Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.112 no.4 Pretoria Abr. 2022
http://dx.doi.org/10.7196/samj.2022.v112i4.16166
RESEARCH
Predictors of mortality in acute hospitalised COVID-19 pneumonia patients: A retrospective cohort study at two tertiary-level hospitals in Zambia
J BandaI, II; A MweembaIII; T BulayaIV; N ChengaV; S SiziyaVI
IMMed (Int Med), PhD; Internal Medicine, Ndola Teaching Hospital, Zambia,
IIMMed (Int Med), PhD; Department of Medical Sciences, Vacuity of Health Sciences and Veterinary Medicine, University of Namibia
IIIMB ChB, MMed (Int Med); Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia
IVMB ChB, MMed (Int Med); Internal Medicine, Ndola Teaching Hospital, Zambia,
VMBBS; Internal Medicine, Ndola Teaching Hospital, Zambia,
VIMSc, PhD; Michael Chilufya Sata School of Medicine, Copperbelt University, Ndola, Zambia
ABSTRACT
BACKGROUND: The global COVID-19 pandemic has resulted in increased acute hospitalisations, a high demand for intensive care and high in-hospital mortality, placing a huge burden on healthcare systems
OBJECTIVES: To assess in-hospital mortality outcomes and associated factors in acute hospitalised COVID-19 pneumonia patients in Zambia
METHODS: We performed a retrospective cohort review of patients admitted to two tertiary-level hospitals in Zambia from 1 March 2020 to 28 February 2021. We examined the factors (demographic, clinical and laboratory) that were associated with in-hospital mortality using multivariate logistic analysis. Adjusted odds ratios with their 95% confidence intervals (CIs) are reported
RESULTS: Of 350 patients, 59.4% were aged >55 years and 52.6% were male. The commonest comorbidities were hypertension, diabetes mellitus (DM), HIV/AIDS and chronic kidney disease (49.6%, 28.5%, 22.0% and 8.1%, respectively). The overall in-hospital mortality rate was 42.6%, and mortality was significantly increased in patients aged >55 years (52.0% v. 48.0%) and in those with DM (52.1% v. 47.9%), cardiac disease (68.0% v. 32.0%), a Quick Sequential (Sepsis-Related) Organ Failure Assessment (q-SOFA) score >2 (75.4% v. 24.6%), and admission blood glucose levels >7.0 mmol/L (66.3% v. 33.7%). Compared with patients who survived, who spent a median (interquartile range) of 6 (3 - 10) days in hospital, the median time between admission and death in those who died was 2.5 (1 - 6) days. In multivariate logistic analysis, age >55 years, a q-SOFA score >2 and a random blood sugar level >7.0 mmol/L were predictors of in-hospital mortality, with adjusted odds ratios of 1.54 (95% CI 1.09 - 2.17), 2.17 (95% CI 1.40 - 3.38) and 1.65 (95% CI 1.18 - 2.30), respectively. Raised serum creatinine was not associated with in-hospital COVID-19 mortality after adjusting for other confounders
CONCLUSIONS: This study highlights that high in-hospital COVID-19 mortality was associated with a high q-SOFA score, hyperglycaemia on admission and older age. The study reinforces the need to invest in emergency healthcare services for optimal management of COVID-19 patients presenting with high q-SOFA scores in resource-limited countries
COVID-19 has stretched healthcare systems globally[1] since its identification. It has resulted in increased acute hospitalisations, a high demand for intensive care and a high in-hospital mortality rate.[2]
Available data from around the globe show that SARS-CoV-2 infection is associated with increased but varied mortality rates in persons with underlying comorbidities.[3,4] Some studies in developed countries have reported increased mortality rates as high as 70%.[3,5] A meta-analysis that included 60 studies with 51 225 patients reported a 24% in-hospital mortality rate among COVID-19 patients, with older age, kidney disease and diabetes mellitus (DM) being listed as risk factors for increased mortality.[6]In a study of hospitalised elderly patients with COVID-19, Becerra-Munoz et al.[5]found almost 40% in-hospital COVID-19 mortality, with kidney disease, lymphopenia and hypoxaemia as independent positive predictors of mortality. In this study, a Quick Sequential (Sepsis-Related) Organ Failure Assessment (q-SOFA) score >1 was associated with an 8.3-fold increase in the mortality rate. Recently, studies have also reported hyperglycaemia and high C-reactive protein (CRP) to be poor prognostic markers in patients hospitalised with COVID-19.[7-9]
Despite a high COVID-19 disease burden, sub-Saharan Africa, including Zambia, has a dearth of information on poor prognostic markers associated with in-hospital deaths. Mwananyanda et al.[10]recently reported a high prevalence of SARS-CoV-2 among bodies examined post mortem at the largest teaching hospital in Zambia. Sub-Saharan African countries have a poor supply of emergency commodities such as oxygen,[11] a critical first-line treatment in severe COVID-19 pneumonia.
Objectives
To examine clinical and laboratory markers linked with poor prognosis in acute hospitalised COVID-19 pneumonia patients, and thus to lay a foundation for planning during future SARS-CoV-2 waves.
Methods
Study design and setting
In a retrospective cohort study, we extracted and examined nonelectronic medical records of all adult patients (>18 years) acutely hospitalised with severe COVID-19 pneumonia at two tertiary-level hospitals in Zambia (Ndola Teaching Hospital and Kitwe Teaching Hospital) from 1 March 2020 to 28 February 2021. The two hospitals were designated as the main referral isolation centres in the Copperbelt region. Ethical approval for the research was obtained from the Tropical Diseases Research Centre Ethics Review Committee (ref. no. #TRC/C4/05/2021) and the National Health Research Authority (ref. no. #NHRA00004/8/06/2021). Excluded were 109 medical patients' files that were missing data or lacked clinical information.
Study procedures
The following information was extracted from the non-electronic medical records: demographic data, comorbidity data on HIV/AIDS, hypertension (HTN) and DM, and laboratory data (CRP, D-dimers, haematology, kidney function, etc.). We also recorded admission vital signs (respiratory rate, blood pressure, etc.) and discharge outcomes. Confirmation of COVID-19 was based on a positive reverse transcription polymerase chain reaction test for SARS-CoV-2[12] or a positive antigen test, in line with the Ministry of Health (MoH) of Zambia guidelines. Both of the designated tertiary-level hospitals were equipped with a 5-bed COVID-19 intensive care unit (ICU) with 3-4 ventilators, and more wards were opened to accommodate increased numbers of patients.
However, the COVID-19 ICUs at both hospitals lacked a pressure swing adsorption oxygen plant, so the oxygen source was delivered via an F-cylinder manifold reticulation piping system to the COVID-19 ICUs and via F-cylinders (without an oxygen piping system) and oxygen concentrators to the COVID-19 main isolation wards. The major limitation was lack of oxygen delivery equipment in the face of increased oxygen consumption.
The two tertiary COVID-19 designated centres admitted moderate to severe/critical COVID-19 patients who were referred from primary health centres and from the other eight COVID-19 isolation units in the Copperbelt region. Patients who were triaged as severe/critical were expected to require more oxygen or intensive care management. The reason for triaging to the two facilities was to provide high-flow oxygen via C-circuits, high-flow nasal cannulas, and non-invasive and/or invasive ventilation that were not available in the lower COVID-19 facilities. After triage, patients were managed according to the MoH COVID-19 guidelines, which were in line with the World Health Organization (WHO) therapeutic guidelines.[13,14]All severe and critically ill patients were escalated on oxygen based on the MoH and WHO therapeutic guidelines.[13] In addition, all patients routinely received dexamethasone or any available steroid, anticoagulation, and antibiotics when indicated and as outlined in the MoH guidelines. Remdesivir was also routinely ordered for all severely and critically ill patients with <10 days of symptoms or from the day of the SARS-CoV-2 result. However, owing to cost limitations, D-dimers and CRP were measured in 33.0% and 36.0% of patients, respectively, while remdesivir was administered in 21.8% of the eligible patients. Nurse-patient ratios were 1:3 in the COVID-19 ICU and 1:9 in the main COVID-19 wards during the period under review.
Study definitions and outcomes
In this study, chronic kidney disease and new-onset DM definitions were based on the Kidney Disease Improving Global Outcomes (KDIGO)[15] and American Diabetes Association (ADA)[16] criteria, respectively. Patients with a clinical diagnosis of heart failure, hypertensive heart disease and ischaemic heart disease were defined as having cardiovascular disease. The outcome measures were in-hospital outcome (discharge or mortality), and exposures were markers associated with in-hospital COVID-19 mortality.
Statistical analysis
Data analysis was conducted using SPSS Statistics, version 20 (IBM Corp., USA). Pearsons x2test was used to establish associations at the 5% significance level. Independent factors associated with mortality were established using binary logistic regression analysis considering the backward (likelihood ratio) variable selection method, probability of stepwise entry of 0.050 and probability of removal of 0.051. Adjusted odds ratios and their 95% confidence intervals are reported, together with p-values.
Results
In total, 350 COVID-19 medical files of hospitalised patients were included in the study. Almost 60% were aged >55 years and 52.6% were male. The main comorbidities were HTN (49.6%), DM (28.5%) and HIV/ AIDS (22.0%) (Table 1). Compared with women, men were more likely to have had a stroke, to have chronic kidney disease and to have raised serum creatinine. The most common presenting symptoms were shortness of breath (75.9%), cough (74.5%) and fever (56.5%).
In-hospital mortality
Clinical characteristics of patients who survived and those who died are presented in Table 2. The medical files reviewed showed that in-hospital mortality was 42.6%. Mortality was significantly increased in patients aged >55 years (52.0% v. 48.0%) and in those with DM (52.1% v. 47.9%) or cardiac disease (68.0% v. 32.0%). The patients who died were likely to have a raised q-SOFA score and raised admission blood glucose, D-dimers, CRP and serum creatinine. The median (interquartile range (IQR)) time between admission and death in the deceased group was shorter than the median time to discharge for the survivors (2.5 (1 - 6) days v. 6 (3 - 10) days; p<0.001) (Table 2 and Fig. 1). The rate of hyperglycaemia was nearly twice as high in patients with DM compared with those without (81.4% v. 44.9%: p=0.000). Of the 44.9% of patients with hyperglycaemia who did not have DM, 38.7% were subsequently diagnosed with DM. Remdesivir was ordered for 294 patients, of whom 64 (21.8%) accessed the drug, with no significant survival benefit. The median (IQR) duration of admission in the remdesivir group v. the non-remdesivir group was 6 (2 - 10) days v. 4 (2 - 8) days (p=0.070). In multivariate logistic analysis, a q-SOFA score >2, a high admission blood sugar level and age >55 years were associated with in-hospital mortality. In multivariate analysis, raised serum creatinine was not associated with in-hospital mortality despite showing significance in unadjusted analysis.
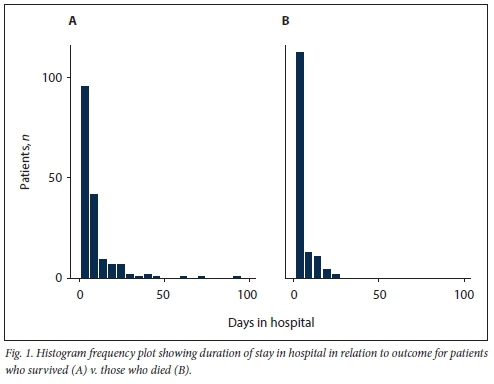
Discussion
This study revealed a high in-hospital COVID-19 mortality rate at nearly 43%, and admission hyperglycaemia, a high q-SOFA score and older age were predictors of in-hospital mortality. The in-hospital overall mortality in this study was higher than reported in previous studies in Europe, China and the USA.[2,17,18] A meta-analysis consisting of 33 studies drawn from Europe, Asia and USA found a 17% overall in-hospital mortality rate.[17] In other studies carried out in tertiary-level hospitals, the in-hospital COVID-19 mortality rates were 26%, 30.1% and 29.7% in Saudi Arabia, Mexico and Italy respectively[19-21] A study from the SEMI-COVID-19 registry in Spain reported 21% overall in-hospital mortality[22] In a retrospective examination of 127 inpatients with COVID-19 in China, Liu et al.[23] found a 16% hospital death rate. In-hospital mortality in a study using the Premier Healthcare Database in the USA was found to be 20.3% after analysis of 35 302 inpatients.[18]
It should be noted that the high in-hospital COVID-19 mortality rate reported in our study confirms the recent findings from the African COVID-19 Critical Care Outcomes Study (ACCCOS)[2]In this multicentre observational study of COVID-19 patients admitted to intensive care and high-care units in 10 African countries, an almost 50% in-hospital mortality was observed, translating to almost 23% more deaths than in developed countries.[2] In the ACCCOS study delayed admission due to shortages of human resources, equipment, etc. was associated with 2-fold odds of in-hospital mortality.[2] The high death rate observed in the present study may therefore also be explained by lack of regional oxygen supply, and limited human resources for an optimal clinician to acute patient care ratio.
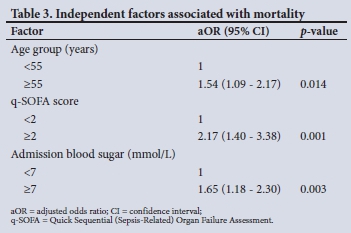
Older age was associated with almost 2-fold odds of COVID-19 in-hospital death in the present study, a finding consistent with previously observed epidemiological and clinical data.[24-26] In previous studies, older age was associated with 6-fold, 1-fold and 1-fold odds of in-hospital death in the Italian, Spanish and African ACCCOS studies, respectively.[2,22,24] Various reasons have been put forward to explain the association of age and COVID-19 death, including underlying comorbidities in the elderly, and impaired lung function and muscle weakness as age progresses.[24]
In the present study, admission hyperglycaemia was associated with almost 2-fold odds of in-hospital mortality, a finding consistent with previous studies.[9,27-28] A retrospective analysis conducted in Boston among patients aged >18 years with and without DM observed that hyperglycaemia in this study was associated with almost 4.5-fold, 4.5-fold, 3.7-fold, 1.9-fold and 4.4-fold odds of in-hospital mortality, intubation, ICU admission, adult respiratory distress syndrome (ARDS) and septic shock, respectively.[27] In the Spanish SEMI-COVID-19 registry, Carrasco-Sánchez et al[9]reported a 15.7% in-hospital death rate in patients with blood glucose levels <140 mg/dL compared with 41% in those with levels > 180 mg/dL. In a meta-analysis of 16 studies examining the association between hyperglycaemia and outcomes, hyperglycaemia was linked with 3.5-fold and 2-fold odds of in-hospital death and severe disease, respectively[28]
Several studies have examined the association between COVID-19, hyperglycaemia and patient outcomes. The hyperglycaemia in patients with COVID-19 results from both direct and indirect mechanisms. Hyperglycaemia has been found to be positively associated with increased levels of inflammatory markers.[27-29] The increased levels of tumour necrosis factor, interleukin-6 and CRP have been observed to increase peripheral insulin resistance,[9,28,29] leading to the release of Cortisol by stimulation of the paraventricular nucleus and epinephrine/norepinephrine excretion post stimulation of the locus coeruleus norepinephrine.[29] SARS-CoV-2 also directly infects the pancreatic beta cells via the angiotensin-converting enzyme 2 (ACE2) receptors,[28] leading to apoptosis and hyperglycaemia
Hyperglycaemia has been observed to be a poor prognostic marker in COVID-19 patients.[30] Hyperglycaemia up-regulates levels of ACE2 receptors and enhances its glycosylation, thereby speeding up internalisation of the virus itself.[30,31] The increased ACE2 expression in various tissues promotes endothelial dysfunction, thromboembolic events, hyperviscosity, coagulation, ARDS, interstitial fibrosis and multisystem failure.[30,31] Furthermore, hyperglycaemia has been shown to impair both adaptive and innate immunity.[31,32]
Consistent with previous studies.[2,23] q-SOFA scores in our study were higher in the patients who died than in those who survived and high scores predicted 2-fold odds of in-hospital mortality. In the ACCCOS multicentre study, a q-SOFA score consisting of two and three factors was associated with respective 2-fold and 4-fold odds of in-hospital COVID-19 death.[2] In resource-limited countries, q-SOFA remains an optimal tool for early identification of patients with severe COVID-19 disease.[23]
We observed a non-significant association between use of remdesivir and patient mortality outcomes, a finding that was observed in previous studies.[33] However, unlike previous studies that showed a quick recovery time for patients in the remdesivir cohort,[34-36] patients on remdesivir in our study showed a trend towards a longer hospital stay. In the presence of severe COVID-19 pneumonia, the clinicians could opt to prescribe remdesivir for longer than 5 days, as was observed in a US veterans study.[37] We also observed no association between HIV and patient outcomes, unlike the ACCCOS study,[2] in which HIV was associated with 1.9-fold odds of in-hospital death. The patients in our study may have been immune competent; however, owing to the retrospective nature of this study, the CD4 cell counts were lacking.
Study strengths and limitations
To our knowledge, the present study is the first to address factors associated with poor in-hospital COVID-19 patient outcomes in Zambia. The study had an adequate sample size, and it has laid a critical foundation in planning for incoming COVID-19 waves in resource-limited countries. However, the study had limitations; it was retrospective in nature, and laboratory markers of renal disease (proteinuria, haematuria) and acute kidney injury (due to non-availability of baseline serum creatinine), and the impact of inadequate delivery of oxygen equipment, were not assessed. Our two hospital COVID-19 sites are tertiary hospitals located in urban areas, and the outcomes may not be representative of outcomes from other hospitals owing to differences in resource capacity, patient characteristics and treatment protocols. It is possible that other confounders that we did not investigate could have contributed to the number of deaths, though this effect is expected to be minimal.
Conclusions
This study has highlighted a high in-hospital COVID-19 mortality rate and the importance of early identification of COVID-19 patients presenting with a high q-SOFA score and hyperglycaemia.
Declaration. None.
Acknowledgements. We thank the nursing teams at the two tertiary-level hospitals who ensured that the medical files were kept well.
Author contributions. All the authors contributed to the conceptualisation of the study, data interpretation, and approval of the final version in line with the internal committee of medical journal editors. SS and JB performed the statistical analysis.
Funding. None.
Conflicts of interest. None.
References
1. Ranzani, OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil A retrospective analysis of nationwide data. Lancet Respir Med 2021;9(4):407-418. https://doi.org/10.1016/s2213-2600(20)30560-9 [ Links ]
2. Biccard BM, Gopalan PD, Miller M, et al. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS). A multicentre, prospective observational cohort study. Lancet 2021;397(10288):1885-1894. https://doi.org/10.1016/S0140-6736(21)00441-4 [ Links ]
3. Rothan HA Byrareddy SN. file epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. https://doi.org/10.1016/j.jaut.2020.102433 [ Links ]
4. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology. A review Clin Immunol 2020;215:108427. https://doi.org/10.1016/j.clim.2020.108427 [ Links ]
5. Becerra-Munoz VM, Nunez-Gil IJ, Eid CM, et al. Clinical profile and predictors of in-hospital mortality among older patients admitted for COVID-19. Age Ageing 2021;50(2):326-334. https://doi.org/10.1093/ageing/afaa258 [ Links ]
6. Tian W. Jiang W, Yao J, et al. Predictors of mortality in hospitalised COVID-19 patients. A systematic review and meta-analysis. J Med Virol 2020;92(10):1875-1883. https://doi.org/10.1002/jmv.26050 [ Links ]
7. Sharifpour M, Rangaraju S, Liu M, et al. C-reactive protein as a prognostic indicator in hospitalised patients with COVID-19. PLoS ONE 2020;15(11):e0242400. https://doi.org/10.1371/journal.pone.0242400 [ Links ]
8. Bannaga AS, Tabuso M, Farrugia A et al. C-reactive protein and albumin association with mortality of hospitalised SARS-CoV-2 patients. A tertiary hospital experience. Clin Med 2020;20(5):463-467. https://doi.org/10.7861/clinmed.2020-0424 [ Links ]
9. Carrasco-Sánchez FJ, López-Carmona MD, Martínez-Marcos FJ, et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status. Data from the Spanish SEMI-COVID-19 Registry Ann Med 2021;53(1):103-116. https://doi.org/10.1080/07853890.2020.1836566 [ Links ]
10. Mwananyanda L, Gill CJ, MacLeod W. et al. Covid-19 deaths in Africa. Prospective systematic postmortem surveillance study BMJ 2021;372.:n334. https://doi.org/10.1136/bmj.n334 [ Links ]
11. Stein F, Perry M, Banda G, et al. Oxygen provision to fight COVID-19 in sub-Saharan Africa. BMJ Glob Health 2020;5(6):e002786. https://doi.org/10.1136/bmjgh-2020-002786 [ Links ]
12. Chipimo PJ, Barradas DT, Kayeyi N, et al. First 100 persons with COVID-19 - Zambia, March 18 - April 28, 2020. MMWR Morb Mortal Wkly Rep 2020;69(42):1547-1548. https://doi.org/10.15585/mmwrmm6942a5 [ Links ]
13. Rochwerg B, Agarwal A, Siemieniuk RAC, et al. A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379. https://doi.org/10.1136/bmj.m3379 [ Links ]
14. World Health Organization. Therapeutics and COVID-19. Living guideline, 20 November 2020. Geneva. WHO, 2020. https://apps.who.int/iris/handle/10665/336729 (accessed 15 December 2020). [ Links ]
15. Levey AS, Eckardt KU, Tsukamoto Y et ai Definition and classification of chronic kidney disease. A position statement from Kidney Disease. Improving Global Outcomes (KDIGO). Kidney Int 2005;67(6):2089-2100. https://doi.org/10.1111/j.l523-1755.2005.00365.x [ Links ]
16. American Diabetes Association. Classification and diagnosis of diabetes. Standards of medical care in diabetes - 2020. Diabetes Care 2020;43(Suppl 1).s14-s31. https://doi.org/10.2337/dc20-S002 [ Links ]
17. Macedo A, Goncalves N, FebraC. COVID-19 fatality rates in hospitalised patients. Systematic review and meta-analysis. Ann Epidemiol 2021;57:14-21. https://doi.org/10.1016/j.annepidem.2021.02.012 [ Links ]
18. Rosenthal N, Cao Z, Gundrum J, et al. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open 2020;3(12):2029058. https://doi.org/10.1001/jamanetworkopen.2020.29058 [ Links ]
19. Khamis F, Memish Z, Al Bahrani M, et al. Prevalence and predictors of in-hospital mortality of patients hospitalised with COVID-19 infection. J Infect Public Health 2021;14(6):759-765. https://doi.org/10.1016/j.jiph.2021.03.016 [ Links ]
20. Olivas-Martínez A, Cárdenas-Fragoso JL, Jiménez JV, et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City. Causes of death, risk factors and the impact of hospital saturation PLoS ONE 2021;16(2):e0245772. https://doi.org/10.1371/journaipone.0245772 [ Links ]
21. Bellan M, Patti G, Hayden E, et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalised COVID-19 patients. Sci Rep 2020;10(1):2073. https://doi.org/10.1038/s41598-020-77698-4 [ Links ]
22. Artero A, Madrazo M, Fernández-Garces M, et al. Severity scores in COVID-19 pneumonia. A multicenter, retrospective, cohort study. J Gen Intern Med 2021;36(5):1338-1345. https://doi.org/10.1007/s11606-021-06626-7 [ Links ]
23. Liu S, Yao N, Qiu Y, et ai. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am J Emerg Med 2020;38(10):2074-2080. https://doi.org/10.1016/j.ajem.2020.07.019 [ Links ]
24. Ho FK, Petermann-Rocha F, Gray SR, et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 2020;15(11):e0241824. https://doi.org/10.1371/journal.pone.0241824 [ Links ]
25. Shi C, Wang L, Ye J, et al. Predictors of mortality in patients with coronavirus disease 2019. A systematic review and meta-analysis. BMC Infect Dis 2021;21(1):663. https://doi.org/10.21203/rs.3.rs-33164/vl [ Links ]
26. Sarfaraz S, Shaikh Q, Saleem SG, et al. Determinants of in-hospital mortality in COVID-19. A prospective cohort study from Pakistan. PLoS ONE 2021;16(5):e0251754. https://doi.org/10.1101/2020.12.28.20248920 [ Links ]
27. Charoenngam N, Alexanian SM, Apovian CM, et al. Association between hyperglycemia at hospital presentation and hospital outcomes in COVID-19 patients with and without type 2 diabetes. A retrospective cohort study of hospitalised inner-city COVID-19 patients. Nutrients 2021;13(7):2199. https://doi.org/10.3390/nu13072199 [ Links ]
28. Yang Y, Cai Z, Zhang J. Hyperglycemia at admission is a strong predictor of mortality and severe/critical complications in COVID-19 patients: A meta-analysis. Biosci Rep 2021;41(2):BSR20203584. https://doi.org/10.1042/bsr20203584 [ Links ]
29. Marik PE, Beliomo R. Stress hyperglycemia. An essential survival response! Crit Care 2013;17(2):305-305. https://doi.org/10.1186/ccl2514 [ Links ]
30. Lim S, Bae JH, Kwon H, et al. COVID-19 and diabetes mellitus. From pathophysiology to clinical management. Nat Rev Endocrinol 2021;17(1):11-30. https://doi.org/10.1038/s41574-020-00435-4 [ Links ]
31. Wang J, Meng W. COVID-19 and diabetes. The contributions of hyperglycemia. J Mol Cell Biol 2020;12(12):958-962.https://doi.org/10.1093/jmcb/mjaa054 [ Links ]
32. Xiu F, Stanojcic M, Diao L. et al. Stress hyperglycemia, insulin treatment, and innate immune cells. Int ] Endocrinol 2014;2014:486403. https://doi.org/10.1155/2014/486403 [ Links ]
33. Singh S, Khera D, Chugh A, et al. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2. A systematic review and meta-analysis. BMJ Open 2021;11(6):e048416. https://doi.org/10.1136/bmjopen-2020-048416 [ Links ]
34. Beigel JH, TomashekKM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl I Med 2020;383(19):1813-1826. https://doi.org/10.1056/NEJMoa2007764 [ Links ]
35. Rezagholizadeh A, Khiali S, Sarbakhsh P, et al. Remdesivir for treatment of COVID-19: An updated systematic review and meta-analysis. Eur J Pharmacol 2021;897:173926. https://doi.org/10.1016/j.ejphar.2021.173926 [ Links ]
36. Reddy Vegivinti CT, Pederson JM, Saravu K, et al. Remdesivir therapy in patients with COVID-19. A systematic review and meta-analysis of randomised controlled trials. Ann Med Surg 2021,62:43-48. https://doi.org/10.1016/j.amsu.2020.12.051 [ Links ]
37. Ohl ME, Miller DR, Lund BC, et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalised with COVID-19. JAMA Netw Open 2021;4(7):e2114741. https://doi.org/10.1001/jamanetworkopen.2021.14741 [ Links ]
 Correspondence:
Correspondence:
J Banda
katusib@yahoo.co.uk
Accepted 12 January 2022














