Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.9 Pretoria Set. 2021
http://dx.doi.org/10.7196/SAMJ.2021.v111i9.15634
RESEARCH
Investigating the need for therapeutic drug monitoring of imipenem in critically ill patients: Are we getting it right?
B MittonI, II ; F ParukIII; A GousIV; J ChausseV; M MilneIV; P BeckerV; M SaidVI, VII
IMB ChB; Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria, South Africa
IIMB ChB; Tshwane Academic Division, National Health Laboratory Service, Pretoria, South Africa
IIIPhD; Department of Critical Care, Faculty of Health Sciences, Steve Biko Academic Hospital and University of Pretoria, South Africa
IVPhD; School of Pharmacy, Sefako Makgatho Health Sciences University, Pretoria, South Africa
VMB ChB; Department of Critical Care, Faculty of Health Sciences, Steve Biko Academic Hospital and University of Pretoria, South Africa
VIPhD; Research Office, Faculty of Health Sciences, University of Pretoria, South Africa
VIIFC Path (Microbiol); Tshwane Academic Division, National Health Laboratory Service, Pretoria, South Africa
ABSTRACT
BACKGROUND. The drug levels and clearances of imipenem in critically ill patients are not comprehensively described in current literature, yet it is vital that adequate levels be achieved for therapeutic success.
OBJECTIVES. To determine the proportion of critically ill patients treated with imipenem/cilastatin with sub-therapeutic imipenem plasma levels, and to compare the clinical outcomes of those patients with therapeutic levels with those who had sub-therapeutic levels.
Methods. Trough imipenem plasma levels of 68 critically ill patients from a surgical intensive care unit were measured using a validated high-performance liquid chromatography method. Imipenem trough levels were compared with the minimum inhibitory concentration (MIC) of the causative bacterial agents, based on a target value of 100% time above MIC (/T >MIC).
RESULTS. The proportion of participants with sub-therapeutic imipenem levels was 22% (95% confidence interval (CI) 13% - 34%). The 14- and 28-day mortality rates in the sub-therapeutic group were 33% and 40%, respectively, compared with 19% (p=0.293) and 26% (p=0.346), respectively, in the therapeutic group. Sub-therapeutic imipenem plasma levels are associated with adjusted hazard ratio of 1.47 (95% CI 0.55 - 3.91).
CONCLUSIONS. The lower proportion of critically ill patients with sub-therapeutic imipenem plasma levels in this study compared with previous studies may be attributed to the practice of higher dosages and the administration method of extended infusions of imipenem/ cilastatin in our setting. The results demonstrate a trend of higher mortality in patients with sub-therapeutic imipenem levels, although the results were not statistically significant at this sample size.
Globally, bacterial infections are a major contributor to morbidity and mortality in critically ill patients[1,2] In this patient population, an infection may rapidly progress to sepsis and if inadequately managed, be fatal[3,4] It is therefore imperative to administer optimal antimicrobial therapy to critically ill patients with a bacterial infection[3,5] Antibiotic dosage guidelines are usually determined by pharmacokinetic and pharmacodynamic (PKPD) studies conducted among healthy volunteers.[5] However, the pharmacokinetics of drugs are drastically altered in critically ill patients compared with their healthy counterparts^3,6 The DALI study[7] found that 16% of critically ill patients had inadequate beta-lactam levels, and that these patients were 32% less likely to have a positive clinical outcome compared with those with adequate levels. Furthermore, under-dosing of antibiotics contributes to the development of antimicrobial resistance.® Individualised drug dosing, guided by therapeutic drug monitoring (TDM), offers a solution to these issues, and is becoming a necessity to ensure efficacy and safety. TDM is used to measure the plasma concentration of a drug at specific time intervals[9] Conventionally, TDM was used only for drugs with a narrow therapeutic range where the risk of toxicity was high.[10] However, owing to the increasingly rapid development of antibiotic resistance and our improved understanding of altered pharmacokinetics in critically ill patients, TDM is now in vogue for use in all classes of antibiotics.[11]
Imipenem/cilastatin is a combination of a hydrophilic antibiotic of the carbapenem class of beta-lactam agents and a dehydropeptidase-1 inhibitor.[12] It exhibits a broad spectrum of activity and is generally effective against Gram-positive, Gram-negative, aerobic and anaerobic bacteria.[13] Under normal conditions, imipenem is rapidly degraded by kidney dehydropeptidase-1.[14] To counteract this, it is combined with cilastatin, which inhibits this enzyme.[14] Due to its wide spectrum of activity, tolerability and safety profile, it is often used to treat infections in critically ill patients.[15] In general, imipenem plasma levels are not routinely measured; rather, standard dosage guidelines are relied upon.[5] These guidelines are based on PKPD studies in healthy volunteers.[5] The package insert recommends using a dosage range between 500 and 1 000 mg every 6 hours, depending on the site and severity of infection.[16] The drug is most effective at a concentration four times the minimum inhibitory concentration (MIC) of the causative organism.[17] As imipenem plasma levels are not routinely measured, the agent's pharmacokinetics in critically ill patients are poorly described in the current available literature[3,5,18,19]Available reports show that sub-therapeutic imipenem levels are found in 0% - 70% of critically ill patients.[5,20-23] This wide variation and the unacceptably high proportions of sub-therapeutic imipenem levels in this population group served as an impetus for this study.
The aim of this study was to investigate the need for imipenem TDM in our critically ill patients. The primary objective was to determine the proportion of critically ill patients treated with imipenem/cilastatin with sub-therapeutic imipenem plasma levels. The secondary objective was to compare the clinical outcomes of those patients with therapeutic levels with those who had sub-therapeutic levels.
Methods
The present prospective, observational, cohort study was conducted among critically ill patients in the surgical intensive care unit (ICU) at the Steve Biko Academic Hospital (Pretoria, South Africa (SA)) from March 2018 to October 2019. The study was approved by the Faculty of Health Sciences Research Ethics Committee of the University of Pretoria (ref. no. 473/2017). Written informed consent was obtained from each participant, or in cases of incapacitation, from the participant's next of kin. Eligibility for inclusion in the study required that a participant be >18 years, critically ill (defined as admission to the ICU), have an infection with a clinical or microbiological indication for imipenem/ cilastatin therapy and initiated on imipenem/cilastatin at the discretion of the responsible clinician. The unit in which the study was done uses individualised dosing schedules of imipenem/cilastatin ranging between 500 and 1 000 mg (based on renal function) 6 - 12-hourly, with each dose infused over 3 hours. Patients with no renal impairment and estimated creatinine clearance >70 mL/min/1.73m2 received 1 000 mg 6-hourly. Patients with mild renal impairment and estimated creatinine clearance 41 - 70 mL/min/1.73m2 received 750 mg 8-hourly. Patients with moderate renal impairment and estimated creatinine clearance 21 - 40 mL/min/1.73m2 received 500 mg 8-hourly. Patients with severe renal impairment and estimated creatinine clearance <20 mL/min/1.73m2 received 500 mg 12-hourly. All doses were infused over 3 hours. Patients who withheld informed consent were excluded from the study.
A trough blood sample was obtained from each participant prior to re-dosing, taken once steady state had been achieved. Steady state is defined as a situation where the overall intake of the drug is in a dynamic equilibrium with its elimination.[24] Each participant received at least four prior doses before the study sample was taken. Each sample was transferred into a heparinised collection tube and transported to the microbiology laboratory immediately after collection, where it was centrifuged at 5 000 revolutions per minute for 10 minutes. After centrifugation, 2 mL of plasma was removed and stabilised with 2 mL of 2-N-morpholine-ethane sulfonic acid and ethylene glycol solution (1:1) and stored at -70°C until analysis.[15] Imipenem levels were measured using a validated high-performance liquid chromatography (HPLC) method, the details of which have been published previously.[25] Analytical-grade imipenem was obtained from the European Directorate for the Quality of Medicines & HealthCare. The HPLC was done utilising a Shimadzu ultra-fast liquid chromatography system.
Imipenem trough levels were compared with the MIC of the causative bacterial agents, based on a target value of 100% time above MIC (/T >MIC). Studies suggest that for beta-lactam antibiotics to be effective in non-critically ill patients, the unbound (free) drug concentration should be maintained above the MIC (/T >MIC) for 40% - 70% of the dosing interval.[26,27] However, in critically ill patients, recent reports suggest a PKPD target of 100% /T >MIC, and this target was used in the present study[24,28] MICs were determined as part of routine laboratory procedure by either the Vitek2 system or by Etest. A limitation of the Vitek2 system is that the lowest reported MIC of imipenem is <0.25 mg/L, and the highest reported value is >16 mg/L. In all calculations performed, MIC values of <0.25 mg/L where rounded to 0.25 mg/L, and values of >16 mg/L where rounded to 16 mg/L. Where MIC data were not available, the highest MIC breakpoint in the susceptible range of the bacterial species cultured was used, as published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST).[24,29] In cases where no organism was cultured, a proxy MIC value of 4 mg/L was used in calculations, as this is the highest susceptible EUCAST breakpoint for any organism.
Participants were allocated to one of two groups according to their measured plasma drug concentration in relation to the MIC of the causative bacterial pathogen: 'therapeutic' and 'sub-therapeutic'. The two groups were compared in terms of length of stay and mortality.
Demographic and clinical data were captured from each participant's hospital chart and entered electronically using Excel (Microsoft Corporation, USA). Infection sites were defined according to the criteria published by Garner et al.[30] Creatinine clearance was estimated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to calculate estimated glomerular filtration rates.[31,32] The clinical course of each participant was followed up for at least 28 days. Clinical outcomes were measured by length of hospital and ICU stay (in days) and 14- and 28-day mortality. To ensure that the comparison between the two groups was robust, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated for each participant on admission.[33]
Statistical analysis employed Stata version 15 software (StataCorp, USA). Comparison of imipenem plasma level groups (therapeutic and sub-therapeutic) with respect to continuous variables was done using Student's two-sample t-test, Welch's t-test with unequal variances and the Wilcoxon rank sum test. For discrete variables, Fisher's exact test was used. The above methods were repeated in the comparison with respect to mortality categories (survived and deceased). Imipenem plasma level categories were compared with respect to their survivor functions using the log-rank test and Kaplan-Meier methods. Survival was modelled using Cox regression with outcome mortality and time as length of hospital stay. Variables for which mortality groups were significantly different at the liberal significance level of 0.15 were included, along with the categorical variable for imipenem therapeutic plasma levels, into a Cox regression analysis. Testing was done at the 0.05 level of significance.
Results
Participant recruitment took place from 1 March 2018 to 31 October 2019. During this period, 69 patients were eligible for recruitment; 1 patient withheld consent and was excluded from the evaluation. Fig. 1 depicts the distribution of study participants. In total, 68 participants were included in the analysis. The demographics and description of the participant population are summarised in Tables 1 and 2. Briefly, 63% of participants were male. The mean age was 47 (range 18 -81) years, and the mean weight was 78.8 (range 40 - 140) kg. Renal function varied widely between participants. The mean creatinine level was 142.4 (range 33 - 840) umol/L, and the mean estimated creatinine clearance was 91 (range 6 - 180) mL/min/1.73m3. Of the 68 participants, 24 (35%) had estimated creatinine clearance levels <60 mL/min/1.73m3, and 20 (29%) had estimated creatinine clearance levels above 130 mL/min/1.73m3. The most common comorbidity was cardiovascular disease (37%), and 16% of participants were HIV-positive. On admission to ICU, 44% of participants had sepsis, 32% were admitted for general surgical conditions and 24% for trauma. The APACHE II scores on admission to ICU ranged between 4 and 39 (mean 18). The most common site of infection was bloodstream infection (62%). The majority (84%) of participants were treated for hospital-acquired infections. The mean length of stay was 16 (range 2 - 58) ICU days, and 41 (range 5 - 167 days) hospital days. The 14- and 28-day mortality rates were 22% and 29%, respectively. The mean imipenem trough plasma level was 11.5 (range 3.6 -92.2) mg/L . Table 3 shows the bacterial organisms cultured from the participants. It also provides a breakdown of the MICs of imipenem determined for each organism. The most common organism cultured was Klebsiella pneumoniae, of which the mean MIC was 2.5 mg/L.
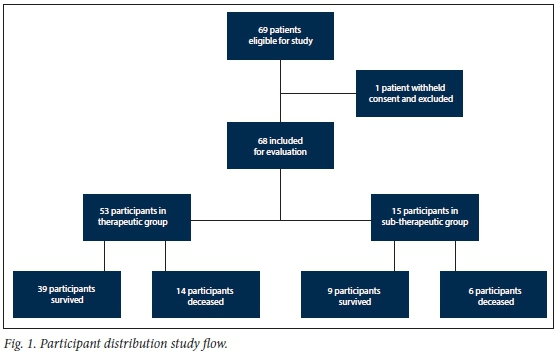
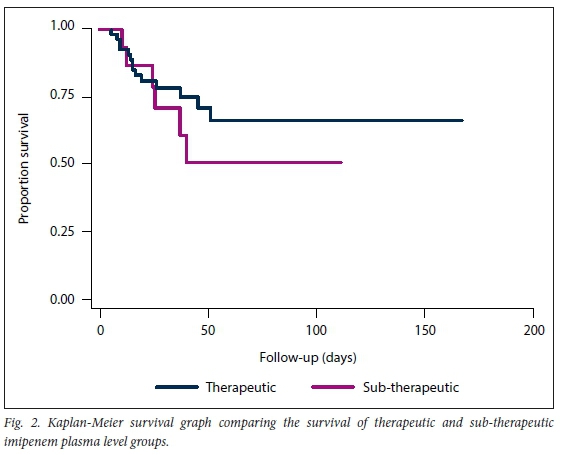
Imipenem-resistant Gram-negative bacilli were cultured in 23 (34%) patients. The isolates cultured in these patients included 21 imipenem-resistant Acinetobacter baumannii, 4 imipenem-resistant Enterobacterales, and 1 imipenem-resistant Pseudomonas aeruginosa. Combination Gram-negative antibiotic therapy was used in 6 of these patients, 5 patients received colistin in combination with imipenem/cilastatin and 1 received piperacillin/tazobactam in combination with imipenem/cilastatin. Of the 68 participants evaluated, 15 (22%; 95% confidence interval (Cl) 13% - 34%) had sub-therapeutic imipenem plasma levels. The mean imipenem trough level in the sub-therapeutic group was 6.8 (range 3.6 -10.8) mg/L, compared with 12.8 (range 4.0 -92.2) mg/L in the therapeutic group. There were no significant differences between the two groups in terms of age, weight, severity of illness (APACHE II score), sex, admission category, infection type, estimated creatinine clearance, creatinine and albumin levels. However, there was a statistically significant difference between the two groups concerning the mean MIC of bacterial pathogens cultured (3.9 mg/L v. 16.3 mg/L, p<0.001), with a higher mean MIC in the sub-therapeutic group. There was also a statistically significant difference in terms of cardiovascular disease (43% v. 13%, /)=0.038), with a higher prevalence in the therapeutic group. Other statistically significant differences between the two groups occurred in the prevalence of lower respiratory tract infections (15% v. 53%, p=0.004), with higher prevalence in the sub-therapeutic group, and in catheter-related bloodstream infections (6% v. 27%, p=0.038), with higher prevalence in the sub-therapeutic group. The details of these differences may be found in Tables 1 and 2. The mean lengths of hospital stay and ICU stay in the sub-therapeutic group were 37 days and 16 days, respectively, compared with 42 days (p=0.639) and 16 days (p=0.886), respectively, in the therapeutic group. In terms of mortality, the 14" and 28-day mortality rates in the sub-therapeutic group were 33% and 40%, respectively, compared with 19% (p=0.293) and 26% (p=0.346), respectively, in the therapeutic group. The log rank test predicted 15.6 mortalities in the therapeutic group, against the 14 observed mortalities in this group, compared with 4.4 predicted and 6 observed mortalities in the sub-therapeutic group (p=0.372). Fig. 2 illustrates the result of the Kaplan Meier survival analysis. Of the 68 participants included in the study, 48 were alive 28 days post inclusion. A comparison between the groups of participants who survived and those who died is presented in Tables 1 and 2. There was no statistically significant difference between the two groups in terms of age, weight, APACHE II scores, length of ICU stay, sex, admission category, site of infection, infection type, MICs, estimated creatinine clearance, creatinine and albumin levels. However, there was a statistically significant difference between the two groups in terms of length of hospital stay (49 days v. 22 days, p=0.001), with a longer stay in the surviving group. There was also a statistically significant difference in terms of the prevalence of malignancy (6% v. 25%), with a higher prevalence in the deceased group. The Cox regression analysis found an unadjusted hazard ratio of 1.54 (95% Cl 0.59 - 4.02; p=0.377) for sub-therapeutic imipenem plasma levels in terms of 28-day mortality. After considering variables for which mortality groups were significantly different at the liberal 0.15 level of significance, for inclusion into the Cox regression analysis, a hazard ratio of 1.47 (95% Cl 0.55 - 3.91; p=0.441) was found for sub-therapeutic imipenem plasma levels in terms of 28-day mortality. The 28-day mortality rate in the 23 participants who had infections with imipenem-resistant Gram-negative bacilli was 34%. There was no statistically significant difference in the 28-day mortality rate of those participants who received combination Gram-negative antibiotic therapy and those who received imipenem mono therapy (33% v. 35%; p=0.932).
Discussion
The pharmacokinetics of antibiotics are drastically different in critically ill patients compared with healthy volunteers[3,5,6] The most important reasons for these differences include the changes in physiology of the critically ill patient, capillary leak syndrome and end organ dysfunction.[34] In addition, patients with sepsis frequently become haemodynamically hyper-dynamic.[34] This leads to increased renal blood flow, with a resultant increase in the glomerular filtration rate and increased clearance of drugs, including beta-lactam antibiotics. This augmented renal clearance (ARC) is well described in the literature.^] In our study, 29% of participants had estimated creatinine clearance levels above 130 mL/min/1.73m3 and would qualify as having ARC. Protein binding is another important feature of drugs as only the unbound fraction is pharmacodynamically active.[3] Hypoalbuminaemia is a frequent finding in critically ill patients.[3] It is further associated with increased volume of distribution and increased drug clearance of highly protein-bound hydrophilic drugs, leading to low plasma drug levels of these agents.[3] Since imipenem's protein binding is approximately 10% - 20%, the albumin concentrations affect imipenem to a lesser extent compared with more highly protein-bound drugs.[14] Fluid accumulation due to hypoalbuminaemia may, however, still increase the volume of distribution of imipenem and result in lower plasma levels. Furthermore, owing to endothelial damage and increased capillary permeability in sepsis, the volume of distribution of hydrophilic agents, such as imipenem, is increased, leading to decreased serum levels.[35] To aid clinicians with drug dosage decision-making, dosing nomograms were developed, but they have proven to be inaccurate and unhelpful in many circumstances.[34]
This study found a lower proportion of critically ill patients with sub-therapeutic imipenem plasma levels than in previous studies, i.e. Belzberg et al.[5] (46%), Fournier et al.[20] (48%) and Huttner et al.[21] (77%). This may be attributed to higher dosages of imipenem/cilastatin used in our setting than in previous studies.[20,21] The use of extended infusion of the imipenem/cilastatin over 3 hours in our study, compared with 30 minutes in the three aforementioned studies, may also have impacted on more favourable pharmacokinetics. Other authors have also reported improved pharmacokinetics when using higher doses and prolonged infusions with beta-lactam antibiotics.[36,37] Differences in study populations in terms of age, sex, race, weight and renal function may also have contributed to the disparity between the studies. However, the finding that more than one in five critically ill patients in this study have sub-therapeutic imipenem levels is nonetheless a significant result considering the impact of inadequate levels on patient outcomes and resistance levels.
This observation alone may be considered evidence to necessitate TDM of imipenem in this population, and is aligned with the recommendations of previous authors.[5,20,21,24]
Interestingly, no significant differences between the two groups were observed in terms of length of hospital or ICU stay. This may well be due to the sample size being too small to detect statistically significant differences. Alternatively, many confounding factors impact on length of stay, such that the impact of imipenem plasma levels might not be evident on outcome. With respect to mortality, our results show a definite trend in that patients with sub-therapeutic imipenem levels have higher mortality (40%) than patients with therapeutic plasma levels (26%), although this result was not statistically significant (p=0.346). Furthermore, sub-therapeutic imipenem plasma levels were found to have an adjusted hazard ratio of 1.47 (95% Cl 0.55 - 3.91; p=0.441) in terms of 28-day mortality. However, as in previous studies, these results were not statistically significant at this sample size, and a larger study is necessary to further investigate the impact of sub-therapeutic imipenem plasma levels on clinical outcome. Due to the study design and PKPD target, sub-therapeutic imipenem plasma levels invariably reflected high MIC values, rather than low imipenem concentrations, suggesting that MIC might be a better indicator of treatment failure than imipenem concentration. In the ideal setting, individualised therapy guided by TDM will be preferred. However, therapeutic drug monitoring of beta-lactams is not routine, and this study reinforces the concept that MIC values should be used to determine treatment outcomes for the present. This study additionally highlights the increasing number of infections with carbapenem-resistant Gram-negative bacilli in the ICU setting, and the difficulties associated with managing these patients. TDM of imipenem or other beta-lactam antibiotics ought to be worthwhile in all patients with multidrug-resistant organism infections. The unfortunate reality is that in many countries, including SA, TDM is not only an expensive intervention but also not easily accessible. Further research is certainly required to ascertain in which specific patient population subsets beta-lactam TDM would be most useful.
This study has important limitations that should be considered. It was conducted on participants from a single centre, and the sample size was small. As such, the results may not be globally applicable. Since this study only analysed a single plasma sample from each patient, it does not adequately reflect the variability of imipenem plasma levels in critically ill patients, which may change on a daily or even hourly basis. Although consensus guidelines for beta-lactam TDM do not exist, recent studies recommend that at minimum, two blood samples be taken, as a single sample would be insufficient to appropriately inform dose adjustment[11,38] We evaluated only one sample per patient, taking into account that this was a non-interventional study. Interpreting data regarding clinical outcomes in patients with sepsis is difficult, with numerous confounding factors to consider. In the present study infection source control was not documented, and this makes outcome data difficult to interpret.
The study also has a number of strengths. The study population is diverse and includes a varied distribution in terms of demographics and clinical conditions. By including patients with a wide range of renal function, the results reflect a realistic representation of patients treated in the ICU setting. To our knowledge, this is the first study on TDM of imipenem in critically ill patients done in Africa.
Conclusion
With the rapid increase in antimicrobial resistance globally, it is becoming ever more important to ensure adequate antimicrobial treatment for bacterial infections. This is imperative not only to ensure satisfactory clinical outcomes, but also to prevent further development of antimicrobial resistance. Mortality in critically ill patients with sepsis is exceedingly high, and sub-therapeutic antibiotic levels may be an important contributing factor. In this study, 22% (95% CI, 13% - 34%) of critically ill patients treated with imipenem/cilastatin had sub-therapeutic imipenem plasma levels. We also found a trend toward higher mortality in patients with sub-therapeutic imipenem plasma levels compared with patients with therapeutic plasma levels. This study is important because it highlights the fact that, even when using high dosages and prolonged infusions, one-fifth of critically ill patients had sub-therapeutic imipenem plasma levels. TDM offers a solution to detect patients with sub-therapeutic antibiotic plasma levels in order to adjust the dose accordingly. It is the authors' view that the future of antibiotic dosing, particularly in critically ill patients, is to aim for individualised dosing, and TDM is vital to achieve this.
The intricacy and heterogeneity of critically ill patients, as well as confounding factors, make associations between antibiotic blood levels and clinical outcomes difficult to establish in studies with small sample sizes. Larger, multicentre studies are required to definitively investigate the clinical impact of sub-therapeutic beta-lactam blood levels in critically ill patients with sepsis.
Declaration. None.
Acknowledgements. The authors would like to thank Dr Edward Bassey, who assisted with the analysis of specimens.
Author contributions. BM designed the study, developed the protocol, recruited participants, collected specimens, processed specimens, collected data, analysed data, drafted and revised the article. He is the guarantor. FP designed the study, recruited participants, provided clinical oversight and revised the article. AG designed the study, analysed the specimens, did the pharmacological calculations and revised the article. JC recruited participants, collected specimens, processed specimens and collected data. MM analysed the specimens. PB did the statistical analysis. MS designed the study, revised the protocol, reviewed the data analysis and revised the article.
Funding. This project was partially funded by a Federation of InfectiousDiseases Societies of South Africa (FIDSSA) - GlaxoSmithKline (GSK) Research Fellowship. The funding body had no input into the design of the study, the collection, analysis or interpretation of the data or the writing of the manuscript.
Conflicts of interest. None.
References
1. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016;193(3):259-272. https://doi.org/10.1164/rccm.201504-0781OC [ Links ]
2. Vincent J, Marshall J, Anzueto A, Martin CD, Gomersall C. International study of the prevalence and outcomes of infection in intensive care units. J Am Medica 2009;302(21):2323-2329. https://doi.org/10.1001/jama.2009.1754 [ Links ]
3. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient -concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014;77:3-11. https://doi.org/10.1016/j.addr.2014.07.006 [ Links ]
4. Junior CT, Franca ISA, Okamoto IVN, Joa II, Salge M, Roberto IC. Infection as an independent risk factor for mortality in the surgical intensive care unit. Clinics 2013;68(8):1103-1108. https://doi.org/10.1164/rccm.201504-0781OC [ Links ]
5. Belzberg H, Zhy J, Cornwell EE, et al. Imipenem levels are not predictable in the critically ill patient. J Trauma-Injury Infect Crit Care 2004;56( 1): 111-117. https://doi.org/10.1097/01.TA.0000056164.26493.28 [ Links ]
6. Asín-prieto E, Rodríguez-gasc A. Applications of the pharmacokinetic/pharmacodynamic (PK/ PD) analysis of antimicrobial agents. J Infect Chemother 2015;21:319-329. https://doi.org/10.1016/j.jiac.2015.02.001 [ Links ]
7. Roberts JA, Paul SK, Akova M, et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014;58(8):1072-1083. https://doi.org/10.1093/cid/ciu027 [ Links ]
8. Nweneka CV, Tapha-Sosseh N, Sosa A. Curbing the menace of antimicrobial resistance in developing countries. Harm Reduction J 2009;6(1):31-35. https://doi.org/10.1186/1477-7517-6-31 [ Links ]
9. Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med 2009;24(1):1-10. https://doi.org/10.3904/kjim.2009.24.L1 [ Links ]
10. Hessen MT, Kaye D. Principles of use of antibacterial agents. Infect Dis Clin North Am 2004;18:435-450. https://doi.org/10.1016/j.idc.2004.04.002 [ Links ]
11. Muller AE, Huttner B, Huttner A. Therapeutic drug monitoring of beta-lactams and other antibiotics in the intensive care unit: Which agents, which patients and which infections? Drugs 2018;78(4):439-451. https://doi.org/10.1007/s40265-018-0880-z [ Links ]
12. Clissold SP, Todd PA, Campoli-Richards DM. Imipenem/cilastatin. Drugs 1987;33(3):183-241. [ Links ]
13. Buckley MM, Brogden RN, Barradell LB, Goa KL. Imipenem/cilastatin. Drugs 1992;44(3):408-444. [ Links ]
14. Rodloff AC, Goldstein EJC, Torres A. Two decades of imipenem therapy. J Antimicrob Chemother 2006;58(September):916-929. https://doi.org/10.1093/jac/dkl354 [ Links ]
15. Kabbara WK, Nawas GT, Ramadan WH. Evaluation of the appropriateness of imipenem/cilastatin prescription and dosing in a tertiary care hospital. Infect Drug Resist 2015;8:31-38. https://doi.org/10.2147/IDR.S78633 [ Links ]
16. Imipenem [package insert]. Whitehouse Station, NJ: Merck & Co, 2007. [ Links ]
17. Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: A review. Scand J Infect Dis Suppl 1990;74:63-70. https://doi.org/10.3109/inf.1990.22.suppl-74.01 [ Links ]
18. Jaruratanasirikul S, Vattanavanit V, Samaeng M, Nawakitrangsan M, Sriwiriyajan S. Pharmacokinetics of imipenem in critically Ill patients with life-threatening severe infections during support with extracorporeal membrane oxygenation. Clin Drug Investigat 2019;39(8):787-798. https://doi.org/10.1007/s40261-019-00796-3 [ Links ]
19. Chen W, Zhang D, Lian W, et al. Imipenem population pharmacokinetics: Therapeutic drug monitoring data collected in critically ill patients with or without extracorporeal membrane oxygenation. Antimicrob Agents Chemother 2020;64(6). https://doi.org/10.1128/AAC.00385-20 [ Links ]
20. Fournier A, Eggimann P, Pagani J, et al. Impact of the introduction of real-time therapeutic drug monitoring on empirical doses of carbapenems in critically ill burn patients. Burns 2015;41(1):956-968. https://doi.org/10.1016/j.burns.2015.01.001 [ Links ]
21. Huttner A, Renzoni A, Pagani L, Centrale O. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int J Antimicrob Agents 2015;45(4):385-392. https://doi.org/10.1016/j.ijantimicag.2014.12.017 [ Links ]
22. Sakka SG, Glauner AK, Bulitta JB, et al Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 2007;51(9):3304-3310. https://doi.org/10.1128/AAC.01318-06 [ Links ]
23. Abhilash B, Tripathi CD, Gogia AR, et al Pharmacokinetic/pharmacodynamic profiling of imipenem in patients admitted to an intensive care unit in India: A nonrandomized, cross-sectional, analytical, open-labeled study. Indian J Crit Care Med 2015; 19( 10):587-592. https://doi.org/10.4103/0972-5229.167036 [ Links ]
24. Hayashi Y, Lipman J, Udy AA, et al B-lactam therapeutic drug monitoring in the critically ill: Optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int J Antimicrob Agents 2013;41(2):162-166. https://doi.org/10.1016/j.ijantimicag.2012.10.002 [ Links ]
25. Garcia-Capdevila L, Lopez-Calull C, Arroyo C, et al. Determination of imipenem in plasma by high-performance liquid chromatography for pharmacokinetic studies in patients. J Chromatogr B Biomed Sci Appl 1997;692(1):127-132. https://doi.org/10.1016/S0378-4347(96)00498-7 [ Links ]
26. Craig W. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998;26:1-10. [ Links ]
27. Drusano G. Antimicrobial pharmacodynamics: Critical interactions of 'bug and drug. Nat Rev Microbiol 2004;2:289-300. https://doi.org/10.1038/nrmicro862 [ Links ]
28. Roberts JA, Ulldemolins M, Roberts MS, et al. Therapeutic drug monitoring of B-lactams in critically ill patients: Proof of concept. Int J Antimicrob Agents 2010;36(4):332-339. https://doi.org/10.1016/j.ijantimicag.2010.06.008 [ Links ]
29. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_ tables/v_8.0_Breakpoint_Tables.xlsx (accessed 01 March 2018). [ Links ]
30. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16(3):128-140. [ Links ]
31. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604-612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 [ Links ]
32. Eknoyan G, Lameire N, Eckardt K, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3(1):5-14. [ Links ]
33. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med 1985;13(10):818-829. [ Links ]
34. Huttner A, Harbarth S, Hope WW, Lipman J, Roberts JA. Therapeutic drug monitoring of the β-lactam antibiotics: What is the evidence and which patients should we be using it for? J Antimicrob Chemother 2015;70(July):3178-3183. https://doi.org/10.1093/jac/dkv201 [ Links ]
35. Shah S, Barton G, Fischer A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J Intensive Care Soc 2015;16(2):147-153. https://doi.org/10.1177/1751143714564816 [ Links ]
36. Patrier J, Tlmsit JF. Carbapenem use in critically ill patients. Curr Opin Infect Dis 2020;33(1):86-91. https://doi.org/10.1097/QCO.0000000000000622 [ Links ]
37. Abdul-Aziz MH, Portunato F, Roberts JA. Prolonged infusion of beta-lactam antibiotics for Gram-negative infections: Rationale and evidence base. Curr Opin Infect Dis 2020;33(6):501-510. https://doi.org/10.1097/QCO.0000000000000681 [ Links ]
38. Fratoni AJ, Nicolau DP, Kuti JL. A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy 2021;41(2):220-233. https://doi.org/10.1002/phar.2505 [ Links ]
 Correspondence:
Correspondence:
B Mitton
barend.mitton@nhls.ac.za
Accepted 15 April 2021.














