Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 n.4 Pretoria Apr. 2022
http://dx.doi.org/10.7196/samj.2022.v112i4.16146
RESEARCH
Profile, presentation and outcomes of prosthetic valve endocarditis in a South African tertiary hospital: Insights from the Groote Schuur Hospital Infective Endocarditis Registry
P MkokoI; B J CupidoII; J HitzerothIII; A ChinIV; M NtsekheV
IMB ChB, MMed (Med); Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital Cape Town, South Africa
IIMB ChB, MPhil (Cardiol); Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital Cape Town, South Africa
IIIMB ChB; Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital Cape Town, South Africa
IVMB ChB, MPhil (Cardiol); Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital Cape Town, South Africa
VMD, PhD; Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital Cape Town, South Africa
ABSTRACT
BACKGROUND: Prosthetic valve infective endocarditis (PVE) is associated with high morbidity and mortality. The prevalence of PVE in South African retrospective studies ranges between 13% and 17%
OBJECTIVES: To define the clinical profile and outcomes of patients with PVE, and compare them with those of native valve endocarditis (NVE) patients
METHODS: We performed a prospective observational study of patients presenting or referred to Groote Schuur Hospital, Cape Town, with definite or possible infective endocarditis (IE) based on the 2015 European Society of Cardiology IE diagnostic criteria. Consenting adult patients who met the inclusion criteria were enrolled into the Groote Schuur Hospital Infective Endocarditis Registry, which was approved by the University of Cape Town Human Research Ethics Committee. This study is an analysis of the patients enrolled between 1 January 2017 and 31 December 2019
RESULTS: During the study period, a total of 135 patients received a diagnosis of possible or definite IE (PVE n=18, NVE n=117). PVE therefore accounted for 13.3% of the overall IE cohort. PVE patients had a mean (standard deviation) age of 39.1 (14.6) years, and 56.6% were male. PVE occurred within 1 year of valve surgery in 50.0% of cases. Duke's modified diagnostic criteria for definite IE were met in 94.4% of the PVE cohort. Isolated aortic valve PVE was present in 33.3%, and a combination of aortic and mitral valve PVE in 66.6%. Tissue prosthetic valves were affected in 61.1% of cases. Of the PVE cases, 55.6% were healthcare associated. On transthoracic echocardiography, vegetations (61.1%), prosthetic valve regurgitation (44.4%) and abscesses (22.2%) were discovered. Staphylococcus and Streptococcus species accounted for 38.8% and 22.2% of PVE cases, respectively, and 27.8% of cases were blood culture negative. Valve surgery was performed in 38.7% of the PVE patients, and 55.6% of the patients died during the index hospitalisation. Secondary analysis indicated that the PVE patients were sicker than those with NVE, with a higher frequency of septic shock and atrioventricular block (22.2% v. 7%; p=0.02 and 27.8% v. 12%; p=0.04, respectively). In addition, in-hospital mortality was higher in PVE patients than NVE patients (55.6% v. 31.6%; p=0.04
CONCLUSIONS: PVE was uncommon, mainly affecting tissue prosthetic valves and prosthetic valves in the aortic position. Patients with PVE were sicker than those with NVE and had high in-hospital mortality
Infective endocarditis (IE) is relatively infrequent but is associated with high mortality and morbidity[1] The IE-related in-hospital mortality rate is reported to be as high as 22% and the 5-year mortality rate up to 45%.[2-4] The mortality rate has remained stable despite advances in healthcare. For example, the IE-associated global age-standardised mortality rate in 2015 was 1.3 per 100 000 v. 1.4 per 100 000 in 2005.[5] In low- and middle-income countries, IE tends to affect young patients with a high background prevalence of rheumatic heart disease (RHD),[6,7] and the observed IE-related age-standardised mortality rate (1.7 per 100 000)[8] is higher than the global figures.
Prosthetic valve infective endocarditis (PVE) is the most lethal form of IE, with in-hospital mortality ranging between 20% and 40%.[9-13] One of the reasons for the high mortality may be related to the difficulty of making a definitive diagnosis of PVE, a challenging exercise, particularly in the setting of mechanical prosthetic valves.[14,15] Indeed, Duke's criteria have a sensitivity of only 62% for PVE, and use of positron emission tomography (PET)/computed tomography (CT) or Duke's criteria plus PET/CT increases the sensitivity to 84% and 91%, respectively[16] Making an accurate diagnosis of PVE is important because at least 1 - 6% of patients with prosthetic valves will develop PVE.[17] In contemporary studies from Europe and the USA, PVE accounts for up 30% of IE cases, and the incidence is increasing.[18,19]
There are limited prospective data on the approximate proportion of PVE relative to the burden of IE admissions in South African (SA) hospitals. There is also scant information on the patient profile and PVE-related outcomes. Two retrospective hospital reviews from SA[6,7] suggested that between 13.3% and 17% of IE admissions had PVE. However, these studies did not provide detail on patient treatment or PVE-related morbidity and mortality, which remain unknown.
Objectives
The primary objective of this study was to define the clinical profile and outcomes of patients with PVE. The secondary objective was to compare the clinical profile and outcomes of PVE with those of native valve endocarditis (NVE).
Methods
The Groote Schuur Hospital Infective Endocarditis Registry is a prospective observational study of patients presenting or referred to Groote Schuur Hospital, Cape Town, with definite or possible IE based on the 2015 European Society of Cardiology (ESC) IE diagnostic criteria.[14] Consenting adult patients who met the inclusion criteria were enrolled into the registry, which was approved by the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee (ref. no. R037/2017). The present study is an analysis of the cohort of patients who were enrolled between 1 January 2017 and 31 December 2019 (ref. no. HREC 406/2020).
Patient selection and data collection
Adult (>18 years of age) patients with possible or definite IE were included in the study. After informed consent had been obtained, demographic data, clinical presentation, past medical and surgical history, and data on recent hospitalisation, dental procedures and endoscopies (upper and lower gastrointestinal tract) were recorded. We also collected data on clinical findings, electrocardiograms, microbiological findings, echocardiographic findings, use of other imaging techniques (CT scan and magnetic resonance imaging), medical therapy, complications (embolic event, infectious and haemodynamic complications), indications for surgery, and in-hospital mortality. Data were extracted from source documents and captured onto standardised electronic case report forms on Research Electronic Data Capture (REDCap), a secure online database hosted by the University of Cape Town.
Definitions
Early PVE was defined as IE occurring within 1 year of cardiac valve surgery and late PVE as IE occurring >1 year after cardiac valve surgery.[14]
Healthcare-associated PVE was defined as the development of IE symptoms or the presence of a positive blood culture at the time of hospital admission or within 48 hours in a patient who fulfilled any of the following criteria: (i) used intravenous antibiotics at home, or received specialised nursing care within 30 days before the onset of symptoms or positive blood culture; (ii) attended a hospital or haemodialysis clinic or received intravenous chemotherapy within 30 days before the onset of symptoms or positive blood culture: (iii) was hospitalised for >2 days within 3 months before the onset of symptoms or positive blood culture; or (iv) was a nursing home or long-term care facility resident.[l1,20]
Statistical analysis
Categorical variables are presented as numbers and percentages and continuous variables as means (standard deviation (SD)) when normally distributed and medians (interquartile range) when skewed Pearsons x2or Fisher's exact tests were used to compare categorical variables, and the independent-samples t-test to compare continuous variables between patients with PVE and patients with NVE. The Kaplan-Meier and log-rank tests were used to assess the cumulative survival difference, with p<0.05 representing a statistically significant difference. Statistical analyses were performed using SPSS Statistics for Macintosh, version 24.0 (IBM Corp., USA).
Results
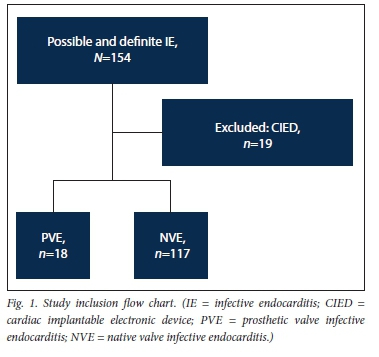
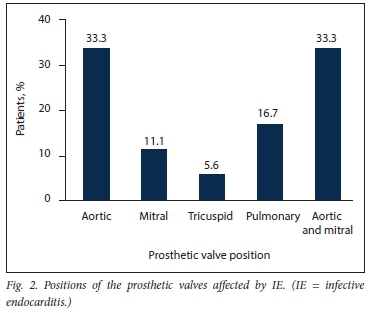
After the exclusion of 19 patients with cardiac implantable electronic device-related infection, 135 patients with possible or definite IE were included in the study (Fig. 1). Eighteen (13.3%) had a diagnosis of PVE and 117 (86.7%) a diagnosis of NVE. The baseline characteristics and demographic details of the overall patient population are presented in Table 1. The patients with PVE had a mean (SD) age of 39.1 (14.6) years, and 56.6% were male. Duke's definite PVE diagnostic criteria were met in 94.4% of cases and Duke's possible PVE criteria in 5.6%. PVE occurred within 1 year of valve replacement surgery (early PVE) in 50.0%. Isolated aortic valve PVE was present in 33.3%, a combination of aortic and mitral valve PVE in another 33.3%, isolated pulmonary valve PVE in 16.7% and isolated mitral valve PVE in 11.1% (Fig. 2). The aortic valve was therefore involved in 66.6% of cases of PVE. Tissue PVE accounted for 61.1% of cases and mechanical PVE for 38.9%. Of the patients with PVE, 27.8% had a history of previous IE, 55.6% had healthcare-associated IE, and 8 (44.4%) had a history of RHD. Transthoracic echocardiography (TTE) was performed in 94.1% of the patients with PVE. Vegetations were documented in 61.1%, prosthetic valve regurgitation in 44.4% and abscesses in 22.2%. Transoesophageal echocardiography was performed in only 2 patients with PVE (11.1%). Vegetations and prosthetic valve regurgitation were documented in both of them and an abscess in one.
Staphylococcus species were responsible for a 38.9% of PVE cases and Streptococcus species for 22.2% (Table 2). Five (27.8%) of the PVE cases were culture negative. All these patients had received antibiotics prior to blood culture sampling, none of them had serum serological tests done, and one patient had a tissue polymerase chain reaction test positive for Aggregatibacter aphrophilus. The main complications in PVE were heart failure (50.0%), acute kidney injury (50.0%), atrioventricular block (28.7%), aortic root abscesses (27.8%) and septic shock (22.2%) (Table 3). After a heart team decision, redo valve surgery was performed in 38.7%. The indications for offering and withholding surgery in PVE are depicted in Fig. 3. The mean (SD) duration of hospital stay for patients with PVE was 28.6 (24.4) days, and their in-hospital mortality rate was 55.6%.
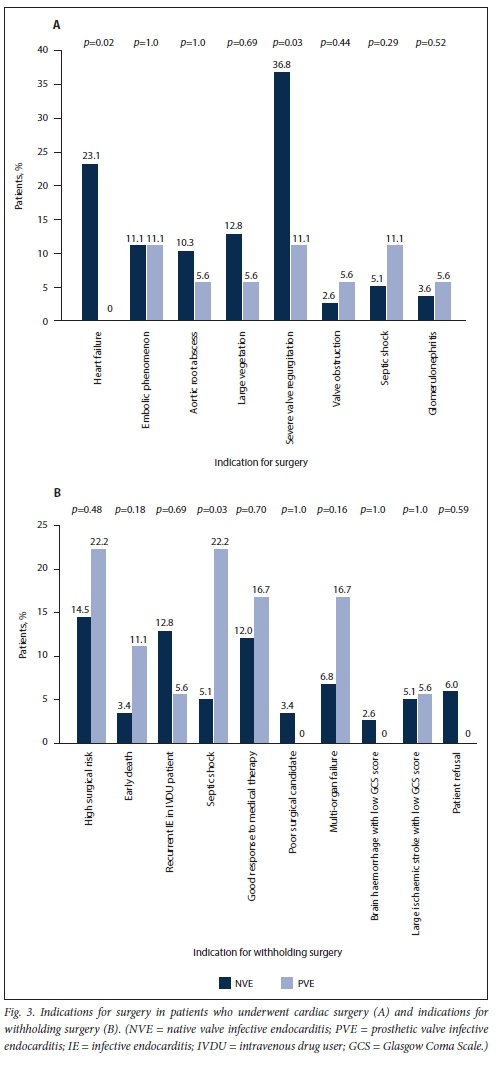
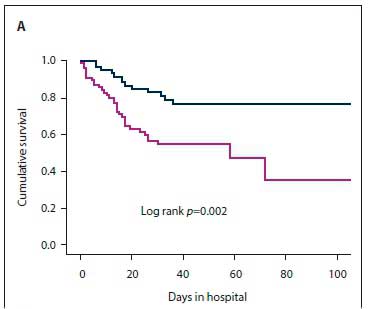
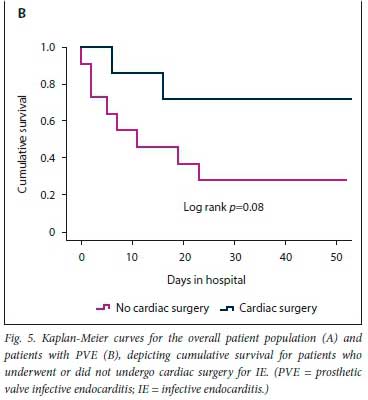
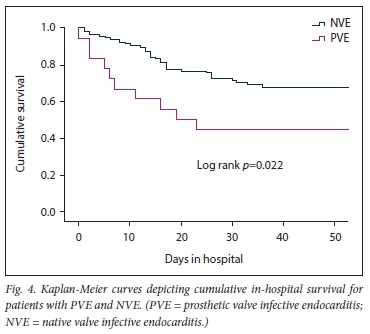
The results of the secondary analysis indicated that patients with PVE were more likely to have a past history of IE, and more likely to have healthcare-associated IE, than those with NVE. Furthermore, in patients with PVE, TTE was less likely to pick up vegetations and valve regurgitation but more likely to pick up abscesses (Table 1). Although there was no statistically significant difference in the frequency of Staphylococcus aureus as the cause of IE between PVE and NVE, coagulase-negative staphylococci (CoNS) were significantly more frequent in PVE than NVE (Table 2). Septic shock and atrioventricular block were more common in PVE than NVE (22.2% v. 6.0%; p=0.02 and 27.8% v. 10.3%; p=0.04, respectively). The decision whether to perform cardiac surgery for IE or to continue with only medical therapy was made by the heart team. The indications for cardiac surgery in patients who underwent surgery and the indications for withholding surgery are presented in Fig. 3. Fig. 4 shows cumulative in-hospital survival for patients with PVE and NVE. For the overall IE patient population, patients who underwent cardiac surgery had better cumulative survival than those who did not (log rank p=0.002) (Fig. 5A). For the PVE patient population, there was a trend towards better cumulative survival for those who underwent cardiac surgery, but this was not statistically significant (log rankp=0.08) (Fig. 5B). The in-hospital mortality rate for PVE was significantly higher than that for NVE (55.6% v. 31.6%; p=0.04).
Discussion
In this prospective observational study, we investigated the patient profile, presentation and outcomes of PVE in a resource-limited setting with a high background prevalence of RHD. The major findings of this study were: (i) the PVE patients were young; (ii) they had a high background prevalence of RHD, congenital heart disease and previous IE; (iii) the majority of PVE cases were healthcare associated; (iv) Staphylococcus species were responsible for the majority of PVE cases, but a substantial number of cases were culture negative; (v) septic shock and atrioventricular block were common complications of PVE; (vi) PVE-associated in-hospital mortality was very high; and (vii) in comparison with NVE, PVE patients were sicker, with more complications and higher in-hospital mortality.
Previous regional reports indicate that the prevalence of PVE in Western Cape Province has been stable over time. For example, in a prospective observational study from 1997 to 2000, Koegelenberg et al.[7]reported a PVE prevalence of 16.6%, and in a retrospective study from 2009 to 2016, De Villiers et al.[6]reported a prevalence of 13.3%. The Western Cape prevalence of PVE is lower than reported for the ESC-EORP EURO-ENDO (European infective endocarditis) registry in 2019, where PVE accounted for 30.1% of IE cases.[18] For the first time in our SA context, the present study found that 25.9% of all IE cases and 55.6% of PVE cases were healthcare associated. The prevalence rate for healthcare-associated PVE of 55.6% in this study is markedly higher than previously reported for PVE. For example, in a prospective multicentre observational study from 2000 to 2005, Wang et al.[11]reported a prevalence of healthcare-associated PVE of 36.5%. IE (both PVE and NVE) affected a young patient population in the present study, and this finding corroborates previous reports from this region.[6,7] Our cohort was at least 20 years younger than the ESC-EORP registry cohort,[18] with a mean (SD) age of 59.2 (18.0) years, and Olmos et al's cohort,[19] with a mean age of 63.8 (17.5) years. This age difference is mainly due to a high prevalence of RHD, which frequently requires surgical treatment at a young age,[21] an important cause of valvular heart disease in our population.
Similar to the ESC-EORP registry, where staphylococci were the most common micro-organisms detected on blood culture in PVE (41%),[18] in the present study, staphylococci remained the leading causative micro-organism for PVE, accounting for 38.9% of cases. However, in the present study, CoNS (33.3%) were cultured more frequently than S. aureus (5.6%). There was no specific mention of CoNS in the ESC-EORP registry[18] This result must be interpreted with caution, as the present study had a high rate of culture-negative IE and a small sample of PVE, and did not adhere to the ESC algorithm for culture-negative IE.[14] The ESC-EORP registry had a lower rate of culture-negative PVE.[18] In addition, our findings are in contrast to the findings of Wang et al.[11]that S. aureus caused 23.0% of PVE cases and CoNS 16.9%, only 11.2% of cases being culture negative. All the blood culture-negative PVE cases in our study had documented receipt of antibiotics prior to the collection of blood samples.
PVE is a difficult diagnosis to make, and there are important technical challenges with the use of the readily available TTE in the context of prosthetic valves.[15] As we found in the present study, there are significant differences in the prevalence of characteristic endocarditis abnormalities such as vegetations, regurgitation and abscesses seen on TTE between patients with PVE and NVE. Contemporary imaging modalities such as 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) with CT have been shown to improve the diagnostic accuracy of the modified Duke's criteria. For example, in a prospective study of 92 patients with suspected PVE, the addition of PET/CT findings to the Duke's criteria improved the sensitivity, specificity, positive predictive value and negative predictive value from 52%, 94.7%, 92.9% and 59.7%, respectively, for the Duke's criteria to 92.1%, 89.5%, 92% and 87.9%, respectively, for the combination of the Dukes criteria and PET/CT[16] PET/CT was used in 25% of PVE patients in the ESC-EORP registry[18] There were important variations in the utilisation of PET/CT for the diagnosis of PVE in the ESC-EORP registry, with more frequent use of PET/CT in Western Europe (33.9%) and less frequent use in South America (2.1%), North America (0%) and Asia (8.5%).[18]Our institution currently does not have access to 18F-FDG PET/CT. There may therefore have been cases in which the PVE diagnosis was missed as a result of absence of this diagnostic modality.
PVE is associated with high in-hospital mortality, ranging between 20% and 40% [11,14,18] Older age (>65 years), healthcare-associated infections, PVE caused by S. aureus, persistent bacteraemia, and the presence of complications such as heart failure, intracranial abscesses, stroke and renal impairment have been identified as poor prognostic markers.[11,14] The combination of surgery and medical therapy in appropriately selected patients is the treatment strategy of choice for PVE.[14,22] In the recent ESC-EORP registry, only 46% of PVE patients received surgical management.[18] In the present study, surgery was performed in 38.7% of PVE cases. In our cohort, after a heart team discussion, surgery was withheld if poor prognostic markers were present, or if the patient was at high surgical risk or responded well to medical therapy. For the overall patient population, cumulative survival was significantly worse in patients who did not receive surgical treatment for IE, and there was a trend towards lower cumulative survival (although this was not statistically significant) for PVE patients who did not receive surgical treatment. The heart team discussion is particularly important in resource-limited settings where there is a high demand for cardiothoracic surgical intervention, to facilitate allocation of this resource to candidates who are most likely to benefit.
Study limitations
The single-centre nature of the present study is an important limitation, and the results are not necessarily generalisable. Furthermore, the number of PVE cases was small. However, we believe that our findings are a true reflection of the prevalence of PVE in Western Cape for two reasons. Firstly, at least two studies, two decades apart, reported a PVE prevalence of <17%.[6,7] Secondly, our centre is the referral centre for all secondary and regional hospitals in the Coastal and Western regions of the Cape, serving -50% by geography and population. Also, provincial referral pathways stipulate that all patients with prosthetic valves who are unwell be referred to tertiary centres with the capacity to investigate and provide optimal treatment for PVE. In this study, we only report overall PVE-associated in-hospital mortality of 55.6%. Long-term follow-up has been severely compromised by the COVID-19 pandemic. We therefore do not have long-term follow-up outcomes.
Conclusions
PVE is relatively uncommon in resource-limited settings and is associated with high in-hospital mortality. Staphylococcus and Streptococcus species are the leading microbiological causes of PVE. However, a large proportion of cases remain culture negative. Diagnosing PVE is difficult, and newer diagnostic modalities that improve the diagnostic yield are not yet available in resource-constrained centres like ours. The selected PVE patients who receive surgical treatment for endocarditis have better in-hospital survival than those who do not. This finding not only reaffirms the importance of surgery as a treatment option for IE but demonstrates the importance of the heart team in selecting appropriate surgical candidates.
Declaration. The research for this study was done in partial fulfilment of the requirements for PM s MPhil (Cardiology) degree at the University of Cape Town.
Acknowledgements. The author would like to thank Dr Charles Viljoen for assistance with the GSH IE registry design.
Author contributions. PM designed the study, collected the data and performed the data analysis. BJC, JH and AC recruited the patients for the GSH IE registry. MN recruited the patients for the GSH IE registry and supervised PM in the study design and data analysis.
Funding. None.
Conflicts of interest. None.
References
1. Bin Abdulhak AA, Baddour LM, Erwin PJ, et al. Global and regional burden of infective endocarditis, 1990 - 2010. A systematic review of the literature. Glob Heart 2014;9(1):131-143. https://doi.org/10.1016/j.gheart.2014.01.002 [ Links ]
2. Sy RW, Kritharides L. Health care exposure and age in infective endocarditis. Results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J 2010;31(15):1890-1897. https://doi.org/10.1093/eurheartj/ehq110 [ Links ]
3. Selton-Suty C, Celard M, le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis. A 1-year population-based survey Clin infect Dis 2012;54(9):1230-1239. https://doi.org/10.1093/cid/cis199 [ Links ]
4. Bannay A, Hoen B, Duval X, et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis. Do differences in methodological approaches explain previous conflicting results? Eur Heart J 2011;32(16):2003-2015. https://doi.org/10.1093/eurheartj/ehp008 [ Links ]
5. Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980 - 2015. A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1459-1544. https://doi.org/10.1016/S0140-6736(16)31012-1 [ Links ]
6. De Villiers MC, Viljoen CA, Manning K, et al. The changing landscape of infective endocarditis in South Africa SAfr Med J2019;109(8):592-596.https://doi.org/10.7196/SAMJ.2019.v109i8.13888 [ Links ]
7. Koegelenberg CFN, Doubell AF, Orth H, Reuter H. Infective endocarditis in the Western Cape Province of South Africa: A three-year prospective study Int J Med 2003;96(3):217-225. https://doi.org/10.1093/qjmed/hcg028 [ Links ]
8. Kwan GF, Mayosi BM, Mocumbi AO, et al. Endemic cardiovascular diseases of the poorest billion. Ckcuiauon2016;133(24):2561-2575.https://doi.org/10J161/circulationaha.116.008731 [ Links ]
9. Wallace SM, Walton BI, Kharbanda RK, Hardy R, Wilson AP, Swanton RH. Mortality from infective endocarditis. Clinical predictors of outcome. Heart 2002;88(1):53-60. https://doi.org/10.1136/heart.88.1.53 [ Links ]
10. Habib G, Tribouilioy C, Ihuny F, et al. Prosthetic valve endocarditis. Who needs surgery? A multicentre study of 104 cases. Heart 2005;91(7):954-959. https://doi.org/10.1136/hrt2004.046177 [ Links ]
11. Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007;297(12):1354-1361. https://doi.org/10.1001/jama.297.12.1354 [ Links ]
12. ALL N, Baig W, Wu J, Blackman D, Giliott R, Sandoe J. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013;173(16):1495-1504. https://doi.org/10.1001/jamainternmed.2013.8203 [ Links ]
13. ALi N, Baig W, Wu J, Blackman D, Giliott R, Sandoe J. Abstract 129. Prosthetic valve endocarditis following transcatheter aortic valve implantation - experience from a UK centre. Heart 2019,105(Suppl 6).A105. https://heart.bmj.com/content/105/Suppl_6/A105 [ Links ]
14. Habib G, Lanceliotti P, Antunes M, etal. 2015 ESC Guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by. European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36(44):3075-3128. https://doi.org/10.1093/eurheartj/ehv315 [ Links ]
15. Zoghbi WA, Chembers JB, Dumesnii JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. A report from the American Society of Echocardiography s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22(9) .975-1014, quiz 1082-1084. https://doi.org/10.1016/j.echo.2009.07.013 [ Links ]
16. Pizzi MN, Roque A, Fernandez-Hidalgo N, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography. Initial results at an infective endocarditis referral center. Circulation 2015;132(12):1113-1126. https://doi.org/10.1161/circulationaha.115.015316 [ Links ]
17. Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med 1996;335(6):407-416. https://doi.org/10.1056/nejml99608083350607 [ Links ]
18. Habib G, Erba PA, lung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis-. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry. A prospective cohort study. Eur Heart J 2019;40(39):3222-3232. https://doi.org/10.1093/eurheartj/ehz620 [ Links ]
19. Olmos C, Vilacosta I, Fernandez-Perez C, et al. The evolving nature of infective endocarditis in Spain. A population-based study (2003 to 2014). J Am Coll Cardiol 2017;70(22):2795-2804. https://doi.org/10.1016/j.jacc.2017.10.005 [ Links ]
20. Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults. A reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002;137(10):791-797. https://doi.org/10.7326/0003-4819-137-10-200211190-00007 [ Links ]
21. Zuhlke L, Karthikeyan G, Engel ME, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries. Two-year follow-up of the Global Rheumatic Heart Disease Registry (the REMEDY Study). Circulation 2016;134(19):.1456-1466. https://doi.org/10.1161/circulationaha.116.024769 [ Links ]
22. Habib G, Thuny F, Avierinos J-F. Prosthetic valve endocarditis. Current approach and therapeutic options. Prog Cardiovasc Dis 2008;50(4):274-281. https://doi.org/10.1016/j.pcad.2007.10.007 [ Links ]
 Correspondence:
Correspondence:
P Mkoko
mkkphi002@myuct.ac.za
Accepted 18 December 2021