Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.9 Pretoria Set. 2021
http://dx.doi.org/10.7196/SAMJ.2021.v111i9.15666
RESEARCH
Magnetic resonance imaging diagnosis of causes of cerebral palsy in a developing country: A database of South African children
M M ElsingergyI; F WoredeI; S VenkatakrishnaI; J CuricII; S AndronikouIII
IMD; Department of Pediatric Radiology, Children's Hospital of Philadelphia, Philadelphia, USA
IIBA, MBA; Graduate MBA Program, Faculty of Health, Education, Medicine and Social Care, Anglia Ruskin University, Cambridge, UK
IIIMD, PhD;Department of Radiology, Perelman School ofMedicine, University of Pennsylvania, Philadelphia, USA
ABSTRACT
BACKGROUND. Cerebral palsy (CP) is a common worldwide disabling disorder. However, data about prevalence and causes of CP in developing countries are deficient because of high cost and limited availability of magnetic resonance imaging (MRI), the gold standard neuro-imaging modality for evaluation and management of CP in neonates.
OBJECTIVES. To determine the frequency of CP causes in children with suspected hypoxic ischaemic injury (HII) involved in medicolegal litigation in South Africa based on MRI report findings.
METHODS. A total of 1 620 MRI reports were categorised into HII, non-HII and normal MRI. None of the patients had prior neuro-imaging records. HII reports were sub-classified according to pattern of brain injury into basal ganglia-thalamus (BGT), watershed (WS), combined BGT-WS, periventricular leukomalacia (PVL) and multicystic encephalomalacia. Non-HII diagnoses were sub-classified into strokes, congenital malformations, kernicterus, hydrocephalus, haemorrhages, atrophies, metabolic causes and infections.
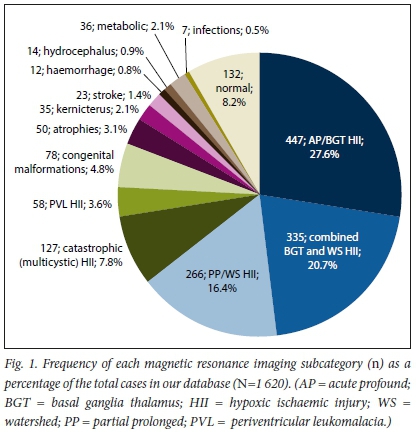
RESULTS. The median age was 6 years. HII reports (n=1 233; 76.1%) showed BGT in 447 (27.6%), WS in 266 (16.4%), combined BGT-WS in 335 (20.7%), PVL in 58 (3.6%) and multicystic in 127 (7.8%). Non-HII diagnoses (n=255; 15.7%) showed 78 (4.8%) congenital malformations, 50 (3.1%) atrophies, 35 (2.1%) kernicterus, 23 (1.4%) strokes, 12 (0.8%) haemorrhages, 14 (0.9%) hydrocephalus, 36 (2.1%) metabolic and 7 (0.5%) infections. Normal exams were 132 (8.2%).
CONCLUSIONS. Despite being performed a relatively long time - median of 6 years - after the suspected perinatal HII, MRI yielded a diagnosis in 92% and showed that only 76% were due to HII, and more importantly, that there was a preterm HII pattern of injury in 15%, which when added to the 16% of non-HII cases, could potentially save on litigation in a total of 31% of cases that are unlikely to be related to malpractice. MRI should be performed wherever possible in CP cases, even if no imaging exam was performed in the perinatal period.
Cerebral palsy (CP) is a neurodevelopmental motor and postural disorder that occurs as a sequela of non-progressive injury of the developing brain, leading to movement restriction and permanent disability.[1] Birth CP is common worldwide, affecting around 2-4 per 1 000 live births[2] Hypoxic ischaemic injury (HII), which is thought to result from decreases in blood and oxygen supply to the brain, is considered to be one of the leading causes for development of CP.[3] Other possible CP causes include stroke,[4] infection, metabolic abnormalities (e.g. kernicterus,[5] hypoglycaemia, hypomyelination syndromes) and genetic abnormalities (mutations) leading to brain malformations. Brain insults can occur in utero (antenatal), during delivery (intranatal), or in the neonatal, infancy and early childhood period (postnatal)J6] Even though the highest risk for development of CP is among preterm and low-birthweight infants (40 - 100 per 1 000 live births), most children with CP are born at term.[7]
Data on the prevalence of CP in many developing African countries, including South Africa (SA), are limited,[8] but some studies suggest that the CP incidence is much higher in these countries, with an estimate of ~10 per 1 000 live births, especially in remote, underserved rural areas where there is a lack of medical resources and obstetric services.[9,10] Unlike in the developed world, postnatal causes account for a significant proportion of CP cases because of increased susceptibility of newborns to contract infections endemic in these regions (e.g. neonatal meningitis, toxoplasmosis and malaria), which is further aggravated by poverty, famine and malnutrition.[8,10]Most causes of CP in developing countries are preventable and treatable through early diagnosis and proper management, at the very least reducing CP-related comorbidities and improving patients' clinical outcomes. A recent survey conducted by the United Nations Children's Fund concluded that in 2018 alone, 43 000 children aged <5 years died in SA, most of them newborns (n= 12 717), because of birth-related complications and neonatal infections, and that a significant proportion of these neonates would have been saved if early and appropriate measures had been taken[11]
Magnetic resonance imaging (MRI) is the most commonly used neuro-imaging modality worldwide for early evaluation and management of the causes of CP.[12] However, MRI availability in many developing countries is limited and the costs are high - more than an average household income can afford - leaving the majority of CP cases undiagnosed during the early course of the disease. As the number of CP cases grows in these countries, so does the amount of medicolegal litigation[13] including in SA, where one recent study showed that 43% of all claims filed against SA state hospitals are birth related.[14,15]
In this study, we aimed to determine causes of CP (HII, non-HII and normal) in children with suspected HII involved in medicolegal litigation in SA based on MRI report findings.
Methods
Study design
We retrospectively reviewed brain MRI reports from a large medicolegal database comprising CP cases referred for medicolegal evaluations long after an alleged perinatal hypoxic ischaemic event occurred. This study is approved by our institutional review board (IRB ref. no 20-017467) and compliant with all Health Insurance Portability and Accountability Act protocols. The requirement for patient informed consent was waived.
All MRI reports were reviewed and evaluated by the study co-ordinators. MRI reports that were duplicated or had inconclusive findings (due to motion artifact or other cause for poor MRI examination quality) were excluded. The remaining reports were categorised according to the MRI diagnosis (impression) into HII, non-HII and normal (Fig. 1). HII cases were further categorised according to the pattern of brain injury as: basal ganglia-thalamus (BGT), watershed (WS), combined BGT/WS, catastrophic multi-cystic and periventricular leukomalacia (PVL). Those without HII were also further classified into broad categories of congenital malformations, atrophies, kernicterus, arterial strokes, haemorrhages, hydrocephalus, metabolic/toxic causes, infections and other.
MRI interpretation
All studies were reported by a sub-specialised paediatric neuro-radiologist with over 20 years of clinical experience, in active clinical practice, and licensed to practise in the USA, UK and SA. The radiologist was blinded to patient clinical findings at the time of reporting, but was aware that the cases were referred for evaluation of the cause for CP. MRI exams were conducted using both 1.5 and 3T scanners at a variety of locations, using a standardised baseline protocol of Tl, T2, T2 fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI) and gradient Echo/susceptibility-weighted imaging sequence performed on the sagittal, axial and coronal planes.
The diagnosis of HII was made according to the reporting radiologist's clinical standards when the distribution of the brain lesions matched one of the common patterns typically observed in neonatal brain hypoxia. Because the MRI scans were performed a long time after the alleged perinatal event, findings were categorised as follows: BGT pattern of hypoxia when there was atrophy, high T2 signal, and/or cavitation present in the thalamus, putamen and peri-rolandic cortex;[16] WS pattern of hypoxia when there was atrophy (with or without ulegyria) or high T2 signal found in the watershed regions of the brain (namely the peri-sylvian, para-sagittal, anterior watershed (AWS) and posterior watershed (PWS) regions);[17] combined BGT/WS pattern of hypoxia when there was an overlap in the pattern of the brain regions affected between BGT and WS; or multicystic (catastrophic) HII when there was bilateral extensive multicystic leukomalacia with visible glial strands involving frontal, temporal, parietal and occipital lobes with preferential sparing of the posterior fossa structures. All of the above hypoxic-ischaemic patterns were considered injury at term gestation. Findings were categorised as PVL when a high T2 signal was reported in a typical distribution involving the white matter immediately surrounding the ventricles with and without changes in the ventricular contour and/or cystic changes, and was considered a pre-term pattern of injury.
Non-HII cases were categorised as: arterial stroke when there was involvement of a major arterial territory; or kernicterus when there was high T2 signal found in the globus pallidus without associated abnormalities in the putamen or thalamus with or without involvement of the hippocampi[18] Finally, a diagnosis of normal MRI was made when there were no findings suggestive of HII or any other brain abnormality.
Results
A total of 1 680 MRI reports in children with CP ranging in age from 0 to 18 years were reviewed. Of these, 60 reports were excluded because they were duplicated (n;1=14) or because the MRI exam was of low quality, e.g. exams with significant motion artifact, affecting the radiologist's ability to offer a report (n;1=46).
The reports of the remaining study population of 1 620 (median age 6 years, interquartile range (IQR) 4-9 years) were classified according to the MRI categories described above.
Of the total 1 620 MRI examinations, there were 1 233 (76.1%) with HII findings (992 (61.2%) with a term pattern of injury and 241 (14.9%) with a preterm pattern of injury), 255 (15.7%) with non-HII findings and 132 (8.2%) categorised into the normal group.
In the HII cases, the basal ganglia and the thalamus were affected in a total of 782 cases (48.3% of total study population), made up of the BGT and combined BGT-WS groups, while the WS regions of the cerebral hemispheres were affected in a total of 601 (37.1% of total study population), made up of the WS and combined BGT-WS groups.
Detailed frequency of MRI patterns of injury in the HII-diagnosis group can be found in Table 1, and findings are summarised both as percentages of the total study population and as percentages of the HII group. The most common pattern of injury in HII was the isolated BGT pattern (447; 36.33% of HII cases), followed by a combined BGT-WS pattern (335; 27.1% of HII cases), isolated WS (266; 21.6% of HII cases), catastrophic multicystic encephalomalacia (127; 10.3% of HII cases) and PVL (58; 4.7% of HII cases).
The non-HII diagnosis group comprised: 78 (4.8% of total study population) congenital malformations; 50 (3.1% of total study population) with atrophy; 35 (2.1% of total study population) with features associated with kernicterus/bilirubin toxicity, 23 (1.4% of total study population) with stroke; 12 (0.8% of total study population) with intracranial haemorrhage; 14 (0.9% of total study population) with hydrocephalus and ventriculo-peritoneal shunts; and 43 cases which were classified into much broader groups (metabolic, hypomyelination and leukodystrophies in 36 cases (2.1% of total study population) and congenital (in atero) and postnatal infections in 7 cases (0.5% of total study population). Table 2 summarises this non-HII diagnosis group and reflects more granular diagnostic information within specific groups.
Discussion
CP can occur as a result of several different causes, and has a very wide range of clinical manifestations that vary greatly in their degree of functional disability and type of movement disorder, depending on the underlying injury. Neuro-imaging plays a key role in CP diagnosis. The American Academy of Neurology (AAN) and Child Neurology Society (CNS) guidelines recommend performing neuro-imaging for all cases of CP where the aetiology has not yet been established.[42]MRI, owing to its high resolution and superior tissue contrast, is considered to be the imaging modality of choice for obtaining detailed and accurate findings of brain pathologies, which helps in determining appropriate management plans, identifying potential risk factors for disease prevention, predicting long-term neurodevelopmental outcomes and, as for this database, assisting in judicial decisions in CP-related medicolegal litigation[20,21]
However, CP imaging data from the developing world are scarce because of the high cost and shortage of MRI scanners. One publication reports that, in SA, which has more infrastructure than many other African countries, there are only 0.3 MRI scanners available per 1 million population in the public health sector, with some provinces, such as North West and Mpumalanga, having no public MRI service at any of their healthcare facilities[22] Patients are often referred to private settings for MRI exams, where the average cost of one MRI exam is ZAR9 000 (~USD617). That is almost one-third of the average monthly salary of a SA household family (ZAR31 100; ~USD2 130), while 84% of the SA population have no health insurance, and 28% are unemployed.[23,24]
Our database is unique in that it comprises a substantial population of children with CP who underwent MRI scans funded by legal firms a long time after the alleged hypoxic ischaemic event, as none of the patients had MRI scans performed in the neonatal period by the facilities in which they were being cared for. Our study differs from publications where MRI studies were performed early in the neonatal period for suspected HII in the developed world. Not only congenital abnormalities but also prior HII can be diagnosed by delayed MRI exams. HII findings can appear more pronounced on delayed scans because there is localised/ regional volume loss that accompanies the established signal abnormality.[25]
Sensitivity of MRI in determining the causes of CP
The sensitivity of MRI in detection of the causes of CP worldwide is between 86% and 89%[26] We showed comparable results where the MRI resulted in a diagnosis in 91.8% (1 488 patients), while only 132 (8.2%) MRI scans were reported as normal - or negative for pathology. HII was the major underlying cause for CP in our patient population involved in medicolegal investigation, with a frequency of 76.1%, while all other causes combined accounted for only 15.7%. The incidence of HII diagnosed in our study is much higher than that in previous publications from regional SA hospitals, such as those by Mahlaba et al[27] and van Toorn et al.,[28] who reported incidences of perinatal asphyxia of 48.2% and 38%, respectively, in their sample of CP patients. This is also different from the epidemiological data generated by the developed world, where a systematic review conducted by McIntyre et al[29] suggested that intrapartum birth asphyxias accounted for <10% of CP causes,[28] likely because of the high quality of obstetric health services provided. One possible reason that our incidence is so much higher than that in other SA studies is that we have used a medicolegal database that has likely already filtered out potential causes, other than HII, that were proven clinically. In addition, the majority of patients imaged in the medicolegal database reflect births in underserved provinces such as the Eastern Cape of SA.
Timing of HII
Many cerebral pathologies tend to affect certain parts of the brain at different time points during the development process. MRI, through its reliable superiority in tissue characterisation, has the potential to differentiate between the physiological and pathological morphological changes, which often give rise to characteristic patterns that occur during the various stages of brain maturation.[30]
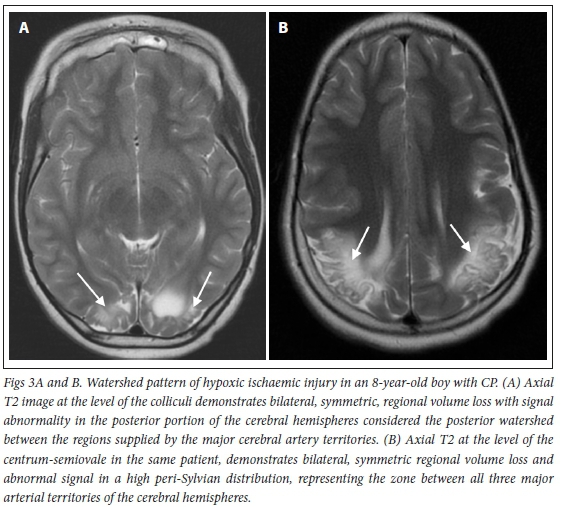
The BGT pattern of HII often occurs after 34 weeks' gestation following exposure to profound perinatal asphyxia, where metabolically active structures of the deep nuclei (e.g. putamen and ventrolateral nucleus of the thalamus, hence BGT pattern), peri-rolandic cortex, hippocampus, dorsal brain stem and superior vermis of the cerebellum are predominantly affected, as they have a high oxygen demand (Fig. 2).[31]Milder and more prolonged brain hypoxia in term infants results in a different pattern of HII involving the WS. In WS injury, the brain shunts blood to metabolically active structures at the expense of less active and less well supplied 'end zones' between the main arteries supplying the cerebral hemispheres (Fig. 3). Para-sagittal, anterior, posterior and peri-sylvian watershed regions are affected either individually or as a continuum in this patternJ17] When imaged long after the event, WS HII is commonly associated with ulegyria (Fig. 4), which results from volume loss due to cortical scarring that occurs at the base of cerebral brain gyri, giving them a characteristic mushroom appearance. This phenomenon is referred to as a 'watershed within a watershed' and occurs while this region of the gyrus is vulnerable to hypoxia, which is helpful in timing injury as having occurred after term gestation. In catastrophic forms of HII, spongiotic transformation may occur, leading to development of large cysts with ultimate destruction of significant portions of the cerebral hemispheres, a pattern known as multicystic leukomalacia. The presence of gliotic strands in multicystic encephalomalacia is helpful in timing the injury, as it can only occur after maturity was at a level where gliosis is possible (i.e. after approximately 34 weeks' gestation) (Fig. 5)[32-34]Based on these radiological findings, there were 992 cases (61.2%) with a presumed term pattern of HII.



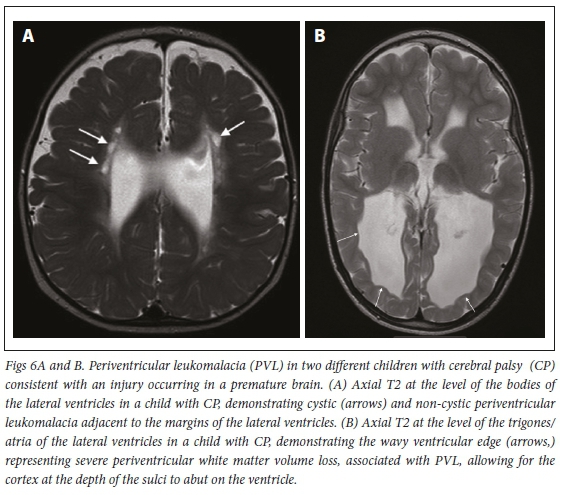
When severe hypoxia occurs preterm, it can result in a pattern that can be confused with the BGT pattern, but the basal ganglia and peri-rolandic cortex are relatively spared, with preferential involvement of the thalami. Milder forms of pre-term HII show the PVL pattern where white matter surrounding the trigones of the lateral ventricles are commonly injured - hence the name periventricular leukomalacia (Fig. 6)[31] In our sample, only 241 cases (14.9%) showed a preterm pattern of HII.
MRI timing of the injury is critical in malpractice litigation when it is alleged that intrapartum asphyxia at term caused permanent neurological damage, giving rise to CP. The onset of the injury is key when the defense argues that the insult happened pre- or postnatally, owing to causes other than malpractice.[35,36]
Differentiating between clinically similar causes of CP
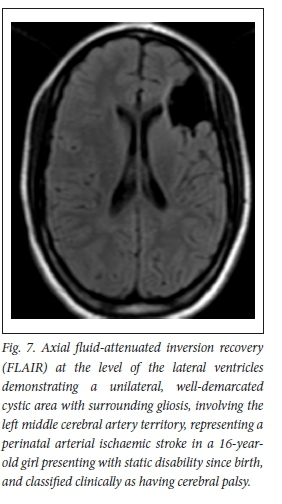
Alternative causes of CP can have similar clinical presentations, given the overlap in the brain territories affected by these insults. However, these diseases are different in their epidemiology, onset, pathophysiology, risk factors and management plans[37]A common example is the difference between perinatal/neonatal strokes and HH.[4] Perinatal strokes involving middle cerebral artery (MCA) territory (the most common site for neonatal stroke), BGT and WS HII can all present with spastic CP.[38] MRI plays a critical role in differentiation between these insults, as each has its own imaging pattern.[6] In our study, strokes accounted for only 1.4% of CP cases - almost 50 times less than HII (76.1%). This is closely reflective of the worldwide incidence of these two pathologies, where the incidence of HII, 1 000 per 1000 000 live births, is 40 times the incidence of perinatal strokes, which is 25 per 100 000 live birthsJ39] Perinatal strokes are more commonly found in neonates with cardiac anomalies, infections and coagulation disorders. They are more commonly observed in term infants, but unlike HII, most patients have routine/ uncomplicated deliveryJ37] On MRI, neonatal strokes are usually differentiated from HII by their involvement of a true arterial region (rather than the regions between two vascular territories in WS HII), and are most often unilateral, compared with HII, which is characteristically (but not universally) bilateral and symmetric (Fig. 7). In scenarios where neonatal strokes are bilateral, they are unlikely to be in symmetrical locations.[40]
Building CP MRI registers
Most CP cases diagnosed in developing countries, including SA, are under-reported because of lack of available CP registers. CP registers are medical databases developed by collaborations between physicians, researchers, and epidemiologists guided by specific definitions for the term 'cerebral palsy' including strict inclusion and exclusion criteria[41] The scarcity of medical registers in developing countries makes an accurate estimation of the true prevalence of disabling diseases very difficult. African countries currently rely on registers created by countries in the developed world, e.g. the 'Life expectancy project in San Francisco' register, to project the survival and life expectancy of CP patients involved in medicolegal litigation. However, the difference in the epidemiological data between developed and resource-limited countries questions the credibility of the prognostic outcome generated from these databases.[42] Our data and findings could form part of a specific section of such a CP register that reflects the selection bias inherent in using a medicolegal database in children with suspected HII.
Unique aspects of this research
The present study is unique in that it has classified findings from a large medicolegal database of MRI reports from more than 56 different legal firms scattered throughout SA. Because there are few sub-specialised paediatric neuroradiologists interpreting medicolegal MRI exams and generating reports in SA, this MRI database represents a centralised source that could be combined with the few other available databases to build the foundation for developing a CP register, which can also be expanded to include other African countries. Generating reliable data regarding the causes of CP in developing countries could help to redirect the attention of government officials and international humanitarian agencies. This would allow them to allocate resources for children with disability, and redirect funding to improve obstetric practices and prevent further instances of avoidable perinatal HII.
Study limitations
There are two unavoidable limitations of our study. First is the lack of clinical and laboratory data in our sample. These were of limited availability, quality and reliability. We have presented only the interpretation of the diagnostic imaging findings on MRI in these children with CP, and hence we draw no conclusions regarding the pathogenesis, pathophysiology or appropriateness of the management in those children with HII. The second is the selection bias accounting for the much higher incidence of HII in our patient sample than those reported by other regional studies (76.1% v. 48.2%%27] and 38%t28]), as the database, by its nature, is made up of those cases undergoing medicolegal litigation for suspected HII. Therefore, while we can conclude that HII makes up the highest proportion of CP causes in medicolegal cases in SA, the proportions may differ when taking the whole CP population into account, i.e. including those not undergoing medicolegal litigation because the cause for CP may be known either clinically, based on laboratory testing, or from prior MRI scans. Our database has no instances where early MRI scans from the first 2 weeks of life were provided.
Conclusions
MRI in children with CP undergoing medicolegal litigation for suspected perinatal HII in SA demonstrated a 92% diagnostic yield, despite being performed long after the perinatal period, at a median age of 6 years. MRI showed that only 76% of cases were due to HII, and more importantly that there was a preterm HII pattern of injury in 15%, which when added to the 16% of non-HII cases could potentially save on litigation in a total of 31% of cases that are unlikely to be related to malpractice. We therefore recommend performing delayed MRI in children with CP with suspected perinatal HII if no imaging was possible during the perinatal period, as it can inform ongoing management, allow for fair litigation and inform the health services regarding allocation of resources both for children with disability and for obstetric services to prevent new cases of CP.
Declaration. None.
Acknowledgements. We would like to thank Colin Mancini for his assistance in the IRB approval process, and Leona Hobbs for setting up the data use agreement between our institution and the medical providers enabling the access to the brain MRI reports.
Author contributions. MME: inception, planning, data collection, analysis, writing. FW: data collection, analysis, draft review. SV: data collections, analysis, draft review. J C: inception, data collection. SA: inception, planning, data collection, data analysis, writing, and supervision.
Funding. None.
Conflicts of interest. None.
References
1. Richards CL, Malouin F. Cerebral palsy: Definition, assessment and rehabilitation. Handb Clin Neurology 2013;111:183-195. https://doi.org/10.1016/b978-0-444-52891-9.00018-x [ Links ]
2. Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral palsy - trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr 2017;5:21. https://doi.org/10.3389/fped.2017.00021 [ Links ]
3. Izbudak I, Grant PE. MR imaging of the term and preterm neonate with diffuse brain injury. Magn Reson Imaging Clin N Am 2011;19(4):709-731. https://doi.org/10.1016/j.mric.2011.08.014 [ Links ]
4. Adami RR Grundy ME, Poretti A, Felling RJ, Lemmon M, Graham EM. Distinguishing arterial ischemic stroke from hypoxic-ischemic encephalopathy in the neonate at birth. Obstet Gynecol 2016;128(4):704-712. https://doi.org/10.1097/aog.0000000000001631 [ Links ]
5. Wu YW, Kuzniewicz MW, Wickremasinghe AC, et al Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: A population-based study. JAMA Pediatr 2015;169(3):239-246. https://doi.org/10.1001/jamapediatrics.2014.3036 [ Links ]
6. Wimalasundera N, Stevenson VL. Cerebral palsy. Pract Neurol 2016;16(3):184-194. [ Links ]
7. Appleton RE, Gupta R. Cerebral palsy: Not always what it seems. Arch Dis Child 2019;104(8):809-814. https://doi.org/10.1136/archdischild-2018-315633 [ Links ]
8. Donald KA, Kakooza AM, Wammanda RD, et al. Pediatric cerebral palsy in Africa: Where are we? J Child Neurol 2015;30(8):963-971. https://doi.org/10.1177/0883073814549245 [ Links ]
9. Gladstone M. A review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settings. Ann Trop Paediatr 2010;30(3):181-196. https://doi.org/10.1179/146532810x12786388978481 [ Links ]
10. Burton A. Fighting cerebral palsy in Africa. Lancet Neurol 2015;14(9):876-877. https://doi.org/10.1016/s1474-4422(15)00189-1 [ Links ]
11. United Nations Children's Fund. The challenge: Thousands of children die from treatable and preventable conditions. New York: UNICEF, 2018. https://www.unicef.org/southafrica/health (accessed 17 September 2018). [ Links ]
12. Accardo J, Kammann H, Hoon AH, Jr. Neuroimaging in cerebral palsy. J Pediatr 2004;145(Suppl 2):S19-S27. https://doi.org/10.1016/j.jpeds.2004.05.018 [ Links ]
13. Capazorio B. More than 5 500 medical negligence claims against the state since 2014. Times Live South Africa, 2017. https://www.timeslive.co.za/news/south-africa/2017-10-30-more-than-5500-medical-negligence-daims-against-the-state-since-2014/ (accessed 16 August 2021). [ Links ]
14. James W. South Africa: Medical malpractice - Health Department Spends R1.2 Billion On Litigation. AllAfrica News. https://allafrica.com/stories/201506090606.html (accessed 09 June 2015). [ Links ]
15. Law HP. Is medical malpractice to blame for SAs shocking cerebral palsy statistics? Go legal industry news and insight. https://www.golegal.co.za/medical-malpractice-cerebral-palsy (accessed 20 July 2020). [ Links ]
16. Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. Am J Neuroradiol 1992;13(3):959-972. [ Links ]
17. Chacko A, Andronikou S, Mian A, Gonçalves FG, Vedajallam S, Thai NJ. Cortical ischaemic patterns in term partial-prolonged hypoxic-ischaemic injury - the inter-arterial watershed demonstrated through atrophy, ulegyria and signal change on delayed MRI scans in children with cerebral palsy. Insights Imaging 2020;11(1):53. https://doi.org/10.1186/s13244-020-00857-8 [ Links ]
18. Ribeiro BNdF, Lima GdA, Ventura N, Gasparetto EL, Marchiori E. Chronic kernicterus: Magnetic resonance imaging findings. Radiol Bras 2016;49(6):407-408. https://doi.org/10.1590/0100-3984.2015.0190 [ Links ]
19. Ashwal S, Russman BS, Blasco PA, et al. Practice parameter: Diagnostic assessment of the child with cerebral palsy: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurol 2004;62(6):851-863. https://doi.org/10.1212/01.wnl.0000117981.35364.1b [ Links ]
20. Staudt M. Imaging cerebral palsy. Handbook Clin Neurol 2013;111:177-181. https://doi.org/10.1016/b978-0-444-52891-9.00017-8 [ Links ]
21. Tharmapoopathy P, Chisholm P, Barlas A, et al. In clinical practice, cerebral MRI in newborns is highly predictive of neurodevelopmental outcome after therapeutic hypothermia. European J Paediatr Neurol 2020;25:127-133. https://doiorg/10.1016/j.ejpn.2019.09.005 [ Links ]
22. Kabongo JM, Nel S, Pitcher RD. Analysis of licensed South African diagnostic imaging equipment. Pan Afr Med J 2015;22:57. https://doi.org/10.11604%2Fpairj.2015.22.57.7016 [ Links ]
23. Khumalo J. The bitter pill of medical costs in SA. 2014; https://www.news24.com/news24/Archives/City-Press/The-bitter-pill-of-medical-costs-in-SA-20150429 (accessed 16 February 2014). [ Links ]
24. Writer S. The 2019 budget in a nutshell https://businesstechco.za/news/budget-speech/300786/the-2019-budget-in-a-nutshell/ (accessed 20 February 2019). [ Links ]
25. Shroff MM, Soares-Fernandes JP, Whyte H, Raybaud C. MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics 2010;30(3):763-780. https://doi.org/10.1148/rg.303095126 [ Links ]
26. Novak I, Morgan C, Adde L, et al Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr 2017;171(9):897-907. https://doi.org/10.1001/jamapediatrics.2017.1689 [ Links ]
27. Mahlaba N, Nakwa FL, Rodda JR. A descriptive study of children with cerebral palsy at Chris Hani Baragwanath Academic Hospital. S Afr J Child Health 2020;14(1):4-9. https://doi.org/10.10520/EJC-2027524696 [ Links ]
28. Van Toorn R Laughton B, van Zyl N, Doets L, Elsinger F. Aetiology of cerebral palsy in children presenting at Tygerberg Hospital. S Afr J Child Health 2007;1(2): 74-77. https://doi.org/10.10520/EJC64664. [ Links ]
29. McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 2013;55(6):499-508. https://doi.org/10.1111/dmcn.12017 [ Links ]
30. Krageloh-Mann I, Horber V The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: A systematic review. Dev Med Child Neurol 2007;49(2):144-151. https://doi.org/10.1111/j.1469-8749.2007.00144.x [ Links ]
31. De Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology 2010;52(6):555-566. https://doi.org/10.1007%2Fs00234-010-0674-9 [ Links ]
32. Triulzi F, Parazzini C, Righini A. Patterns of damage in the mature neonatal brain. Pediatr Radiol 2006;36(7):608-620. https://doi.org/10.1007/s00247-006-0203-5 [ Links ]
33. Korzeniewski SJ, Birbeck G, DeLano MC, Potchen MJ, Paneth N. A systematic review of neuroimaging for cerebral palsy. J Child Neurol 2008;23(2):216-227. https://doi.org/10.1177/0883073807307983 [ Links ]
34. Ghei SK, Zan E, Nathan JE, et al MR imaging of hypoxic-ischemic injury in term neonates: Pearls and pitfalls. Radiographics 2014;34(4):1047-1061. https://doi.org/10.1148/rg.344130080 [ Links ]
35. Donn SM, Chiswick ML, Fanaroff JM. Medico-legal implications of hypoxic-ischemic birth injury. Semin Fetal Neonatal Med 2014;19(5):317-321. https://doi.org/10.1016/j.siny.2014.08.005 [ Links ]
36. Tan S. Fault and blame, insults to the perinatal brain may be remote from time of birth. Clin Perinatol 2014;41(1):105-117. https://doi.org/10.1016/j.clp.2013.10.006 [ Links ]
37. Lehman LL, Rivkin MJ. Perinatal arterial ischemic stroke: Presentation, risk factors, evaluation, and outcome. Pediatr Neurol 2014;51(6):760-768. https://doi.org/10.1016/j.pediatrneurol.2014.07.031 [ Links ]
38. Noritz GH, Murphy NA. Motor delays: Early identification and evaluation. Pediatrics 2013;131(6):e2016-2027. https://doi.org/10.1542/peds.2013-1056 [ Links ]
39. Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr 2001;13(6):499-505.https://doi.org/10.1097/00008480-200112000-00002 [ Links ]
40. Bernson-Leung ME, Rivkin MJ. Stroke in neonates and children. Pediatr Rev 2016;37(11):463-477. https://doi.org/10.1542/pir.2016-0002 [ Links ]
41. Katangwe TJ, Van Toorn R Solomons RS, et al. A South African cerebral palsy registry is needed. S Afr Med J 2020;110(5):353-354. https://doiorg/10.7196/sairg.2020.v110i5.14504 [ Links ]
42. Steel S, Botha AS, Khalawan T. Cerebral palsy and delictual compensation - is there a need for a register? http://www.derebus.org.za/cerebral-palsy-and-delictual-compensation-is-there-a-need-for-a-register/ (accessed 1 September 2019). [ Links ]
 Correspondence:
Correspondence:
S Andronikou
doctor.andronifau@gmail.com
Accepted 12 April 2021














