Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.12 Pretoria Dez. 2021
http://dx.doi.org/10.7196/samj.2021.v111i12.15682
RESEARCH
Stroke: A retrospective review of the incidence and epidemiology in a South African academic hospital emergency department
S TribelhornI; F MotaraII; C M LewisIII
IBSc, MB BCh; Department of Emergency Medicine, School of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIBA, MB BCh, FCFP, ACEM, Dip HIV Man (SA), CAHM; Department of Emergency Medicine, School of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIFCEM (SA), MMed (Emerg Med), Dip PEC (SA), Dip ROM (RCSEd); Department of Emergency Medicine, School of Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Stroke is a leading cause of morbidity and mortality. Most deaths occur in low- and middle-income countries, with the incidence predicted to increase as populations undergo socioeconomic and epidemiological changes. Knowledge of contributing factors in a South African (SA) population can be used to drive healthcare initiatives to modify this burden of disease
OBJECTIVES: To analyse epidemiological data on patients with stroke presenting to an emergency department in Johannesburg, SA
METHODS: The study was a 12-month descriptive, retrospective review of medical records, undertaken at a tertiary-level hospital. Patients' records were selected based on the presumptive diagnosis of stroke. Data collected included ethnicity, age, gender, risk factors, signs and symptoms at presentation, and computed tomography (CT) brain scan findings
RESULTS: Of 312 records reviewed, 160 were eligible for inclusion. The mean age of the patients included was 57.7 years, and 64 patients (40%) had CT-confirmed haemorrhagic strokes. Hypertension was the most common comorbidity/risk factor identified, in the sample as a whole (n=93; 58%) and in both patients with haemorrhagic (n=33; 52%) and ischaemic strokes (n=56; 61%). Diabetes was the second most common comorbidity/risk factor (n=23; 14%), and was three times more likely in patients with ischaemic strokes (n=19; 20%) compared with haemorrhagic strokes (n=4; 6%
CONCLUSIONS: Stroke is an important healthcare concern for SA. Our study demonstrated a lower mean age of patients presenting with stroke compared with high- and upper middle-income countries, and a higher proportion of haemorrhagic strokes. Several modifiable risk factors such as hypertension and diabetes were identified. Data collection on a regional and national level is important to drive targeted healthcare initiatives
Stroke is the second leading cause of mortality globally, resulting in >6 million deaths per year, and is the third leading cause of adult physical disability.[1-4] The majority of deaths resulting from stroke occur in low- and middle-income countries (LMICs).[5] The incidence of stroke is increasing in sub-Saharan Africa, including in South Africa (SA), as the region undergoes socioeconomic and epidemiological changes resulting in an increased burden of non-communicable diseases and an ageing population.[6,7]
Stroke was declared a catastrophic illness in SA at the Joint World Congress of Stroke in 2007.[6] In SA, stroke is the second leading cause of death after HIV/AIDS.[5] Although community-based studies and population surveillance data are lacking, the crude stroke mortality was estimated to be 127/100 000 in people aged >35 years in rural SA.[7]
Stroke is largely preventable with appropriate control of modifiable comorbidities and/or risk factors that contribute to this disease.[6] The most commonly associated modifiable comorbidities and/or risk factors that contribute to stroke are hypertension, smoking, obesity, high cholesterol, physical inactivity, low fruit and vegetable intake, diabetes and alcohol consumption.[6] Other contributing factors include increasing age and HIV infection. Africa has the highest burden of hypertension worldwide, which is generally considered the strongest risk factor for stroke.[8,9] People with hypertension are three to four times more likely to have a stroke.[10] In a study of patients attending general practice, it was found that hypertension affects all race groups in SA and was present in more than half of the patients attending general practice.[9] Furthermore, this study found that black patients were more likely to have hypertension compared with other race groups.[9] Similar studies in the USA found that African American patients were more likely to have hypertension than other race groups.[9]
Stroke incidence and management in SA are further complicated by the high burden of HIV infection, with an estimated prevalence of 20% and the majority of people infected aged <50 years.[11] HIV is an important comorbidity/risk factor for stroke with multiple mechanisms implicated, including vasculopathy, coagulopathy and an increased likelihood of opportunistic infections.[12]
Advances in the acute management of stroke as well as in rehabilitation and long-term care have resulted in lower mortality and dependence, especially in high-income countries (HICs).[13] The most recent SA stroke care guidelines (2010) recommend that patients with a suspected stroke should be managed by a dedicated stroke unit, including medical staff, physiotherapists, occupational therapists, speech therapists and social workers.[6] There is evidence that outcomes improve when stroke patients are prioritised and receive early brain imaging, when their candidacy for thrombolysis is assessed early, and when ongoing hospital management is by a multidisciplinary team. There has been a drive towards establishment of stroke units across the country to improve the care of stroke patients, but the reality remains that most people who suffer a stroke in SA will have limited access to stroke care and rehabilitation.[14]
The primary imaging modality used in stroke diagnosis is the computed tomography (CT) scan. The goal of early CT scanning is to assist in the diagnosis of the patient who presents with signs and symptoms consistent with a stroke, to determine the presence of intracranial haemorrhage, and to rule out space-occupying lesions and stroke mimics.[15] The rapid performance of a CT scan enables timely decision-making with regard to potential thrombolysis and ongoing management.
While the exact cost of stroke care in SA is not known, it is reasonable to infer that the high stroke burden places great pressure on the country's healthcare system, leading to significant healthcare expenditure in addition to the social costs incurred by patients and their families. A recent estimate is that stroke accounts for 1.6 - 3% of healthcare expenditure, with 80% of that incurred for inpatient care.[16] To improve stroke care and mitigate the effect that strokes have on the SA healthcare system, local data are needed to effectively plan the delivery of stroke services and prevention strategies.[8]
There is a paucity of data on stroke in SA. The healthcare discrepancies between the public and private sectors as well as geographically between urban and rural areas make it difficult to determine the actual burden of disease. Stroke registries could potentially bridge this lack of knowledge. They provide epidemiological data and data on current healthcare initiatives and could guide new policy making, not only to keep up with global standards but also to tailor policy making for our very heterogeneous population. National and regional stroke registries are used in many countries around the world, but SA has yet to develop and implement one.
Objectives
To analyse recent epidemiological data on patients presenting to a tertiary-level government hospital in Johannesburg, SA, with acute neurological deficit suggestive of a stroke.
Methods
The study was a descriptive, retrospective review of medical records, conducted in the emergency department (ED) at Helen Joseph Hospital, a tertiary academic hospital. The ED mostly attends to adult patients because there is a mother and child hospital nearby. Stroke is primarily managed by ED physicians, with admission to and further management by the internal medicine department. There is no dedicated stroke unit. The sample was collected over a 1-year period from 1 January to 31 December 2018.
Patients were selected from ED registers based on the recorded diagnoses. Diagnoses used to select patients included stroke or cerebrovascular accident (CVA), focal neurological signs (such as hemiparesis and gait abnormalities, visual disturbances and pupillary findings, aphasia and seizures) or altered mental status. ED clerking sheets were obtained from the records department, and further information was gathered from CT scan reports.
A standardised data collection sheet was used to gather raw data. This was solely done by the principal investigator (ST). Data collected from the ED clerking sheet included age, gender, comorbidities and risk factors for stroke, presenting signs and symptoms (including triage blood pressure (BP) or the first BP reading), and time from onset of the neurological deficit to presentation to the ED. This information was recorded by the ED doctor who assessed the patient at the time of presentation. All patients who were included had a CT scan from the ED, and these results were also collected.
Exclusion criteria included missing files and ED clerking sheets or missing records of CT scan results, patients <18 years of age, patients who presented to the ED but did not have a CT scan at the time of initial consultation, and any patient seen for a primary trauma-related injury or whose final diagnosis was something other than stroke.
Raw data were captured by the researcher and were analysed using SPSS version 22 (IBM, USA). Descriptive statistics were presented as frequencies and percentages for categorical variables and as means, standard deviations (SDs), medians and ranges for continuous numerical data. Normality of distribution was examined by testing the skewness of data for gender (0.2), age (0.1) and stroke types (0.4). The skewness value for each of these variables was <0.5 and therefore an indication of sufficiently normally distributed data. Parametric statistical methods were therefore used in the subsequent analysis. The prevalence of stroke types by patient demographics was compared using x2 and Fisher's exact tests. The independent-samples f-test was used to do the same according to patient age. A binomial logistic regression model was run to determine the odds ratios (ORs) for ischaemic v. haemorrhagic stroke in terms of underlying stroke risk factors. Significance testing was set at the 95% confidence level (95% CI), and a p-value <0.05 therefore indicated statistically significant differences.
The study was assessed and approved by the postgraduate assessors meeting of the University of the Witwatersrand, and final ethics approval was obtained from the Human Research Ethics Committee of the University (ref. no. M1911155).
Results
Of a total of 312 ED patient records that were reviewed based on triage diagnosis, 160 were eligible for the study and were included in the analysis. The remainder of the records were excluded because of missing clerking sheets, a final diagnosis other than stroke, patients who did not receive a CT scan from the ED, or missing CT scan data.
Demographic data
Of the 160 patients included in the study, 87 (54%) were female and 73 (46%) were male. The age range was 28 - 90 years (mean (SD) 57.7 (14.9) years). When the mean age of stroke patients was compared between race groups, the mean age for black patients was significantly younger than that for white patients (p<0.01) and Indian patients (p=0.04), but was not significantly different from that for coloured patients (p=0.97). Demographic data are set out in Table 1.
CT scan findings demonstrated that ischaemic stroke was more prevalent in the sample (n=96; 60%) compared with haemorrhagic stroke (n=64; 40%). Mean age of stroke type and type by gender are presented in Table 2. Ischaemic stroke was more prevalent among females (n=56) compared with males (n=40), i.e. 64% v. 55%, while haemorrhagic stroke was more prevalent among males (n=33) compared with females (n=31), i.e. 45% v. 36%. However, these differences based on gender were not statistically significant (p=0.22).
Patients with haemorrhagic stroke (mean (SD) 53.1 (13.2) years) were significantly younger than patients with ischaemic stroke (mean (SD) 60.7 (15.3) years) (p=0.01).
Males were more likely than females to have a stroke at a younger age in both the ischaemic (p=0.01) and haemorrhagic groups (p=0.02).
Comorbidities, risk factors and stroke
The comorbidities and risk factors noted in our sample are presented in Table 3.
Hypertension was the most common comorbidity/risk factor identified in the sample. The proportion of hypertensive ischaemic stroke patients (n=59; 61%) was higher than that of hypertensive haemorrhagic stroke patients (n=33; 52%). However, this was a marginal and statistically non-significant difference (p=0.94).
Diabetes was the second most common comorbidity/risk factor identified in the sample. The proportion of diabetic ischaemic stroke patients (n=19; 20%) was higher than that of diabetic haemorrhagic stroke patients (n=4; 6%). In this sample, patients with diabetes were more likely to experience an ischaemic stroke than a haemorrhagic stroke (p=0.02).
Likelihood ratio tests were performed for each comorbidity for ischaemic v. haemorrhagic stroke. Diabetes was three times more likely in ischaemic stroke patients compared with haemorrhagic stroke patients (OR 3.42; 95% CI 1.03 - 11.33; p=0.04). There were no significant differences in the ORs for the other risk factors noted in Table 3. Comorbidities and risk factors that are associated with stroke, such as obesity and hypercholesterolaemia, were not included in this sample.
Signs and symptoms at presentation
Signs and symptoms were recorded on the ED clerking sheet. BP and Glasgow Coma Scale (GCS) score were not documented for all patients, but the data available were analysed. Missing data is therefore a limitation of the study in this regard. In our sample it was noted that patients who presented with a higher BP and suspected stroke were more likely to have a haemorrhagic stroke as opposed to an ischaemic stroke, as seen in Table 4 (p=0.04). Although this finding is strongly significant, it should be interpreted with caution in view of the missing data.
Patients who presented with a lower GCS score and a stroke were more likely to have a haemorrhagic stroke than an ischaemic stroke, as seen in Table 4 (p=0.01). Again, this significant finding should be interpreted in the light of limited available data.
Most patients who presented to the ED had some focal neurological signs that assisted in making the diagnosis of stroke, most commonly left or right hemiplegia. Other signs that were present in the sample included visual disturbances, confusion, vomiting and headache, but these symptoms were neither common nor significant.
Timing of symptom onset
The majority of patients who presented to the ED with a stroke were beyond the thrombolysis window (which extends to 3 - 4.5 hours), as seen in Table 5.
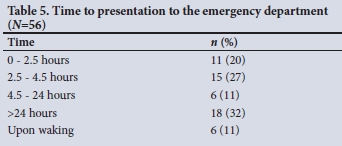
CT scan findings
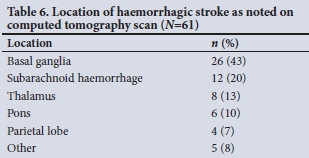
The CT scan findings were reported by radiologists. The locations of intracerebral pathology for haemorrhagic strokes are presented in Table 6.
In 64 of the CT scans that demonstrated haemorrhagic strokes, the extent of the intracranial bleed was commented on by the radiologist: 37 (56%) had evidence of intraventricular extension of the haemorrhage on the CT scan, 27 (39%) had evidence of midline shift, and 8 (13%) had evidence of tonsillar herniation.
The location of ischaemic stroke was described in 67 CT scans. The parietal lobe was most frequently affected (n=24; 36%). The affected vessel was described more readily on the CT scans that noted an ischaemic stroke (n=95) compared with the description on the location. The middle cerebral artery was most commonly affected (n=85; 89%).
The radiologists commented on the temporal nature of 73 ischaemic lesions noted on CT scans. Of these lesions, 41% were acute, 55% were subacute, and 4% were mixed subacute and chronic.
Discussion
The ED at Helen Joseph Hospital attends to ~60 000 patients per year. Our study sample of 160 patients (0.27%) is comparable to the crude incidence of stroke estimated in a population-based study in the Agincourt subdistrict in Mpumalanga Province, a rural part of SA (244 per 100 000).[5]
The incidence of stroke increases with increasing age. It is estimated that the risk of stroke doubles every decade above 55 years.[11] In sub-Saharan Africa, stroke commonly affects people aged >60 years, while in developed countries the incidence increases most rapidly between 70 and 75 years.[11] The mean age of patients in our study was 57.7 years, which is comparable to the 59.8 years in a similar study recently conducted in KwaZulu-Natal Province,[17] showing that stroke occurs at younger ages in sub-Saharan Africa. It was also noted that males had strokes at younger ages than females. Having a stroke at a relatively young age means more disease-adjusted life-years and years lived with disability for stroke survivors, as well as higher socioeconomic and psychosocial costs for survivors who are no longer able to provide for family members and dependants.[18]
The majority of patients in the present study were black (54%). While the study reflected some of the ethnic diversity seen in urban areas in SA, in more rural areas the population is predominantly black and little diversity exists. The mean age of stroke in black patients was younger than in the other races in this study.
The incidence of ischaemic v. haemorrhagic stroke differs depending on the population that is studied. It is generally considered that ischaemic stroke accounts for 80 - 85% of all stroke cases, and haemorrhagic stroke for 15 - 20%.[6,10] In HICs, such as the USA, ischaemic strokes contribute to 87% of the stroke burden.[19] The highest incidence of haemorrhagic stroke cases and deaths occur in LMICs.[13,20] Previous studies showed that haemorrhagic stroke accounted for a larger proportion of strokes in Africa (34% of stroke cases).[10] In our sample, haemorrhagic strokes accounted for 40% of total cases.
Hypertension was the most common comorbidity/risk factor, being present in 45% of the patients in our sample. This diagnosis was based on history at presentation to the ED. This figure is comparable to a study that examined vascular comorbidities/risk factors in general practice, in which it was found that 55% of all patients attending general practice had hypertension.[9] We expected a high proportion of patients to have hypertension. While most of the cases of high BP measured in the ED are due to premorbid hypertension, an acute hypertensive response may occur in some patients following a stroke. This response occurs in the first 24 hours after the stroke and is defined as an elevated BP above premorbid values.[21] The pathophysiology is multifactorial, but centres around disruption of autoregulatory pathways that leads to increased sympathoadrenal drive while stifling parasympathetic mechanisms, perpetuated stress response, and increased intracranial pressure. However, in some cases it may be due to underlying undiagnosed hypertension.[21]
There is a trend, as noted in this study, towards hypertension, previous CVA and cardiovascular disease being more prevalent in coloured and Indian population groups presenting with new-onset stroke, but this warrants further investigation and is beyond the scope of our current study.
Hypertension is an important comorbidity/risk factor for haemorrhagic stroke and is more likely to result in deep intracerebral haemorrhage (ICH) rather than lobar ICH.[20] The mean age of patients who experienced a haemorrhagic stroke in our study was younger, 53.1 years, compared with the mean age of 60.7 years for ischaemic stroke. We also noted that younger males were more likely to have haemorrhagic stroke than ischaemic stroke.
Half of the haemorrhagic strokes were subarachnoid haemorrhages (SAHs). In the present study, we did not differentiate between aneurysmal v. non-aneurysmal SAH. Most of the haemorrhagic strokes were located in the basal ganglia. The basal ganglia is the most common site for spontaneous ICH and is associated with the worst prognosis.[22] Of the patients with haemorrhagic stroke, more than half had evidence of intraventricular extension, which is a poor prognostic factor. Other poor prognostic factors for ICH include low initial GCS score, large volume of the bleed (>30 cm3), infratentorial origin of the ICH, old age (>80 years), advanced white-matter lesions, being underweight at admission, hyperglycaemia at admission, and chronic kidney disease.[20,22] The outcomes of patients with ICH are beyond the scope of this study.
Diabetes is an independent comorbidity/risk factor for stroke and is more commonly associated with ischaemic stroke.[23] Diabetes causes microvascular and macrovascular changes that can ultimately result in stroke.[24] Of our sample, 14% had diabetes. We found that diabetes was three times more likely in ischaemic stroke than in haemorrhagic stroke.
HIV contributes to an increased risk of stroke.[24] The present sample included 12% of patients who had HIV. Considering the high burden of HIV in SA, this is likely to be an underestimate, which we attribute to poor history taking, inadequate record keeping and patients being unaware of their diagnosis at the time of presentation. It was also not always clear whether patients with known HIV infection were on antiretroviral therapy. A recent study in KwaZulu-Natal found a high prevalence of HIV in stroke of 20.58%.[17]
The timing of symptom onset was not recorded for every patient. It was noted that some patients presented to the ED within 4.5 hours, the current window in which thrombolysis could be considered, but the majority presented beyond this window. A study done at the same hospital, analysing the delays to CT scan in stroke patients, noted that the average time to ED presentation was 33 hours and the average time to CT scan 6 hours.[25] There are many reasons for delays in hospital presentation, ranging from failure to recognise the symptoms of stroke to transport issues, while in hospital the doctor may not initially recognise the symptoms of stroke, or the CT scan may be delayed.[25] This study did not take into account barriers to and delays in patients getting a CT scan once in the hospital, but undoubtedly these may have increased the time it took to make a radiological diagnosis, even if the patient initially presented within the intervention window for thrombolysis.
Study limitations
This study is limited by variable accuracy of completion of healthcare records by doctors and the storage and maintenance of the clinical files in order to review clinical notes. Data required for the study may not have been present in the files. Future interventions such as detailed templates, clinical auditing and feedback, dictation of records to clerks and electronic-based records could be implemented to improve overall medical record keeping. Furthermore, hospital-based and nationally based databases could yield much more information on stroke patients, with the aim of optimising their care.
Conclusions
This study reflects the burden of stroke faced in SA, particularly for individuals with underlying comorbidities and risk factors such as hypertension and diabetes. On average, stroke in our study occurred at a younger age than in the developed world. Male patients had first strokes at younger ages than female patients. There was a high proportion of CT-confirmed haemorrhagic strokes, many of which had both clinical and radiological features indicating poor prognosis.
Stroke remains an important healthcare burden, with its incidence predicted to increase. It is clear that small hospital-based studies are not adequate to describe the growing epidemic of stroke in SA. Furthermore, it is clear that primary healthcare initiatives need to be the focus of ongoing stroke care in SA, with early recognition of comorbidity and early treatment.
Information such as that from this study, along with other similar studies, could be used to develop a national stroke registry. National stroke registries may be the key in providing up-to-date epidemiological data on stroke, and could therefore be used to guide future healthcare initiatives.
Declaration. The research for this study was done in partial fulfilment of the requirements for ST's MMed (Emergency Medicine) degree at the University of the Witwatersrand.
Acknowledgements. Successful conduct of this study was made possible by the Helen Joseph Hospital ED and radiology staff.
Author contributions. The study idea was conceived by ST. Data were collected and analysed by ST. All authors revised the manuscript and approved the final version.
Funding. None.
Conflicts of interest. None.
References
1. Xing L, Jing L, Tian Y, et al. High prevalence of stroke and uncontrolled associated risk factors are major public health challenges in rural northeast China: A population-based study. Int J Stroke 2020;15(4):399-411. https://doi.org/10.1177/1747493019851280 [ Links ]
2. Feign VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120(3):439-488. https://doi.org/10.1161/CIRCRESAHA.116.308413 [ Links ]
3. GBD 2016 Mortality Collaborators. Global, regional and national under-5 mortality, adult mortality, age-specific mortality, and life expectance, 1970 - 2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1084-1150. https://doi.org/10.1016/S0140-6736(17)31833-0 [ Links ]
4. Rangani E, Matizirofa L. An analysis of recent stroke cases in South Africa: Trend, seasonality and predictors. S Afr Med J 2020;110(2):92-99. https://doi.org/10.7196/SAMJ.2020.v110i2.013891 [ Links ]
5. Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: An estimate of incidence, mortality and disability adjusted life years. BMC Neurol 2015;15(54):1-12. https://doi.org/10.1186/s12883-015-0311-7 [ Links ]
6. Bryer A, Conner MD, Haug P, et al. South African guideline for management of ischaemic stroke and transient ischaemic attack 2010: A guideline from the South African Stroke Society (SASS) and the SASS Writing Committee. S Afr Med J 2010;100(11):747-778. https://doi.org/10.7196/SAMJ.4422 [ Links ]
7. Conner MD, Walker R, Modi G, et al. Burden of stroke in black populations in sub-Saharan Africa. Lancet Neurol 2007;6(3):269-278. https://doi.org/10.1016/S1474-4422(07)70002-9 [ Links ]
8. Owalabi M, Olowoyo P, Popoola F, et al. The epidemiology of stroke in Africa: A systematic review of existing methods and new approaches. J Clin Hypertens 2018;20:47-55. https://doi.org/10.1111/jch.13152 [ Links ]
9. Conner M, Rheeder P, Bryer A, et al. The South African Stroke Risk in General Practice Study. S Afr Med J 2005;95(5):334-339. https://www.ajol.info/index.php/samj/article/view/134606 [ Links ]
10. Donkor ES. Stroke in the 21st century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat 2018;2018:3238165. https://doi.org/10.1155/2018/3238165 [ Links ]
11. Avert. HIV and AIDS in South Africa. https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/south-africa (accessed 20 November 2020). [ Links ]
12. Conner M. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 2007;78(12):1291. https://doi.org/10.1136/jnnp.2007.116103 [ Links ]
13. Brainin M, Teuschl Y, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol 2007;6(6):553-561. https://doi.org/10.1016/S1474-4422(07)70005-4 [ Links ]
14. Ntsiea MV. Current stroke rehabilitation services and physiotherapy research in South Africa. S Afr J Physiother 2019;75(1):a475. https://doi.org/10.4102/sajp.v75i1.475 [ Links ]
15. Broder J, Preston R. An evidence-based approach to imaging of acute neurological conditions. EBMedicine.net 2007. https://www.ebmedicine.net/topics/imaging/imaging-neurologic-disorders (accessed 13 February 2019). [ Links ]
16. Maredza M, Chola L. Economic burden of stroke in a rural South African setting. eNeurological Sci 2016;3:26-32. https://doi.org/10.1016/j.ensci.2016.01.001 [ Links ]
17. Ferris SG, Naicker B. Acute stroke in the emergency department: A chart review at KwaZulu-Natal hospital. S Afr Fam Pract 2020;62(1):a5126. https://doi.org/10.4102/safp.v62i1.5126 [ Links ]
18. Bertram MY, Katzenellenbogen J, Vos T, et al. The disability adjusted life years due to stroke in South Africa in 2008. Int J Stroke 2013;8(Suppl A100):76-80. https://doi.org/10.1111/j.1747-4949.2012.00955.x [ Links ]
19. Ovbiagele B, Nguyen-Huynh MN. Stoke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics 2011;8:319-329. https://doi.org/10.1007/s13311-011-0053-1 [ Links ]
20. An SJ, Kim TK, Yoon B. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke 2017;19(1):3-10. https://doi.org/10.5853/jos.2016.00864 [ Links ]
21. Qureshi AI. Acute hypertensive response in patients with stroke. Circulation 2018;118:176-187. https://doi.org/10.1161/CIRCULATIONAHA.107.723874 [ Links ]
22. Kwon SM, Choi KS, Yi HJ, et al. Impact of brain atrophy on 90-day functional outcome after moderate-volume basal ganglia hemorrhage. Sci Rep 2018;8:4819. https://doi.org/10.1038/s41598-018-22916-3 [ Links ]
23. Chen R, Ovbiagele B, Feng W Diabetes and stroke: Epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci 2016;351(4):380-386. https://doi.org/10.1016/j.amjms.2016.01.011 [ Links ]
24. Tipping B, de Villiers L, Wainwright H, et al Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 2007;78(12):1320-1324. https://doi.org/10.1136/jnnp.2007.116103 [ Links ]
25. Khalema D, Goldstein LN, Lucas S. A retrospective analysis of the time delays in patients presenting with stroke to an academic emergency department. S Afr J Radiol 2018;22(1):a1319. https://doi.org/10.4102/sajr.v22i1.1319 [ Links ]
 Correspondence:
Correspondence:
S Tribelhorn
sophia.tribelhorn@gmail.com
Accepted 12 August 2021














