Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.5 Pretoria Mai. 2022
http://dx.doi.org/10.7196/SAMJ.2022.v112i5.15910
RESEARCH
Echocardiographic features of infective endocarditis in South Africa: A prospective cohort study
A J K PecoraroI; P HerbsttII; L JoubertIII; K HassanIII; C PienaarIV, V; J TaljaardVI; H ProzeskyVI; J JansonVII; A DoubellVIII
IMMed (Int Med), PhD ; Division of Cardiology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
IIMB ChB; Division of Cardiology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
IIIMB ChB, MMed (Int Med); Division of Cardiology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
IVMB ChB, MMed (Microbiol Path); Division of Medical Microbiology, Department of Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
VMB ChB, MMed (Microbiol Path); National Health Laboratory Service, Division of Medical Microbiology, Tygerberg Hospital, Cape Town, South Africa
VIMB ChB, MMed (Int Med); Division of Infectious Diseases, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
VIIMMed (Cardiothoracic), PhD; Division of Cardiothoracic Surgery, Department of Surgery, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
VIIIMMed (Int Med), PhD; Division of Cardiology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND. Historically, infective endocarditis (IE) in South Africa (SA) was associated with the viridans group of streptococci affecting patients with underlying rheumatic heart disease (RHD). A changing IE bacteriological profile raises the question of whether the profile of underlying valvular abnormality has changed.
OBJECTIVES. To investigate the prevalence of underlying structural valve abnormalities and their aetiologies associated with IE in SA, and describe the typical imaging findings.
METHODS. The Tygerberg Endocarditis Cohort study prospectively enrolled patients with IE between November 2019 and April 2021. Patients underwent detailed transthoracic and transoesophageal echocardiography to assess their underlying cardiac and valvular structure.
RESULTS. Among 71 patients included, a predisposing endocardial abnormality was detected in 49.3%, with RHD the most common single identifiable aetiology (16.9%). The in-hospital mortality rate was similar in patients with and without a predisposing endocardial abnormality (20% v. 16.7%; p=0.72), as was the rate of embolic events (20% v. 27.2%; p=0.58). Significantly more patients with a predisposing endocardial abnormality had an indication for surgery (94.3% v. 69.4%; p<0.01). The viridans group of streptococci was more prevalent in patients with a predisposing endocardial abnormality (25.7% v. 2.7%; p<0.01). Left-sided linear vegetation size >10 mm was associated with an increased risk of in-hospital mortality (24% v. 5%; p=0.05).
CONCLUSION. We observed a marked decrease in the prevalence of RHD in this cohort of patients with IE. The viridans group of streptococci was an uncommon cause of IE in patients with no predisposing endocardial abnormality detected. The presence of a predisposing endocardial abnormality was not associated with an increased risk of in-hospital mortality or embolic events. Linear vegetation length >10 mm was associated with an increased risk of in-hospital mortality in patients with left-sided IE.
Infective endocarditis (IE), an infection of the endocardial surface of the heart or any prosthetic material within the heart, continues to be associated with significant morbidity and mortality.[1-4 Current diagnostic algorithms place a high importance on organism identification with a view to optimising antibiotic therapy.[3] A diagnosis of IE is initially suspected on the basis of clinical and imaging (echocardiography in particular) features, with some delay until microbiological confirmation.[3] During the period awaiting identification of the causative organism, patients are treated with empirical antimicrobial therapy targeted at the most common causative organisms.!31 In a number of patients, no causative organism is identified and these patients will remain on empirical therapy for the duration of treatment. The fact that some patients with IE will require empirical therapy that is determined by local causative organisms highlights the importance of accurate local data on these organisms and the need for using additional modalities to predict the most likely causative organism in the absence of microbiological confirmation.[1,3,5]
Historically, IE in South Africa (SA) has been associated with infection by the viridans group of streptococci on structurally abnormal valves.[1,61 Rheumatic heart disease (RHD) has been reported in as many as 75% of cases in cohorts of IE in SA.[6,71 However, recent studies suggest that Staphylococcus aureus and Bartonella species have emerged as the most prevalent causative organisms of IE in the region.[8-101 This change in epidemiology of the causative organism to a profile with some similarity to that of developed countries[111 raises the question of whether a change in the prevalence of structurally abnormal valves, and RHD in particular, has occurred in the SA IE population. Local experience at Tygerberg Hospital in Cape Town suggests that our IE patient cohort exhibits a larger proportion of valves without apparent underlying structural abnormality. Furthermore, referrals of patients for percutaneous mitral commissurotomy, an important local barometer of clinically significant RHD burden in the region, has declined over the past decade (our data) despite improvements in access to healthcare in the region.[121 In contrast to these findings, RHD screening studies do not suggest a decline in subclinical RHD, with a recently completed large-scale screening study of schoolgoing children in the Tygerberg referral network reporting a prevalence of subclinical RHD of up to 28.2/1 000 of the population (combined definite and borderline disease), which is similar to previous studies reporting the prevalence of subclinical RHD in SA.[13-16]
In the majority of cases, the typical imaging findings of IE can be detected by both transthoracic echocardiography (TTE) and transoesophageal echocardiography (TEE).[1] The finding of independently mobile masses or vegetations on heart valves remains the hallmark finding of IE on echocardiography (TTE/TEE).[3] Echocardiography is not only an important diagnostic modality in IE, but is also important in predicting the prognosis and therefore plays an important role in management decisions. Linear vegetation length >10 mm, periannular extension and the presence of acute severe valvular incompetence are some of the validated independent risk factors for in-hospital mortality in patients with IE.[3,17] An additional important role of echocardiography is to identify concomitant structural heart or valve disease, as this predisposes patients to IE, is included in the minor criteria for diagnosis of IE, may predict the likely causative organism, and influences the treatment options for patients who have an indication for surgery.[1,3]
Objectives
We sought to investigate the prevalence of underlying structural valve abnormalities and their aetiologies as well as associated bacteriological profiles in a prospectively sampled cohort of patients presenting with IE in our region. Anticipating different outcomes related to different underlying structural and bacteriological causes of IE, we sought to describe the typical imaging features associated with these, as well as to document important echocardiographic markers of adverse outcome in the local IE population.
Methods
Study design and participants
Consecutive patients presenting with IE[1,3] to the Division of Cardiology in the Department of Medicine at Tygerberg Hospital, Cape Town, between November 2019 and April 2021 were prospectively included in the Tygerberg Endocarditis Cohort study as previously described.[9,10] Patients with known or newly diagnosed malignancy were excluded.
The Division of Cardiology at Tygerberg Hospital is a public sector tertiary referral centre that serves a population of ~2.4 million people.[18] Patients with features of IE presenting to hospitals within the referral network are referred to Tygerberg Hospital for definitive care.[12] The Division of Cardiology is categorised as a high-volume centre for the diagnosis and management of IE.[19] All patients were managed by an endocarditis team that fulfilled all the criteria set out by current guidelines.[3]
Echocardiographic evaluation
Detailed TTE and TEE were performed in the absence of identifiable contraindications to TEE.[20,21] TTE was performed using GE Vivid S70, S5/6 and E95 machines with a 2 - 3.6 MHz transducer probe (GE 3S/4S/5S) (General Electric, USA). TEE was performed using GE Vivid S70/E95 machines with a 3 - 8 MHz probe (GE 6VT-D).
Image clips were stored in an imaging archive (GE EA) and were available for review and post processing using GE EchoPAC software. Studies were systematically reviewed by the primary investigator (AJKP).
All patients underwent detailed assessment of the underlying heart and valvular structure to identify features of structural abnormalities that may have predisposed them to the development of IE, in particular underlying RHD.[13,22-24] In patients who underwent surgery, imaging findings were correlated with the findings at surgery. Patients were assigned to two groups depending on the presence or absence of such a predisposing endocardial abnormality.
Patients in whom a predisposing endocardial abnormality was detected were further categorised into one of three predefined categories, namely RHD, prosthetic valve and non-rheumatic, the latter including congenital heart or valve disease and degenerative valve disease. The identification of underlying RHD was made on the basis of typical morphological valvular and subvalvular features of RHD.[16,23]
To ensure uniformity in measurement of vegetation size and grading of mobility, the following protocol was applied:
• All views acquired were evaluated for the presence of vegetations.
• If a vegetation was detected, the saved clip of the cardiac cycle with the most optimal visualisation of the vegetation(s) was selected.
• The clip was evaluated frame by frame to display the maximal linear length of the vegetation. This usually occurred during peak systole for the tricuspid and mitral valve and mid to end diastole for the aortic and pulmonary valves.
• Measurement of the maximum linear length and circumference was performed on the still frame of the cycle that revealed the maximum linear length.
• Where more than one vegetation was present on a valve, the largest vegetation in terms of linear length was measured and graded in terms of mobility.
• The mobility of the vegetation was graded using a four-point scale as follows:[25]
• 1 - fixed vegetation with no independent movement
• 2 - fixed vegetation at the site of attachment, but with independent movement of the free edge of the vegetation
• 3 - pedunculated vegetation with independent movement, but remaining within the chamber of attachment during systole and diastole
• 4 - prolapsing vegetation that crosses the valvular coaptation surface during the cardiac cycle.
The following predefined echocardiographic features associated with mortality and morbidity were systematically evaluated on both TTE and TEE:
• ejection fraction, left ventricular size, pulmonary artery systolic pressure
• haemodynamic effect of valve lesions[26]
• presence, size and point of attachment of vegetations
• mobility of vegetations
• presence of a flail leaflet segment(s)
• presence of periannular involvement
• presence of a pericardial effusion
• presence of myocardial or aortic wall destruction.
Additional imaging, including cardiac computed tomography with or without 18-fluorodeoxyglucose positron emission tomography (18FDG-PET) and cardiac magnetic resonance imaging, was performed at the discretion of the endocarditis team.
Microbiological assessment
A stepwise protocol for organism detection was utilised to identify the aetiological organisms associated with IE and to minimise the incidence of blood culture-negative IE (BCNIE). Further management and analysis of the samples were performed according to current guidelines.[3,9] A minimum of three sets of blood cultures per patient were collected. Each set included a BacT/ALERT FA Plus (aerobic) (bioMérieux, France) and BacT/ALERT FN Plus (anaerobic) bottle and was drawn from different peripheral sites using an aseptic technique. Additional blood cultures were collected if clinical features of infection persisted. The blood cultures were submitted to the diagnostic microbiology laboratory of the National Health Laboratory Service at Tygerberg Hospital for processing. Patients without an identified organism after 5 days using standard culture techniques were defined as having BCNIE. Broad-range polymerase chain reaction (PCR) and sequencing of 16S rRNA (for bacteria) and ITS2 (for fungi) were performed on negative blood culture bottles.
All BCNIE patients underwent venous blood analysis for additional testing, including:
• serology for detection of IgM and IgG antibodies to Bartonella henselae and Bartonella quintana, Brucella species, Coxiella burnetii, Legionella pneumophila and Mycoplasma pneumoniae
• antibody testing for antinuclear antibodies and anti-cardiolipin antibodies.
A sample of heart valve tissue was collected from all patients who required surgery and submitted for:
• bacterial and fungal culture
• broad-range PCR and sequencing of 16S rRNA and ITS2
• histopathological examination to detect bacteria and fungi, as well as histopathological features of IE.
All patients were treated by an endocarditis team according to the current European Society of Cardiology guidelines.[3,9]
Statistical analysis
Statistical analysis was done using Statistica 14 (Tibco Software, USA), SPSS v27 for iOS (IBM, USA) and JASP (version 0.14.1) for iOS (JASP, Netherlands).
Descriptive statistics were calculated, nominal data were compared via cross-tabulation and χ2 tests, parametric data were compared using independent-sample t-tests (Cohen's d), and non-parametric data were compared using independent-samples t-tests (MannWhitney U or Kruskal-Wallis one-way analysis of variance).
Ethical considerations
The study was approved by the Health Research Ethics Committee of the Faculty of Medicine and Health Sciences, Stellenbosch University (ref. no. S19/08/162), and performed in accordance with the Declaration of Helsinki (2013 version). All patients signed written informed consent, including consent to publication of the data and images. A waiver of consent was granted for patients who died prior to enrolment.
Results
Seventy-one patients with IE were included in this prospective cohort study. The mean patient age was 39 years, and most were male (66.2%) (Table 1). Of the 71 patients, 23.9% had a history of valvular abnormality, with 15.5% having had previous cardiac surgery. All patients had detailed TTE, with the majority (63.9%) undergoing TEE after a median of 3 days. Early mortality and emergency surgery before planned TEE were common reasons for not performing TEE.
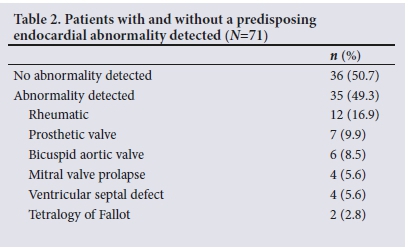
An underlying predisposing endocardial abnormality was detected in 49.3% of patients (Table 2). In patients with a predisposing endocardial abnormality, RHD was the most common (16.9%), with both the mitral and aortic valves involved in the majority of cases (75%). The in-hospital mortality rate was similar in patients with and without a predisposing endocardial abnormality (20%.v. 16.7%; p=0.72), as was the rate of embolic events (20% v. 27.2%; p=0.25). Significantly more patients with a predisposing endocardial abnormality had an indication for surgery (94.3% v. 69.4%; p<0.01).
The viridans group of streptococci was the most common causative organism in patients with a predisposing endocardial abnormality (25.7%), particularly in patients with underlying RHD (50%) (Tables 3 and 4). In patients with no abnormality detected, S. aureus (33.3%) and Bartonella species (27.8%) were the most common causative organisms. The mean linear vegetation length was 12 mm for the aortic valve, 14.3 mm for the mitral valve and 12.4 mm for the tricuspid valve (Table 5). In patients who had both TTE and TEE performed, linear vegetation length was similar (p=0.45/0.09), but with a significant difference in vegetation circumference (p=0.03/<0.04) (Table 5). Linear vegetation length >10 mm (24% v. 5%; p=0.05) and cerebral embolism (54.5% v. 18.4%; p=0.01) were associated with an increased risk of in-hospital mortality. Apart from vegetation-related factors, no other predefined imaging features for predicting in-hospital mortality reached statistical significance (Table 6).
Discussion
In patients with IE, we observed a marked reduction in the prevalence of RHD (16.9%) compared with historical cohorts of IE in SA.[6,7] Coupled with this, we observed a change in the bacteriological profile tracking the presence or absence of a predisposing endocardial abnormality.
Various factors may have contributed to the observed reduction in the prevalence of RHD in this cohort. A decrease in the prevalence of RHD or in the severity of RHD in the population should be considered. Recent data from a large-scale screening project of schoolgoing children within our referral network have revealed a persistently high prevalence of subclinical RHD compared with previous studies reporting the prevalence of subclinical RHD in SA.[13-15] This finding contrasts with the decrease in the proportion of RHD patients in our IE cohort as well as the decrease in patients referred to our division for percutaneous mitral commissurotomy (our data), an important barometer of the burden of severe RHD in our population. It may therefore be that although the prevalence of subclinical RHD has not decreased significantly in our population, a decrease in severity of RHD has contributed to the observed change in the prevalence of RHD in our IE cohort. A possible explanation for this apparent paradox includes improved access to healthcare in the region, so that patients may receive surveillance and treatment (including prophylaxis) earlier in the course of the disease.[12]
The changing bacteriological profile of IE in SA may reflect the lower-than-expected proportion of patients with RHD in our cohort. S. aureus and Bartonella species, both known for their ability to adhere to normal endocardium and cause IE in patients with normal valvular structure, have recently emerged as the most common causes of IE in SA.[7,910,27] In our cohort, S. aureus (33.3%) and Bartonella species (27.8%) were the most common causes of IE in patients in whom no predisposing endocardial abnormality could be detected (Tables 3 and 4). The viridans group of streptococci was an uncommon cause of IE (2.7%) in patients with no predisposing endocardial abnormality. An additional contributing factor when comparing our cohort with previous cohorts in terms of the presence of RHD may be the misclassification of patients with congenital or degenerative valve disease. Previous reports of predisposing abnormalities associated with IE in SA have reported very low rates of congenital heart disease or degenerative valve disease.[6,8,28] The advances in imaging modalities with improved image quality and more frequent use of TEE are likely to have increased clinicians' ability to accurately diagnose predisposing valvular heart disease in patients with IE.[29]
Evaluating the underlying valve structure in patients with IE may aid clinicians in predicting the most likely causative organism.
In patients with no detectable predisposing endocardial abnormality, our data suggest that the viridans group of streptococci is a rare cause of IE (2.7%), in contrast to patients with RHD, where the viridans group of streptococci was the most common cause of IE (50%). We did not identify Bartonella species in cases of prosthetic valve endocarditis, although some case reports of prosthetic valve endocarditis associated with Bartonella species have been reported.[30] The addition of doxycycline to guideline empirical antimicrobial therapy in patients with BCNIE without a underlying endocardial abnormality should be considered.[9,10]
The presence of a predisposing endocardial abnormality was not associated with an increased risk of either embolic events (20% v. 27.3%; p=0.25) or in-hospital mortality (20% v. 16.7%; p=0.72). This unexpected finding may be explained by the lower virulence of the viridans group of streptococci as opposed to S. aureus. However, we did not identify S. aureus as an independent risk factor for in-hospital mortality or cerebral embolism. Patients with a predisposing endocardial abnormality had a significantly higher requirement for surgical intervention (94.3% v. 69.4%; p<0.01). The high prevalence of RHD in patients with IE in SA, with subsequent high rates of mechanical valve replacement, has previously been reported to be a major contributor to the high mortality and morbidity associated with IE in SA.[1,6,8] Our data do not support this and suggest equal short-term outcomes. Prosthetic valve replacement, in particular mechanical prosthetic valve replacement in patients with RHD in SA, has previously been demonstrated to have a significant complication rate,[31] and a difference between the two groups in this study may therefore only become evident after a longer period of follow-up.
The presence of a vegetation or vegetations with a linear length of >10 mm in patients with left-sided IE was associated with an increased risk of in-hospital mortality, which is similar to reports from cohorts in the developed world.[3,19,32] Surprisingly, vegetation size was not associated with an increased risk of cerebral embolism. A common reason for in-hospital mortality in patients with IE is cerebral embolism, and it is conceivable that the cause of death in some patients was undiagnosed cerebral embolism.[1] Cerebral embolism was an independent risk factor for in-hospital mortality in our cohort, which further strengthens this argument. Routine autopsy was not performed in patients who died suddenly in hospital. None of our other predefined risk factors for adverse events reached statistical significance (Table 6). There was a trend towards increased mortality in patients with acute left-sided valvular regurgitation and a pericardial effusion. In terms of the composite outcome of in-hospital mortality and cerebral embolism, the presence of a flail segment was more frequently observed in patients with the combined outcome of cerebral embolism/in-hospital mortality. Perivalvular extension, a validated risk factor for in-hospital mortality in IE, did not reach statistical significance in our study.[3] This has to be seen in the context of access to earlier surgery, which may have offset the otherwise known high mortality from this complication. Furthermore, this study was underpowered to exclude risk factors for in-hospital mortality or cerebral embolism, and the results should be interpreted in this context.
The average linear vegetation size was similar for vegetations attached to the aortic, mitral and tricuspid valves. In patients with left-sided IE who underwent TEE evaluation, the linear vegetation size was similar when comparing measurements by TTE or TEE. This was in contrast to circumferential measurement of vegetation size, which was significantly lower when evaluated by TEE. The difference in circumferential measurement is likely to be due to the better resolution of TEE compared with TTE.[29] This allows more accurate delineation of the circumference of the vegetation through better identification of true vegetation edge, excluding the 'fuzzy lining' from the measurement.[29] TEE was on average performed within 3 days of TTE, and it is also conceivable that the vegetation circumference decreased due to either the effect of antimicrobial therapy or embolisation of the vegetation. This seems an unlikely explanation, given that we did not detect a difference in linear vegetation length when comparing TTE and TEE.
Study limitations
Advanced valvular destruction is often present when patients present with IE, and it is possible that some predisposing valve abnormalities were masked because of this and therefore not detected. Some of the patients assigned to the group without a detectable predisposing endocardial abnormality may have had mitral valve prolapse associated with fibroelastic deficiency. The underlying structural valvular abnormality in patients with fibroelastic deficiency is often quite subtle before the advent of chordal rupture.[26,33] Patients with significant valvular destruction, including chordal rupture, may therefore remain undiagnosed as all the observed features may be ascribed to IE. However, cord rupture due to fibroelastic deficiency is uncommon in the age group of our cohort.[33]
This is an ongoing prospective cohort study and may be underpowered to detect some of the risk factors associated with adverse prognosis. The predefined factors that did not reach statistical significance should therefore be interpreted with caution.
Conclusion
In this cohort of patients with IE, we observed a marked decrease in the prevalence of RHD compared with previously published data from SA. In addition, we observed a change in the bacteriological profile tracking the presence or absence of a predisposing endocardial abnormality.
The likely contributors to the reduced proportion of patients with RHD in this cohort include a possible decrease in prevalence of RHD, in particular severe RHD; the changing bacteriological profile of patients with IE in SA, affecting a higher proportion of normal valves; and the noted effect of improved image quality and new imaging modalities. The presence of a predisposing endocardial abnormality was not associated with an increased risk of in-hospital mortality or cerebral embolism in patients with IE. The viridans group of streptococci was an uncommon cause of IE in patients in whom no predisposing heart or valvular abnormality was detected. Vegetation size by linear length >10 mm in patients with left-sided IE was associated with an increased risk of in-hospital mortality.
Declaration. The research for this study was done in partial fulfilment of the requirements for AJKP's PhD degree at Stellenbosch University.
Acknowledgements. We thank Prof. M Kidd, Centre for Statistical Consultation, Stellenbosch University, and Dr M McCaul, Division of Epidemiology and Biostatistics, Stellenbosch University, for their help with statistical analysis, and Dr E Ngarande, research co-ordinator, Division of Cardiology, Department of Medicine, Stellenbosch University and Tygerberg Hospital.
Author contributions. All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing or revision of the manuscript. AJKP as the primary investigator was responsible for the conception and design of the study, acquisition of data, analysis and interpretation, as well as drafting of the manuscript. CP, PH and AD contributed to the conception and design of the study, acquisition of data, analysis and interpretation, as well as revising the manuscript critically for important intellectual content. KH, LJ, HP, JT and JJ contributed to the acquisition, analysis and interpretation of data, as well as revising the manuscript critically for important intellectual content.
Funding. None.
Conflicts of interest. None.
References
1. Pecoraro AJ, Doubell AF. Infective endocarditis in South Africa. Cardiovasc Diagn Ther 2020;10(2):252-261. https://doi.org/10.21037/cdt.2019.06.03 [ Links ]
2. Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017;69(3):325-344. https://doi.org/10.1016/j.jacc.2016.10.066 [ Links ]
3. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J 2015;36(44):3075-3128. https://doi.org/10.1093/eurheartj/ehv319 [ Links ]
4. Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: Challenges and perspectives. Lancet 2012;379(9819):965-975. https://doi.org/10.1016/s0140-6736(11)60755-1 [ Links ]
5. Pecoraro AJ, Herbst PG, Doubell AF. Infective endocarditis in Africa: An urgent call for more data. Lancet Glob Health 2022;10(1):e8-e9. https://doi.org/10.1016/s2214-109x(21)00489-7 [ Links ]
6. Koegelenberg CFN, Doubell AF, Orth H, Reuter H. Infective endocarditis in the Western Cape Province of South Africa: A three-year prospective study. Q J Med 2003;96(3):217-225. https://doi.org/10.1093/qjmed/hcg028 [ Links ]
7. Nel SH, Naidoo DP. An echocardiographic study of infective endocarditis, with special reference to patients with HIV: Cardiovascular topic. Cardiovasc J Afr 2014;25(2):50-57. https://doi.org/10.5830/CVJA-2013-084 [ Links ]
8. De Villiers MC, Viljoen CA, Manning K, et al. The changing landscape of infective endocarditis in South Africa. S Afr Med J 2019;109(8):592-596. https://doi.org/10.7196/SAMJ.2019.v109i8.13888 [ Links ]
9. Pecoraro A, Herbst P, Pienaar C, et al. Bartonella species as a cause of culture-negative endocarditis in South Africa. Eur J Clin Microbiol Infect Dis 2021;40(9):1873-1879. https://doi.org/10.1007/s10096-021-04239-w [ Links ]
10. Pecoraro AJK, Pienaar C, Herbst PG, et al. Causes of infective endocarditis in the Western Cape, South Africa: A prospective cohort study using a set protocol for organism detection and central decision making by an endocarditis team. BMJ Open 2021;11(12):e053169. https://doi.org/10.1136/bmjopen-2021-053169 [ Links ]
11. Miro JM, Ambrosioni J. Infective endocarditis: An ongoing global challenge. Eur Heart J 2019;40(39):3233-3236. https://doi.org/10.1093/eurheartj/ehz694 [ Links ]
12. Van Deventer J, Doubell A, Herbst P, Piek H, Marcos M, Pecoraro A. Evaluation of the SUNHEARTCardiology Outreach Programme. SA Heart 2015;12(2):82-86. https://doi.org/10.24170/12-2-1723 [ Links ]
13. Hunter LD, Monaghan M, Lloyd G, Pecoraro AJK, Doubell AF, Herbst PG. Screening for rheumatic heart disease: Is a paradigm shin required? Echo Res Pract 2017;4(4):R43-R52. https://doi.org/10.1530/ERP-17-0037 [ Links ]
14. Engel ME, Haileamlak A, Zühlke L, et al. Prevalence of rheumatic heart disease in 4720 asymptomatic scholars from South Africa and Ethiopia. Heart 2015;101(17):1389-1394. https://doi.org/10.1136/heartjnl-2015-307444 [ Links ]
15. Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol 2013;10(5):284-292. https://doi.org/10.1038/nrcardio.2013.34 [ Links ]
16. Hunter LD, Pecoraro AJK, Doubell AF, et al. Screening for subclinical rheumatic heart disease: Addressing borderline disease in a real-world setting. Eur Heart J Open 2021;1(3):oeab041. https://doi.org/10.1093/ehjopen/oeab041 [ Links ]
17. Murdoch DR, Corey RG, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169(5):463-473. https://doi.org/10.1001/archinternmed.2008.603 [ Links ]
18. Western Cape Government. SEP: Socio-economic profile, City of Cape Town 2017. 2017. https://www.westerncape.gov.za/assets/departments/treasury/Documents/Socio-economic-profiles/2017/city_ of_cape_town_2017_socio-economic_profile_sep-lg_-_26_january_2018.pdf (accessed 3 July 2019). [ Links ]
19. Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis: Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur Heart J 2019;40(39):3222-3232. https://doi.org/10.1093/eurheartj/ehz620 [ Links ]
20. Wheeler R, Steeds R, Rana B, et al. A minimum dataset for a standard transoesophageal echocardiogram: A guideline protocol from the British Society of Echocardiography. Echo Res Pract 2015;2(4):G29-G45. https://doi.org/10.1530/ERP-15-0024 [ Links ]
21. Wharton G, Steeds R, Allen J,et aLA minimum dataset for a standard adult transthoracic echocardiogram: A guideline protocol from the British Society of Echocardiography. Echo Res Pract2015;2(1):G9-G24. https://doi.org/10.1530/erp-14-0079 [ Links ]
22. Hunter LD, Monaghan M, Lloyd G, et al Interscallop separations of the posterior mitral valve leaflet: A solution to the 'borderline RHD' conundrum? Open Heart 2020;7(2):e001452. https://doi.org/10.1136/openhrt-2020-001452 [ Links ]
23. Hunter LD, Doubell AF, Pecoraro AJK, et al. The variable spectrum of anterior mitral valve leaflet restriction in rheumatic heart disease screening. Echocardiography 2021;38(5):729-736. https://doi.org/10.1111/echo.15039 [ Links ]
24. Hunter LD, Lombard CJ, Monaghan MJ, et al. Screening for rheumatic heart disease: The reliability of anterior mitral valve leaflet thickness measurement. Echocardiography 2020;37(6):808-814. https://doi.org/10.1111/echo.14751 [ Links ]
25. Sanfilippo AJ, Picard MH, Newell JB, et al. Echocardiography assessment of patients with infectious endocarditis: Prediction of risk for complications. J Am Coll Cardiol 1991;18(5):1191-1199. https://doi.org/10.1016/0735-1097(91)90535-h [ Links ]
26. Falk V, Baumgartner H, Bax JJ, et al 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38(36):2739-2791. https://doi.org/10.1093/eurhear1j/ehx391 [ Links ]
27. Holland TL, Baddour LM, Bayer AS, et al. Infective endocarditis. Nature Rev Dis Primers 2016;2:10059. https://doi.org/10.1038/nrdp.2016.59 [ Links ]
28. Koshy J, Engel M, Human P, Carrara H, Brink J, Zilla P. Long term outcome and EuroSCORE II validation in native valve surgery for active infective endocarditis in a South African cohort. SA Heart 2018;15(2):116-126. https://doi.org/10.24170/15-2-3045 [ Links ]
29. Bai AD, Steinberg M, Showler A, et al. Diagnostic accuracy of transthoracic echocardiography for infective endocarditis findings using transesophageal echocardiography as the reference standard: A meta-analysis. J Am Soc Echocardiogr 2017;30(7):639-646.e8. https://doi.org/10.1016/j.echo.2017.03.007 [ Links ]
30. Papineni P, Carroll A, Radvan J, et al. Management of Bartonella prosthetic valve endocarditis without cardiac surgery. Emerg Infect Dis 2017;23(5):861-863. https://doi.org/10.3201/eid2305.161238 [ Links ]
31. Scherman J, Manganyi R, Human P, et al Isolated mechanical aortic valve replacement in rheumatic patients in a low- to middle-income country. J Thorac Cardiovasc Surg 2019;157(3):886-893. https://doi.org/10.1016/j.jtcvs.2018.06.083 [ Links ]
32. Thuny F, Disalvo G, Belliard O, et al. Risk of embolism and death in infective endocarditis: Prognostic value of echocardiography: A prospective multicenter study. Circulation 2005;112(1):69-75. https://doi.org/10.1161/circulationaha.104.493155 [ Links ]
33. Naili MA, Herbst PG, Doubell AF, Janson JJ, Pecoraro AJK. A retrospective audit of mitral valve repair surgery at Tygerberg Hospital. SA Heart 2018;15(3):182-189. https://doi.org/10.24170/15-3-3182 [ Links ]
 Correspondence:
Correspondence:
A J K Pecoraro
pecoraro@sun.ac.za
Accepted 25 January 2022.














