
Results
Initiations and other programme inflow
Overall, 19 850 children aged <15 years were newly started on ART in Johannesburg from January 2004 to December 2018. The number of initiations peaked in 2010, with 1 812 children initiated that year. Thereafter, initiations plateaued between 1 172 and 1 373 children per year.
The highest proportion of children initiated was in the 1 - <5-year group (30.1%; n=5 981), followed by 5 - <10 years (25.9%; n=5 137), <1 year (24.2%; n=4 813) and lastly 10 - <15 years (19.7%; n=3 919). The proportions varied over time, with the number of children aged <1 year initiating ART increasing from 6.5% to 34.2% and children aged 10 - <15 years increasing from 12.7% to 24.5% between 2004 and 2018, while the proportions of children initiated in the 1 - <5- and 5 " >10"year age groups declined (Fig. 3). In 2018, 58.4% of initiations were taking place in children aged <5 years.
From January 2017 to December 2018, -64% of children who entered the Johannesburg paediatric ART programme were newly initiated (Fig. 4). A high proportion of children also transferred into the ART programme, ranging from 31.5% to 44.8%. A minority of children were restarted on ART (5.2 - 9.8%).

Total children on ART and programme growth
At the end of December 2004, 221 children aged <15 years were receiving ART. By December 2018, the number of children on ART had increased to 7 630 (Fig. 5). This number initially grew rapidly until 2012, with annual growth rates in excess of 10%, and then more slowly until 2016, after which the annual growth rate slowed to <5% (Fig. 5). In 2018, the rate of paediatric programme growth was 1%, with the programme growing by 97 children.
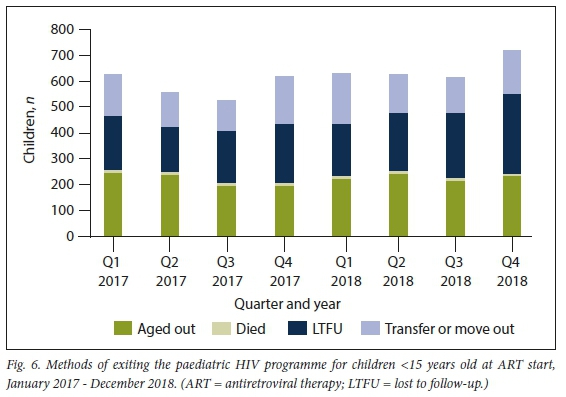
The slow growth, despite the steady rate of initiations, was due to children exiting the programme, a phenomenon that is increasing over time (Fig. 6).
In 2004, the proportion of children aged >10 years receiving ART was low (8.1%; ;1=18) compared with later periods. This proportion changed as the programme matured, and at the end of 2018, 82.5% of the children on ART were 5 15> " years of age (u=6 293), with 54.1% being >10 years old (;1=4 124). The proportions of children on ART who are 10 15> " years old has increased in recent years, while the number aged 5 10> " years has been decreasing, probably as those children age and vertical transmission declines.
Methods of paediatric HIV programme exit
In 2018, 2 581 children left the paediatric ART programme. Of these, 924 children (35.8%) aged out, 639 (24.8%) transferred out, 35 (1.4%) died and 983 (38.1%) were LTFU (Fig. 6). Of the total children on ART in 2018, 12.9% were LTFU, which increased from 10.7% in 2017. While the absolute number of children who aged out increased from the year before (;1=889), the proportion of the outflow due to ageing out did not increase, from 38.2% in 2017 to 35.8% in 2018. The outflow due to ageing out represents -12% of the children who were receiving ART in the paediatric programme in 2018.
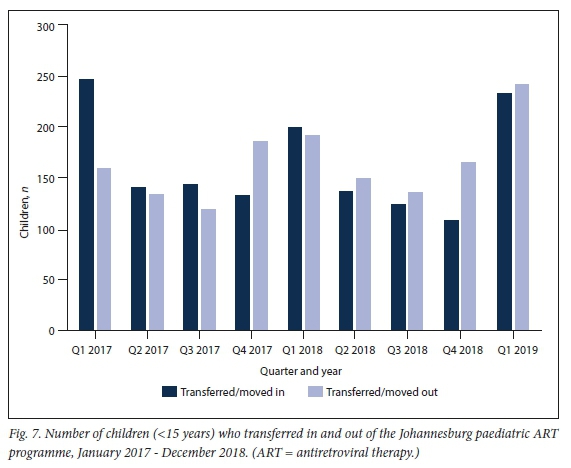
Transfer in and out dynamics
During the period 2017 2018 ", the number of children who transferred in was 1 224, with 1 233 transferring out (Fig. 7).
Outcomes of children who started ART at <15 years and aged out of the paediatric programme
Of children who aged out of the paedia-trie programme (11=7 960), 4 478 (56.3%) remained in care at the same facility at the end of June 2019.
Discussion
Summary of principal findings
• There has been a substantial increase in the number of children in the paediatric ART programme in Johannesburg since the start of the ART roll-out in 2004.
• Since 2011, as a result of children exiting the programme, the number of children on ART in Johannesburg has not increased despite new children being initiated on ART.
• Currently, -80% of children on ART are between 5 and <15 years old, which represents maturing of the paediatric HIV population combined with the successes of the PMTCT programme.
• More than 50% of new initiations still take place in children aged <5 years.
• Of children who aged out of the paediatric ART programme, 56.3% remained in care at the same facility.
With almost 120 000 children and adolescents aged <15 years living with HIV in SA still not accessing ART,[3] particular emphasis on increasing case finding and retention in care for this population is required. Better understanding of the dynamics of the paediatric HIV programme is therefore needed. The present study addresses this need by providing an in-depth description of a large paediatric ART programme over >10 years using routine data. The findings from this study can be used to inform strategies to increase case finding and retention in care for children and adolescents living with HIV.
Initiations and programme inflow
The paediatric HIV programme in Johannesburg has grown substantially since its inception in 2004, when the SA ART roll-out began in the public sector.[2] The programme grew rapidly from 2004 to 2011, after which the number of initiations per year stabilised.
In 2004, most of the children who entered the ART programme were 1 10> " years of age. The small number of infants was probably due to the difficulty of diagnosing infants at that time, as HIV polymerase chain reaction testing was not routinely available.[16] There were also few adolescents aged 10 " <15 years being initiated on ART, as the epidemic in SA was not mature enough for large numbers of perinatally infected children to have reached that age group.
By 2018, with improvements in PMTCT and EID, and the widespread access to and provision of ART, the proportions of infants and adolescents 10 15> " years of age initiated on treatment had increased; however, under half of all initiations still took place in those 5 15> " years old. A gap still remains in identifying and treating CLHIV 5 15 > " years of age, who make up >80% of untreated CLHIV in Johannesburg[15] Case finding in this group requires prioritisation, and healthcare workers need to be aware that older children who are mildly ill or asymptomatic can still be HIV infected and need to be screened and tested for HIV. This need can be addressed using strategies in the health facility, such as index-contact HIV testing services and screening children and adolescents who enter health facilities via key entry points, including acute care and tuberculosis care, as well as in the community, such as community index-contact testing and school-based HIV screening and testing.[15] For all these strategies, it is important that healthcare workers are well equipped to conduct HIV testing in children and adolescents.[17] Self-screening for HIV is another possible modality for improving HIV testing rates in children >12 years of age, as HIV self-screening is not recommended for use in children <12 years old in SA.[18]
Most children and adolescents who enter the ART programme in Johannesburg are newly initiated on treatment (-64% in 2017 and 2018), with fewer children and adolescents with HIV transferring into the programme or being restarted on treatment after having previously been lost to care. Nevertheless, a relatively high proportion of children transfer into the ART programme (between 31.5% and 44.8%), demonstrating the mobility of Johannesburg's population. Unfortunately, our data systems are not adequate to track those remaining in care across facilities. The number of children re-starting ART is relatively low considering the duration of ART access in SA, probably because many children re-initiating ART are captured as newly initiated owing to lack of information provided during consultation or sparse clinical notes about previous ART.[19]
Total number of children on ART
With the considerable growth in the early years of the ART programme, the slowing and then stabilising of growth in recent years has been concerning given the large projected gap in CLHIV who need to be on treatment. The lack of programme growth in recent years is probably due to slowing of case finding, combined with continued programme losses such as ageing out and, more unfavourably, LTFU.
Over 50% of children on ART in Johannesburg were aged 10 15> " years at the end of 2018. This group is largely made up of children who started ART over the past decade, and have now become adolescents who will soon age out of the paediatric ART programme. This finding suggests that paediatric HIV programme outflow due to ageing out will continue to be a considerable factor affecting the total number of children on ART in the coming years. It is therefore recommended that, instead of closely monitoring the total number of children on ART per district, it would be more useful to monitor the gap between the estimated CLHIV and the number of children on ART.
Methods of programme exit for those starting ART at age <15 years
Although ageing out leads to fewer children aged <15 years being on ART, it is the most favourable method for adolescents to leave the paediatric ART programme. Ageing out means that these children have been retained in care and have survived to reach their later adolescent and adult years.
Children may also leave a district paediatric programme by transferring out. In the Johannesburg paediatric ART programme, transfer out and transfer in have balanced out over time, with considerable overall movement in this population.
The number of children who leave the programme through LTFU or death is concerning. Of children who exit the paediatric ART programme, a third are LTFU, with an additional 1 - 2% known to have died. The LTFU cohort, however, may include undisclosed deaths and undocumented transfers, where children do not have the required or adequate documentation to be transferred, as well as disengagement from care.[12] Nyakato et aI.Mfound that 65% of children thought to be LTFU were in fact undocumented transfers to another facility. This finding cannot be verified in other districts such as Johannesburg, as Nyakato et alls study was conducted in Western Cape, the only province in SA where unique patient identifiers have been implemented to enable patient tracking across facilities.
Improving retention in care for children and adolescents on ART is a priority. Children are often more difficult to retain in care owing to their need for age-appropriate support, suboptimal, cumbersome ART formulations, and delayed disclosure of their own HIV status by caregivers.[20] Differentiated service delivery, such as providing ART through alternative pick-up
strategies, which leads to fewer clinic visits and easier ART collections for adherent clients, have been shown to improve retention in adolescents on ART.[21] In addition, losses during down-referral to other treatment sites also influence reported LTFU rates.[19] Strengthening the documentation of transfers and implementing systems to confirm that transfers have been received by the accepting health facilities are needed to reduce falsely high LTFU rates.[21] Lastly, adherence to ART and retention in care are key to reducing morbidity and mortality in treated CLHIV. Healthcare workers need to provide support to CLHIV in care, and their caregivers, to equip them with strategies to adhere to ART regimens and prevent care interruptions.
The number of children or adolescents who have died is likely to be under-represented, as the primary healthcare facility, where ART is usually provided, may not be notified when children or adolescents die in hospital or at home. In these cases, the child or adolescent maybe considered LTFU.[5,12]
Study limitations
This secondary analysis of routine patientlevel data used information collected at healthcare facilities and collated at district level. It is therefore limited to the capacity and accuracy of data capturing by facility staff, as well as recording of visit data by clinicians in client folders. In some cases, there may be concerns about completeness and accuracy.
A more detailed cohort analysis of these data would have provided better insight; however, there are inherent limitations to longitudinally analysing particular groups of clients using TIER.Net. These limitations include that only a cross-sectional analysis is possible using data exported at a time point, as visit data are overwritten at each new client visit, where fields are not available on TIER.Net, or where fields are not routinely captured.
Retention in care cannot be accurately quantified retrospectively, as clients who have disengaged for periods of time may since have returned to the ART programme and be active in care at the time of the analysis. Retention is therefore best viewed as a snapshot at a particular period in time, and is likely to have been underestimated in this study.
Conclusion
Despite considerable growth in the paedia-trie HIV programme in Johannesburg since its inception in 2004, challenges with finding HIV-positive children and adolescents and retaining them in care still remain. Case finding in children >5 years of age needs to be prioritised, considering that there are still many undiagnosed or untreated CLHIV who are at risk of serious morbidity and mortality. In order to find these children, available, evidence-based strategies should be employed, such as index testing, as well as screening and identifying children needing testing at key entry points in health facilities and at schools andwithin high-risk groups in communities such as orphans and vulnerable children and adolescents. The number of CLHIV not on ART has remained unacceptably high despite a stable number of initiations yearly. While ageing out of the paediatric programme is a consideration, the number of children LTFU remains unacceptably high. Retaining children and adolescents started on ART in the paediatric HIV programme is vital to close the gap ofuntreated CLHIV and ensure that case-finding efforts are not in vain. More focus and advocacy are needed around strategies to improve retention, clinically, psychosocially and through the use of better data systems, that will assist in achieving the UNAIDS 90-90-90 goals for children in SA.
Declaration. None.
Acknowledgements. We acknowledge the Johannesburg Health District for the use of TIER.Net data that were analysed, and the health facility managers and staff who provided and continue to care for these clients.
Author contributions. All authors contributed to the analysis and write-up of this research.
Funding. The Anova Health Institute NPC is supported by PEPFAR via USAID under Cooperative Agreement No. AID-674-A-12-00015.
Conflicts of interest. None.
References
1. Sohn AH, Bekker LG. Rethinking the challenges of paediatric HIV diagnosis. Lancet HIV 2021;8(3):e123-e124. https://doi.org/10.1016/S2352-3018(20)30270-8 [ Links ]
2. Johnson L. A model of paediatric HIV in South Africa. Centre for Infectious Disease Epidemiology and Research working paper, December 2010. http://www.cilt.uct.ac.za/sites/default/files/image_tool/images/108/Paediatric_HIV_modelling5.pdf (accessed 29 March 2022). [ Links ]
3. Joint United Nations Programme on HIV and AIDS (UNAIDS). AIDSinfo: South Africa 2020. https:// www.unaids.org/en/regionscountries/countries/southafrica (accessed 19 May 2021). [ Links ]
4. Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359(21):2233-2244. https://doi.org/10.1056/NEJMoa0800971 [ Links ]
5. Archary M, Fairlie L, Slogrove A. Current perspectives on paediatric HIV management from the Mexico International AIDS Society Conference, 2019. South Afr J HIV Med 2019;20(1):a1027. https://doi.org/10.4102/sajhivmed.v20i1.1027 (accessed 29 March 2022) [ Links ]
6. National Department of Health, South Africa. Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients. 6 September 2016. https://sahivsoc.org/Subheader/Index/ndoh-and-who-guidelines (accessed 27 October 2021). [ Links ]
7. Crowley T, Mokoka E, Geyer N. Ten years of nurse-initiated antiretroviral treatment in South Africa: A narrative review of enablers and barriers. South Afr J HIV Med 2021;22(1):a1196. https://doi.org/10.4102/sajhivmed.v22i1.1196 [ Links ]
8. Tsondai PR, Braithwaite K, Fatti G, et al. Describing the characteristics and long-term outcomes of adolescents living with perinatally acquired HIV in the IeDEA-Southern Africa Collaboration: 2004 to 2017 (WEAB0201). In: Oral Abstracts of the 10th IAS Conference on HIV Science, 21 - 24 July 2019, Mexico City, Mexico. J Int AIDS Soc 2019;22(S5):e25327. https://doi.org/10.1002/jia2.25327 [ Links ]
9. Arrivé E, Dicko F, Amghar H, et al HIV status disclosure and retention in care in HIV-infected adolescents on antiretroviral therapy (ART) in West Africa. PLoS ONE 2012;7(3):e33690. https://doi.org/10.1371/journal.pone.0033690 [ Links ]
10. Nasuuna E, Kigozi J, Muwanguzi PA, et al. Challenges faced by caregivers of virally non-suppressed children on the intensive adherence counselling program in Uganda: A qualitative study. BMC Health Serv Res 2019;19:150. https://doi.org/10.1186/s12913-019-3963-y [ Links ]
11. Osler M, Hilderbrand K, Hennessey C, et al. A three- tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc 2014;17(1):18908. https://doi.org/10.7448/IAS.17.L18908 [ Links ]
12. Nyakato P, Boulle A, Wood R, et al. Outcomes of children and adolescents living with HIV (CALHIV) considered lost to follow up (LTFU) at IeDEA-SA cohorts in the Western Cape: Linkage to Western Cape Provincial Health Data Centre records. 10th IAS Conference on HIV Science, 21 - 24 July 2019, Mexico City, Mexico. http://regist2.virology-education.com/presentations/2019/HIVPed/28a_Nyakato.pdf (accessed 29 March 2022). [ Links ]
13. Joint United Nations Programme on HIV and AIDS (UNAIDS). 90-90-90 - An ambitious treatment target to help end the AIDS epidemic. 1 January 2017. https://www.unaids.org/en/resources/documents/2017/90-90-90 (accessed 29 March 2022). [ Links ]
14. Massyn N, Day C, Ndlovu N, Padayachee T. District Health Barometer 2019/20. Durban: Health Systems Trust, December 2020. https://www.hst.org.za/publications/District%20Health%20Barometers/DHB%202019-20%20Complete%20Book.pdf (accessed 29 March 2022). [ Links ]
15. MRC Centre for Global Infectious Disease Analysis at Imperial College London, Centre for Infectious Disease Epidemiology and Research at the University of Cape Town, and Health Economics and Epidemiology Research Office (HE2RO) at the University of the Witwatersrand, in partnership with the South Africa Department of Health, South Africa National AIDS Council, UNAIDS, US Centers for Disease Control, and the Human Sciences Research Council. South Africa District HIV Estimates. HIV Data. 2020. https://www.hivdata.org.za/ (accessed 14 September 2021). [ Links ]
16. Kalawan V, Naidoo K, Archary M. Impact of routine birth early infant diagnosis on neonatal HIV treatment cascade in eThekwini district, South Africa. South Afr J HIV Med 2020;21(1):1-5. https://doi.org/10.4102/SAJHIVMED.V21I1.1084 [ Links ]
17. Van Rooyen HE, Strode A, Slack C. HIV testing of children is not simple for health providers and researchers: Legal and policy frameworks guidance in South Africa. S Afr Med J 2016;106(5):451-453. https://doi.org/10.7196/SAMJ.2016.v106i5.10484 [ Links ]
18. National Department of Health, South Africa. National HIV Self Screening Guidelines - 2018. https:// www.knowledgehub.org.za/elibrary/national-hiv-self-screening-guidelines-2018 (accessed 27 October 2021). [ Links ]
19. Copelyn J, Apolles P, Davies M-A, Eley B. Short-term outcomes of down-referral in provision of paediatric antiretroviral therapy at Red Cross War Memorial Children's Hospital, Cape Town, South Africa: A retrospective cohort study. S Afr Med J 2018;108(5):432-438. https://doi.org/10.7196/SAMJ.2018.v108i5.12855 [ Links ]
20. Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014 - July 2015. BMC Infect Dis 2017;17(1):326. https://doi.org/10.1186/s12879-017-2428-3 [ Links ]
21. Shava A, Masiye K, Chitiyo V, et al. Retention and viral suppression of children and adolescents in HIV Care, Zimbabwe. 10th IAS Conference on HIV Science, 21 - 24 July 2019, Mexico City, Mexico. http://regist2.virology-education.com/posters/2019/HIVPEDIATRICS/Shava.pdf (accessed 29 March 2022). [ Links ]
 Correspondence:
Correspondence:
JL Dunlop
dunlop@anovahealth.co.za
Accepted 26 January 2022















