Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 n.11 Pretoria Nov. 2021
http://dx.doi.org/10.7196/samj.2021.v111i11.15947
IN PRACTICE
ISSUES IN MEDICINE
COVID-19 complications in a small-town hospital in South Africa
G CociI; B L RaynerII
IMB BCh, MRCP (UK); Consultant physician, Plettenberg Bay, South Africa
IIMB ChB, FCP (SA), MMed (Int Med), PhD; Senior Research Scholar, Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
South Africa has experienced three deadly waves of the COVID-19 pandemic with devastating consequences, but little is known about the experiences in small-town hospitals in the country. Between May 2020 and June 2021, author GC treated ~100 confirmed COVID-19 cases. This retrospective case series report describes 10 of these cases, 7 with unusual complications and 3 with sudden death.
This series of case reports describes 10 cases out of a total of ~100 patients with COVID-19 admitted to a hospital in a small coastal town in the Western Cape region of South Africa (SA) from May 2020 to June 2021 and treated by author GC. The nearest tertiary care facilities are 150 km away, so general physicians have to treat many problems that would normally be referred to specialised centres.
The study was approved by the Health Sciences Research Ethics Committee of the University of Cape Town (ref. no. 258/2021).
Case reports
Cases 1 and 2: Exercise after COVID-19 -a cautionary tale
These two cases illustrate the importance of medical review before exercising after COVID-19 infection.
Case 1 was a woman in her forties, who was admitted in July 2020 with severe COVID-19 pneumonia. She had no known comorbidities and exercised regularly. There was a strong family history of diabetes, and during her admission she developed type 2 diabetes. She responded well to diet and oral agents and made an uneventful recovery. Six weeks later, on her own initiative, she decided to report back for a check-up prior to resuming running.
Findings on physical examination were unremarkable apart from the presence of sinus tachycardia of 100 bpm with frequent ectopic beats. Her blood pressure was 135/96 mmHg and her oxygen saturation (sats) 98%. An electrocardiogram (ECG) showed sinus rhythm with multiple ventricular ectopics (Fig. 1). On a multistage treadmill test, there were runs of ventricular tachycardia. Twenty-four-hour Holter monitoring showed frequent ventricular ectopics, bigeminy, couplets and ventricular tachycardia (VT).
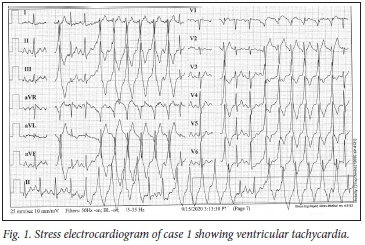
The diagnosis was almost certainly the result of a COVID-19 myocarditis that resulted in scarring of the myocardium. A cardiac electrophysiological study identified a number of arrhythmogenic foci in the left ventricular outflow tract from which VT could be induced. These were ablated, and VT could no longer be induced. The patient is now able to exercise safely.
Case 2 was a fit woman in her thirties, with no previous medical history. She contracted mild COVID-19 pneumonia in July 2020, for which she was treated at home. Her course was uncomplicated and she recovered well. About one month later she started jogging again, and soon noticed that exertion brought on significant dyspnoea and pleuritic chest pain. She consulted her general practitioner, who did not find any clinical or ECG abnormalities and referred her to GC's practice for an echocardiogram. This documented the presence of a small posterior pericardial effusion, probably related to COVID-19 infection (Fig. 2).
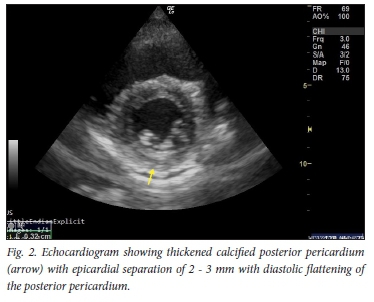
Cardiac complications of COVID-19 include myocardial injury, myocarditis, acute myocardial infarction, heart failure, dysrhythmias, pericarditis and venous thromboembolic events.[1] The prevalence is unclear, but acute myocarditis may account for up to 7% of deaths. It may present with a variety of symptoms including chest pain, dyspnoea, dysrhythmia, and acute left ventricular dysfunction with cardiogenic shock. Serum troponin values are generally abnormal. The ECG can demonstrate a range of findings including nonspecific ST-segment-T-wave abnormalities, T-wave inversion, PR-segment and ST-segment deviations (depression and elevation) and ventricular arrhythmias. The echocardiogram may be helpful in distinguishing pericarditis from acute myocardial infarction. The likely pathophysiology is a combination of the direct viral insult to cardiomyocytes, the immune response to virally infected myocardium, and drugs that prolong the QT interval.[2]
The later cardiac effects of COVID-19 are less well described, and milder cases of myocarditis may go unrecognised and pose potential risks. This possibility understandably leads to caution when advising a return to physical activity after infection. Without evidence from robust studies to inform practice, all current guidance to date is based on consensus or expert opinion.[3] The current consensus is to risk-stratify patients before recommending a return to physical activity. Patients with ongoing symptoms, who had severe COVID-19 or with a history suggestive of cardiac involvement, need further clinical assessment that may include clinical examination, resting and effort ECGs, echocardiography and Holter monitoring, and cardiac magnetic imaging if indicated. In all other cases, after at least 7 days free of symptoms, minimal exertion may be undertaken for 2 weeks before increasing activity.[3] However, case 2 did not meet the criteria for detailed investigations, as a potentially fatal ventricular tachycardia was only detected on Holter monitoring. Perhaps a routine examination and ECG should be advised, as in our case multiple ventricular ectopics were detected that prompted further investigation.
Case 2 represents another cardiac complication of COVID-19, namely pericarditis.[4] She typically presented with pleuritic chest pain. Pericarditis is usually mild, but may occasionally be associated with significant pericardial effusion and tamponade requiring therapeutic pericardiocentesis.[5] It can be present at the time of the initial diagnosis or occur later in the course of the disease. The appearance of an increased cardiac silhouette on the chest radiograph and typical features of ST elevation on the ECG may suggest pericarditis/pericardial effusion, which can usually be confirmed by transoesophageal echocardiography or a computed tomography (CT) scan.
These two cases illustrate that there is a need to consider a basic cardiac assessment before returning to exercise.
Case 3: Sudden severe dyspnoea after recovery from COVID-19 pneumonia
A man in his seventies was admitted in November 2020 with COVID-19 pneumonia and treated with low-molecular-weight heparin (LMWH), antibiotics and supplements. He was discharged 5 days later, but within 72 hours was readmitted with a high fever and severe dyspnoea. At that stage, clinically he had severe pneumonia confirmed on a repeat high-resolution CT scan of the chest. Heparin was restarted and dexamethasone 6 mg daily added. The response was slow but satisfactory, and he was discharged 9 days later feeling quite well.
A few days later, while watching television at home, he had a sudden bout of coughing and slight haemoptysis, followed by severe dyspnoea. He only reported to casualty the following morning, and was found to be in severe respiratory distress with a sats of 80% on room air. Clinically a large left-sided tension pneumothorax was present (confirmed on CT scan of the chest, Fig. 3). An intercostal drain was inserted with dramatic symptomatic improvement. The further course was uncomplicated.
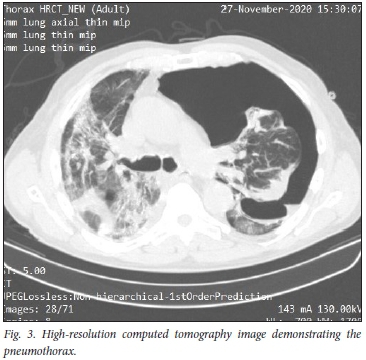
Pneumothorax and tension pneumothorax are uncommon but well-described complications of COVID-19 pneumonia. In a study of 3 368 patients with suspected COVID-19 pneumonia, 902 patients were nasopharyngeal swab-positive, and there were 6 cases (0.66%) of spontaneous pneumothorax.[6] Four of these cases were associated with mechanical ventilation and 2 occurred spontaneously.
Pneumothorax usually presents with increasing dyspnoea and may be confused with worsening pneumonia or thromboembolism. This is an important complication of COVID-19 to recognise, as it is easily remedied by insertion of an intercostal drain as in the case described.
Cases 4 and 5: Neurological complications
Case 4 was a healthy woman in her thirties who presented to the emergency room 140 minutes after experiencing right-sided paralysis and aphasia. She had had mild pre-eclampsia 7 years previously, with no sequelae or continuing hypertension. There was no history of cardiac pathology, atrial fibrillation, diabetes mellitus, thyroid disease, vascular/collagen disorders or dyslipidaemia. Her HIV status was negative and she was not on hormone replacement therapy. On physical examination she was apyrexial and in sinus rhythm with a sats of 98%. Her blood pressure was 128/75 mmHg and there was a dense right hemiplegia with expressive aphasia.
A diagnosis of acute ischaemic stroke was made, and in view of the presentation within 3 hours, after a CT scan of the brain confirmed no cerebral haemorrhage, she received thrombolysis with dramatic improvement of both the hemiplegia and aphasia. Extensive investigation showed no cause for her ischaemic stroke other than a positive COVID-19 test on admission. She had no other symptoms of COVID-19. She was anticoagulated with rivaroxaban and later low-dose aspirin. She made a very good recovery, with slight residual weakness in the right leg and arm.
Several neurological manifestations of COVID-19 are increasingly being recognised.'7' These include encephalopathy, encephalitis, Guillain-Barré syndrome, anosmia, ageusia, transverse myelitis and acute cerebrovascular disease. Various studies report stroke in 1.6 - 2.4% of patients hospitalised with COVID-19. Cryptogenic stroke (patients such as the case reported above, in whom no typical underlying cause of stroke is found) is being reported more frequently. The proposed mechanisms for these cerebrovascular events include a hypercoagulable state from systemic inflammation and cytokine storm, post-infectious immune-mediated responses, and viral-induced vasculitis potentially leading to thrombosis. Neurological problems caused directly or indirectly by the virus must be distinguished from nonspecific complications of severe disease (e.g. hypoxic encephalopathy and critical care neuropathy).
Case 5 was a woman in her thirties. In July 2020, she was hospitalised with severe bilateral pneumonia highly suggestive of COVID-19. Interestingly, the first three COVID-19 swabs were negative, and she only tested positive on the fourth occasion. Her treatment included dexamethasone 6 mg intravenously daily. Six days after admission she requested to be discharged, but was re-admitted the next day with severe dyspnoea at rest. Treatment was restarted and the dosage of dexamethasone increased from 6 mg to 8 mg intravenously daily. She improved slowly, and 8 days later was discharged on high-dose prednisone. Shortly thereafter she became confused, aggressive and disorientated. This settled spontaneously, and she returned for follow-up 2 weeks later. At that stage, her mental state and the findings on detailed neurological examination were normal. A CT scan of the brain and routine blood tests were all unremarkable. She was reviewed by a psychiatrist and a diagnosis of probable steroid-induced psychosis was made.
The Association of British Neurologists launched a portal in April 2020 to report neurological and neuropsychiatric complications of COVID-19.[8] Complete clinical datasets were available for 125 (82%) of 153 patients. Of these, 77 (62%) presented with a cerebrovascular event and 39 (31%) with altered mental status. The latter comprised 9 patients (23%) with unspecified encephalopathy, 7 (18%) with encephalitis, and 23 (59%) with altered mental status fulfilling the clinical case definitions for psychiatric diagnoses. The relationship of the neuropsychiatric events to use of high-dose steroids was not reported. Although the psychiatrist reported that case 5 had steroid-induced psychosis, it is possible that this was directly related to
COVID-19 infection.
Case 6: Pulmonary sequelae of COVID-19 pneumonia
Case 6 was a woman in her seventies with the following comorbidities: atrial fibrillation due to longstanding hypertension, mild asthma, dyslipidaemia, hypothyroidism and depression. A chest radiograph performed in 2013 was normal. In September 2020 she presented with mild COVID-19 pneumonia and was admitted to hospital. A high-resolution CT chest scan showed multifocal ground-glass opacification in keeping with the diagnosis. She was treated according to the standard protocol, but was not given corticosteroids. She was anxious to go home and requested to be discharged. However, she was re-admitted 9 days later with increasing dyspnoea. The chest radiograph showed a bilateral coarse reticulonodular pattern with increased interstitial markings, and spirometry revealed a mild restrictive pattern.
Echocardiography documented a normal left ventricle with an ejection fraction of 63% and features of pulmonary hypertension. Two days later, the patient again requested to be discharged and was sent home on a short course of prednisone and a budesonide/ formoterol inhaler. A repeat high-resolution CT scan of the chest done as an outpatient showed good resolution of the ground-glass opacification and confirmed the presence of the reticulonodular pattern and interstitial changes noted on the chest radiograph. Shortly thereafter the patient left the area, and we were unable to do further investigations.
This case probably represents post-COVID-19 lung fibrosis, but a pre-existing interstitial lung disease could not be excluded. Post-COVID-19 fibrosis is one of the emerging complications of COVID-19 pneumonia. It is estimated to affect around one-third of COVID-19-infected hospitalised patients.[9] The predictors of lung fibrosis are advanced age, illness severity, length of intensive care unit (ICU) stay and mechanical ventilation, smoking and chronic alcoholism. Data regarding long-term clinical outcomes are not yet available, predictions for long-term outcome are speculative at best, and treatment with antifibrotic therapy with either pirfenidone or nintedanib is anecdotal.
Case 7: Renal infarction after COVID-19
Five weeks prior to presentation, a woman in her fifties had had mild COVID-19 with myalgia, fever, a non-productive cough and anosmia. She had been in contact with two couples on a trip, who all tested positive for COVID, but was not tested herself for logistical reasons and isolated at home. The symptoms settled down after 10 days. A week prior to admission she developed severe left flank pain, possibly suggestive of renal calculus. A COVID antibody test was positive. Findings on ultrasound examination of the abdomen were completely normal, and a follow-up CT scan showed an area of wedge-like hypoattenuation within the medial interpolar aspect of the left kidney, with non-enhancement of the overlying renal cortex. The features were consistent with a renal infarct involving the medial interpolar aspect of the left kidney. Kidney function was normal (estimated glomerular filtration rate 86 mL/min) and D-dimers were not elevated at 448 ng/mL (normal <500 ng/mL). No other cause was identified for the thrombosis or systemic embolisation.
Renal infarction post COVID-19 is a rare but well-described complication and probably a result of a hypercoagulable state as described below.[10] It could potentially contribute to some cases of acute kidney injury described in COVID-19 infections.
Cases 8, 9 and 10: The reality of COVID-19
Case 8 was a woman in her seventies, who was admitted in December 2020 with moderately severe COVID-19 pneumonia. She was treated according to the standard protocol, which included LMWH and dexamethasone.
At the time of admission, the patient was in mild respiratory distress. Four days later she felt better, but of concern, her D-dimers went up to 3 786 ng/mL from 831 ng/mL 2 days previously. Her pulse rate was 96 bpm, her blood pressure 138/76 mmHg and her sats 92% on oxygen via a nasal cannula at 4 L/min. She reported pain in the right shoulder to the nursing staff, and 45 minutes later was found dead. The likely cause of death was an acute pulmonary embolus despite appropriate anticoagulation.
Patients with COVID-19 are at increased risk of venous thromboembolism, and systemic inflammation, abnormal coagulation status, multiorgan dysfunction and critical illness are all potential contributing factors. Elevated D-dimers are an important predictor of risk, and the use of LMWH may be associated with reduced mortality in severe COVID or in patients with D-dimers >6 times elevated.[1] In a recent meta-analysis, anticoagulation (either prophylactic or therapeutic), mainly with heparin, was associated with 50% reduced in-hospital mortality risk.[11] Both anticoagulant regimens reduced in-hospital all-cause mortality compared with no anticoagulation, particularly in ICU cases. However, therapeutic anticoagulation was associated with a 2.53 times increased risk of bleeding, suggesting that prophylactic dosages of anticoagulant are probably to be preferred in non-critically ill COVID-19 patients.
Case 9 was a man in his fifties who developed COVID-19 pneumonia while on holiday in the area. He was hospitalised in December 2020 with moderate to severe COVID-19 pneumonia confirmed on CT scan.
Although the sats were low (84% on room air and 89% on oxygen via a nasal cannula at 8 L/min), the patient was not in respiratory distress. He was treated according to the standard protocol together with chest physiotherapy, and his oxygen saturation improved to 94% on oxygen via nasal prongs at 8 - 10 L/min. Initially he refused to use a rebreather mask.
Six days later the patient's saturation dropped and settled at 88% on oxygen via a rebreather mask at 8 L/min. The inflammatory markers were still elevated. The C-reactive protein level was 268.4 mg/mL (normal <5 mg/mL), the lactate dehydrogenase level 1 055 ng/mL (normal <500 ng/mL) and the white cell count 18.3 x 109/L (normal 3.92 - 9.88 x 109/L). The D-dimer level was particularly high at 3 791 ng/mL. That evening, the family requested that he be moved to the ICU of a tertiary care hospital 250 km away to escalate the level of care. He was transferred by advanced life support ambulance with a full paramedic team and was on oxygen with cardiac monitoring throughout the 3-hour journey. Ten minutes before arriving at the hospital he had a cardiac arrest, and resuscitation was unsuccessful. The cause of death was not established but was probably a pulmonary embolus or the effects of severe hypoxaemia related to COVID-19 pneumonia.
Case 10 was a woman in her fifties who developed COVID-19 pneumonia in December 2020 while on holiday in the area. She was a longstanding insulin-dependent diabetic with hypertension. On admission she was severely distressed and confused with her glucose out of range. Sats on room air was 83%. A CT scan demonstrated features consistent with severe COVID-19 pneumonia, and she was admitted to the ICU and treated with the COVID-19 protocol together with remdesivir.
Her diabetes stabilised, and oxygen was given via an Airvo 2 nasal high-flow device (Fisher & Paykel, New Zealand) and chest physiotherapy was commenced. Over the next few days her mental state improved and her sats remained between 88% and 92% on the Airvo 2. Non-invasive ventilation was then commenced, alternating with the Airvo 2. An echocardiogram was normal apart from a small pericardial effusion.
A repeat chest radiograph 8 days later showed no deterioration, and her sats stayed at ~90%. Inflammatory markers remained high, but she started to feel better. Four days later her electrolytes were normal and D-dimers only slightly elevated.
Unfortunately, in the early hours of the morning 13 days after admission the patient suddenly went into ventricular fibrillation. Attempts at resuscitation were unsuccessful. The likely cause of death was COVID-19 myocarditis or acute myocardial infarction.
Conclusions
A retrospective case series is presented from a small hospital in the Western Cape region of SA between May 20 and June 2021. Out of a total of ~100 admissions under the direct care of author GC, 7 cases with unusual complications and 3 cases of sudden death are presented. This case series provides insights into the challenges faced by physicians during the COVID pandemic outside the major centres of SA.
Declaration. None.
Acknowledgements. None.
Author contributions. GC provided the clinical information and wrote the initial draft. BLR provided critical review and revised the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med 2020;38(7):1504-1507. https://doi.org/10.1016/j.ajem.2020.04.048 [ Links ]
2. Wang Y, Wang Z, Tse G, et al. Cardiac arrhythmias in patients with COVID-19. J Arrhythm 2020;36(5):827-836. https://doi.org/10.1002/joa3.12405 [ Links ]
3. Salman D, Vishnubala D, le Feuvre P, et al. Returning to physical activity after covid-19. BMJ 2021;372:m4721. https://doi.org/10.1136/bmj.m4721 [ Links ]
4. Kumar R, Kumar J, Daly C, Edroos SA. Acute pericarditis as a primary presentation of COVID-19. BMJ Case Rep 2020;13(8):e237617. https://doi.org/10.1136/bcr-2020-237617 [ Links ]
5. Hua A, O'Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J 2020;41(22):2130. https://doi.org/10.1093/eurheartj/ehaa253 [ Links ]
6. Zantah M, Domínguez Castillo E, Townsend R, Dikengil F, Criner GJ. Pneumothorax in COVID-19 disease - incidence and clinical characteristics. Respir Res 2020;21(1):236. https://doi.org/10.1186/s12931-020-01504-y [ Links ]
7. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand 2020;142(1):14-22. https://doi.org/10.1111/ane.13266 [ Links ]
8. Maratharaj A, Thomas N, Ellul MA, et al., on behalf of the CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020;7(10):875-882. https://doi.org/10.1016/S2215-0366(20)30287-X [ Links ]
9. Ahmad Alhiyari M, Ata F, Islam Alghizzawi M, Bint I Bilal A, Salih Abdulhadi A, Yousaf Z. Post COVID-19 fibrosis, an emerging complication of SARS-CoV-2 infection. ID Cases 2020;23:e01041. https://doi.org/10.1016/j.idcr.2020.e01041 [ Links ]
10. Ammous A, Ghaffar MA, El-Charabaty E, El-Sayegh S. Renal infarction in COVID-19 patient. J Nephrol 2021;34(1):267-268. https://doi.org/10.1007/s40620-020-00866-2 [ Links ]
11. Parisi R, Costanzo S, di Castelnuovo A, de Gaetano G, Donati MB, Iacoviello L. Different anticoagulant regimens, mortality, and bleeding in hospitalized patients with COVID-19: A systematic review and an updated meta-analysis. Semin Thromb Hemost 2021;47(4):372-391. https://doi.org/10.1055/s-0041-1726034 [ Links ]
 Correspondence:
Correspondence:
B Rayner
brian.rayner@uct.ac.za
Accepted 23 August 2021














