Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.9 Pretoria Set. 2021
http://dx.doi.org/10.7196/SAMJ.2021.v111i9.15583
RESEARCH
Patterns of leprosy at Chiis Hani Baragwanath Academic Hospital, Johannesburg, South Africa, and review of current clinical practice
L J NkehliI, II; C N MenezesIII, IV; J M L TsitsiV, VI
IMB ChB, FC Derm (SA), MMed (Derm); Division of Dermatology, Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IIMB ChB, FC Derm (SA), MMed (Derm); Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMD, MMed (Int Med), Dip HIV Man (SA), DTM&H, FCP (SA), Cert ID (SA), PhD; Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVMD, MMed (Int Med), Dip HIV Man (SA), DTM&H, FCP (SA), Cert ID (SA), PhD; Division of Infectious Diseases, Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital, Soweto, South Africa
VMB ChB, FCP (SA) Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIMB ChB, FCP (SA) Division of Infectious Diseases, Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital, Soweto, South Africa
ABSTRACT
BACKGROUND. The World Health Organization announced a strategy to eliminate childhood leprosy infections, visible deformities and discriminatory legislation against leprosy patients by 2020. However, challenges in achieving a leprosy-free world and preventing neurological sequelae still exist. HIV infection is a challenge in South Africa (SA). HIV-leprosy co-infection may result in an increase in the frequency of leprosy reactions without affecting the spectrum of leprosy. From 1921 to 1997, the prevalence of leprosy remained <1 patient per 10 000 population. Current SA literature has very scanty information regarding leprosy infections.
OBJECTIVES. To describe the trend of new leprosy patients at Chris Hani Baragwanath Academic Hospital, Johannesburg, SA, from 1999 to 2015, including demographics, clinical spectrum and treatment outcomes.
METHODS. A retrospective review of patients' clinical records was undertaken. Data on demographics, clinical spectrum including the leprosy classification, reactions, neurological involvement, association with HIV infection and treatment outcomes were extracted. Data analysis was performed using descriptive and inferential statistics and a time series analysis.
RESULTS. An upward trend from 1999 to 2001 was followed by a decline in the number of new patients. Eighty patients were registered over a period of 17 years, with a male-to-female ratio of 3:1. Thirty-six patients were immigrants, and 5 were children aged <15 years. Multibacillary leprosy was the most common type (n=71 patients). Thirty-six patients had the lepromatous leprosy subtype, 22 were borderline lepromatous, 13 were borderline tuberculoid, 6 were borderline borderline, and 3 had tuberculoid leprosy. Thirty-one patients presented with reactions, type 1 in 9 patients and type 2 in 21 patients, with both types in 1 patient. Grade 2 neurological deformities were diagnosed in 37 patients, of whom 2 were children. Eight patients were found to have HIV-leprosy co-infection. Of 52 patients who completed treatment, 26 were cured and 26 were lost to follow-up. Twenty-one patients defaulted from treatment, while 3 patients relapsed.
CONCLUSIONS. This study highlights the current status of leprosy in a low-endemic centre with declining numbers of new patients. Multibacillary forms with grade 2 disabilities (G2Ds) are common. The constant emergence of leprosy in our population highlights shortfalls in our control campaigns. Furthermore, a high rate of G2Ds necessitates scrutiny of education directed at early patient detection and follow-up strategies.
Leprosy is a notifiable chronic infectious disease caused by Mycobacterium leprae that primarily affects the skin and peripheral nerves.[1,2] Other organs such as the eyes, mucosae, bones and testes may be affected.[3] Leprosy often results in severe, lifelong disabilities and deformities.[2] Through awareness and early medical intervention, a reduction in disabilities is possible. Neurological damage is irreversible and often requires lifelong care.[4] The World Health Organization (WHO) declared leprosy eliminated as a public health problem in 2000 based on the achievement of a global prevalence of <1 case of leprosy per 10 000 people.[3,4]
There are regions and countries where leprosy is still endemic, and these carry the bulk of global cases.[2,5] In 2015, the highest incidence of the disease was found in India, Brazil and Indonesia, together accounting for 81%, while Africa contributed ~9% to the global incidence.[2,6] In 2016, the WHO committed to strategies of reducing the disease burden to achieve a world free of leprosy.!51 These focused on strengthening government ownership, co-ordination and partnership; strategies to further reduce leprosy and its complications; and tackling discrimination and stigma associated with leprosy.[5]
Middle age is the most common age of presentation, with males most often affected.[7-9] The latter has been attributed to male migration patterns.[10] Data from multiple countries suggest that infections in females are detected late.´[11] The proportion of infected children aged <15 years varies, ranging from 1.9% to 18%.[7,10,12,13]
Risk factors for contracting leprosy include low socioeconomic status, poor education, contact with leprosy, and genetic factors. !3] These may influence endemicity, but they do not fully explain the differences in endemicity that exist between African and Asian regions.[6] Close prolonged contact with an infected patient increases the risk by five to eight times.[8,14] A study found that 62% of new cases had contact history in a low-endemic area compared with 25% in a high-endemic area.[13] In 2016, foreign-born patients contributed 100% of new cases in Qatar and the United Arab Emirates, highlighting the disparity in distribution of leprosy.[2]
Leprosy has a myriad of clinical presentations that depend on cell-mediated immune response to the bacilli. A simple WHO classification is used for the current treatment model (Table 1).[3,15]
The Ridley-Jopling classification (RJC) uses the spectrum of cell-mediated responses to the bacilli, divided into five categories that range from mild tuberculoid leprosy (TT) to the most severe lepromatous leprosy (LL), as summarised in Table 2. In between these extremes lie immunologically unstable forms, borderline tuberculoid (BT), borderline borderline (BB) and borderline lepromatous (BL), which can evolve to either TT or LL poles by downgrading or upgradingJ16-18] A form where an immune response has not yet developed is called indeterminate leprosy.[19]
Leprosy reactions are inflammatory reactions due to T-lymphocyte activation or circulating immune complexes resulting in leuko-cytoclastic vasculitis.!?0 Type 1 reactions, which are delayed-type hypersensitivity, include upgrading (also known as reversal), representing a shift towards the TT pole, and downgrading reactions, which represent a shift towards the LL pole.[3,20-22] Upgrading reactions are often seen during initiation of treatment.[6] Type 1 reactions are characterised by erythema, induration or ulceration of existing skin lesions as well as nerve pain or tenderness, which may be followed by loss of function. Type 1 reactions are frequent in borderline forms (BT, BB and BL), which are immunologically unstable.[3,21,22] Type 2 reactions, also known as erythema nodosum leprosum (ENL), are caused by an acute immune complex vasculitis affecting the skin and other organs, commonly seen in lepromatous subtypes (LL and BL) with high bacillary loads.[3,6,21] These present as new erythematous painful nodules, pustules or ulcers. Other features include fever, lymphadenopathy, arthritis, neuritis, glomerulonephritis, iridocyclitis, orchitis and dactylitis.[6,23]
Lucio's phenomenon is a rare leprosy reaction caused by direct endothelial cell damage, characterised by acute painful necrotic ulcers associated with fever, anaemia, lymphadenopathy and hepatosplenomegaly.l3] In a study where borderline forms predominated, Chhabra ef al.[9] reported that more patients presented with type 1 reactions (n=258/849) than with type 2 (n=60/849). In a review, Kahawita ef al.[22] demonstrated a varying type 1 reaction prevalence rate ranging from 19% to 29%. Leprosy reactions are more frequent in patients co-infected with HIV, and the rapid-phase immune reconstitution seen with the initiation of HIV treatment allows for manifestations of pre-existing leprosy.[24,25] Systemic steroids are used to treat all types of leprosy reactions, with high doses in Lucio's phenomenon[2,26] In addition, analgesia, clofazimine and thalidomide are used in treatment of type 2 reactions[22]
Inclusion of leprosy in the differential diagnosis is a major challenge in non-endemic settings[27] A high index of suspicion and interpretation of clinical signs as shown in Table 2 are the mainstay of the diagnosis. Demonstration of M. leprae by slit-skin smears, histopathology or polymerase chain reaction is useful to confirm the diagnosis. Limited availability of laboratory facilities in some areas makes clinical diagnosis important.[19,26]
Multidrug treatment (MDT) comprising a combination of rifampicin, dapsone and clofazimine for 12 months in multibacillary (MB) leprosy and for 6 months in paucibacillary (PB) leprosy is recommended by the WHO.[3,5,28] Adjusted doses of MDT are used in children.[26] MDT prevents resistance, induces a rapid decline in infectivity, and decreases the rate of recurrence.[26] The Leprosy Mission distributes the leprosy MDT in South Africa (SA) through its partnership with the WHO. Cure rates ranging between 64% and 100% have been reported.[7,28] Reported relapse rates vary between zero and 20% following MDT.[29-32] Relapses tend to occur between 3.5 and 6 years after MDT completion, and are associated with higher bacillary loads.[29,31] Resistance to rifampicin and dapsone as well as to secondary therapies such as ofloxacin has been reported to be as high as 8%.[26]
Understanding of the current leprosy situation in our setting relies on documented information in the literature. This information is currently scarce in SA. Furthermore, despite global efforts to eliminate leprosy, it continues to spread in SA, causing recognisable morbidity. Studying leprosy patterns at Chris Hani Baragwanath Academic Hospital (CHBAH), which is the largest hospital in SA, will therefore assist efforts to understand its patterns in the country.[33]
Objectives
To describe the current leprosy trends, demographics and clinical spectrum, and to determine the treatment outcomes of patients treated for the disease at CHBAH from 1999 to 2015.
Methods
Study sample
We retrospectively reviewed clinical records of 80 adult and paedia-tric patients diagnosed with and treated for leprosy at CHBAH from 1 January 1999 to 31 December 2015. The hospital is a designated referral treatment centre for leprosy patients in Johannesburg, the largest city in SA. The population of Johannesburg is diverse, including immigrants from various parts of Africa and other parts of the world. For the purposes of data analysis, patients aged <15 years were classified as children.
Data collection
The data collection instrument was a data sheet. The diagnosis of leprosy was based on clinical examination, histopathological findings or slit-skin smear results. The demographic data obtained included age at diagnosis, gender, region of origin, and history of leprosy contact. Using the United Nations Sub-Saharan African divisions and the world map, regions of origin were divided into northern, central, eastern, western and southern Africa, and Asia or other, based on where the patients were born. The southern Africa region was further divided into member countries, and SA was divided into provinces. The trend was formulated using the total number of new leprosy patients over the years. Patients who relapsed and were admitted to our centre for retreatment were excluded in formulation of the trend of new cases.
The clinical examinations were carried out by a doctor. The skin-slit smears were done by a technician and stained by modified Ziehl-Neelsen methods. The histopathological findings were obtained from skin biopsy reports analysed and reported by the SA National Health Laboratory Service. HIV-positive status was confirmed on antibody or antigen (rapid or enzyme-linked immunosorbent assay) tests. Initial CD4 counts in cells uL at the time of presentation were used. The information on the clinical spectrum of leprosy included the leprosy classification, reactions, neurological involvement, and association with HIV infection.
Records were classified into WHO categories, PB if the number of skin lesions was <5 and MB if there were >5.[15] In addition, a record was classified into one of the RJC variants, i.e. TT, BT, BB, BL and LL, using a combination of clinical assessment, slit-skin smear results and histopathological findings.[15]
Information on the leprosy reactions the patient presented with during the course of the follow-up period was captured. A type 1 reaction was diagnosed when rapid swelling and erythema of existing skin lesions was noted.[21] Type 1 reactions were classified as downgrading if occurring in untreated patients as borderline forms shifts towards the LL pole, and upgrading if occurring with antibiotic treatment.[20] A type 2 reaction was diagnosed if a patient presented with a new painful erythematous nodule, with histopathological features of neutrophilic leukocytoclastic vasculitis A, or with arthritis, neuritis, glomerulonephritis, iridocyclitis, orchitis and dactylitis.[23]
Information on neurological involvement obtained from the records was represented by disability grade.[11 For peripheral nerve involvement, grade 0 represented no impairment, grade 1 anaesthesia of hands or feet with no visible deformity, and grade 2 presence of both anaesthesia and visible deformities such as trophic ulcers, claw deformities and bone resorption in the extremities.!1! For the eyes, grade 0 represented no vision loss related to leprosy, grade 1 some visual impairment (vision 6/60 or better), and grade 2 severe loss of vision.[11]
The considered treatment outcome variables were treatment completed, and patient cured, transferred, relapsed, died or defaulted. Treatment was deemed completed if a PB patient completed the WHO-recommended 6 months of MDT within a 9-month period, and in MB patients if 1 year of MDT was completed within 18 months.[5,28,34]
Cure was defined as bacillary index (BI) negativity in MB types and complete disappearance of the skin lesion(s) in PB types.[28] Relapse was defined as appearance of a new lesion or an increase in size of the lesion, erythema or swelling where it had subsided, confirmed histologically by granuloma reappearance in PB types, increased macrophage infiltration with solid bacilli in MB types, or 2+ increase in BI following successful treatment.[31] Cure and relapse were assessed every 6 months up to 18 months for PB and up to 24 months for MB, which constituted a period of 1 year's follow-up post treatment.
Patients were considered as having defaulted if they did not take their medication for >3 months (PB infection) or >6 months (MB infection).[34] Patients who were sent to other centres for treatment completion were considered transferred. Those who lost their lives during the study period were recorded as having died.
Statistical analysis
Data analysis and interpretation was conducted using both descriptive and inferential statistics. Descriptive statistics were used to describe the distribution of the data using measures such as means, standard deviations (SDs), minimums and maximums for numerical variables such as age, while frequencies and graphs were used to report categorical variables. The number of leprosy patients was reported as frequencies or percentages. A line graph was used to show the trend in numbers of leprosy patients over time.
For categorical variables such as age category, gender, country of origin, history of contact, classification, leprosy reactions, neurological involvement, association with HIV and treatment outcome, frequencies and percentages were reported. Graphical demonstration to visually display the results was done using pie charts or bar charts. Inferential statistics were used to make judgements based on the sample data collected. The χ2 test was used to ascertain associations between the categorical variables if its assumptions were satisfied; otherwise Fisher's exact test was used. The χ2 test was used to test for association between the RJC and leprosy reaction as well as between HIV status and leprosy reaction.
Ethical considerations
Anonymised data were used, with patients' identity protected, and all collected data were secured. Permission for analysis and publication was obtained from the Human Research Ethics Committee of the University of the Witwatersrand (ref. no. M180951).
Results
Demographic characteristics
A total of 80 leprosy patient records were reviewed in the study. The majority (n=70) were new patients, while 10 (12.5%) were relapses after MDT. The sociodemographic features of all patients are summarised in Table 3. There was a preponderance of males (76.2%), with a male-to-female ratio of 3:1. Only 5 patients (6.2%) were children aged <15 years. The minimum and maximum ages of the participants were 4 and 73 years, respectively, with the mean (SD) age 36 (15.27) years.
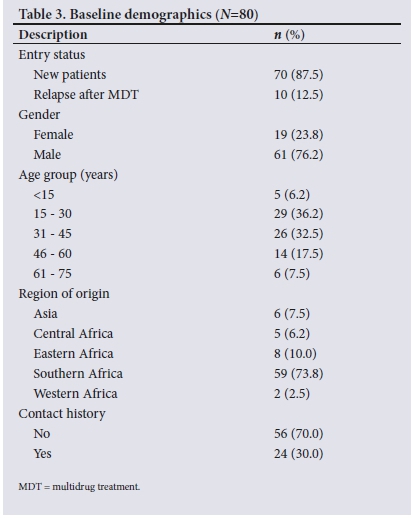
The majority of the patients (73.8%; n=59) were from the southern African region. A total of 56 patients (70.0%) did not have any history of contact with a leprosy patient (Table 3).
Further division of the southern African region showed that SA-born individuals comprised the highest number (/7=44) of leprosy patients compared with migrant groups from this region, i.e. Mozambique (n=10), Swaziland (n=2), and Lesotho, Namibia and Zambia (n=1 each).
Of the 44 SA-born patients, most were originally from Gauteng and KwaZulu-Natal (KZN) provinces, with 17 patients each, 4 were from Mpumalanga, 3 were from Limpopo, 2 were from Free State and 1 was from North West.
Trend
The number of new leprosy patients showed a decline after 2001 (Fig. 1). Before this period, from 1999 to 2001, an upward trend was recorded. After 2004 the number of new patients stabilised, with very little change until 2015.

Clinical spectrum
Classification
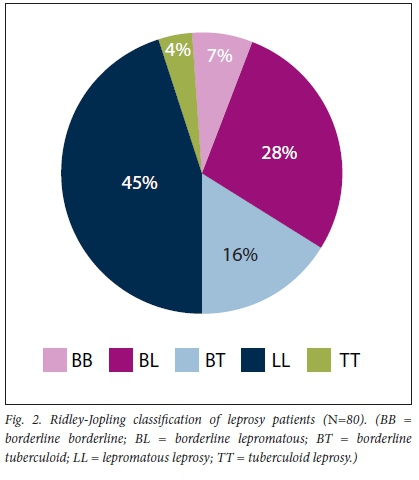
As shown in Table 4, 71 patients (88.8%) had MB leprosy and 9 (11.2%) PB. According to the RJC, the majority (45.0%; n=36) were classified as having LL (Fig. 2).
Leprosy reactions
Most patients (/7=49) had no leprosy reaction, and the breakdown of leprosy reactions is shown in Table 4. All type 1 reactions were upgrading reactions. Of the 21patients with ENL, 17 had the LL variant. The upgrading reaction was found in the LL (n=1), BL (n=4), BB (n=1) and BT (n=3) groups. One patient with BL had both ENL and upgrading reactions, as shown in Table 5.
Neurological involvement
From the available data, 37 patients (46.2%) had grade 2 neurological disability, 2 of whom were children, as shown in Table 4. Patients with the LL variant formed the majority (n=26) of those with neurological disability, 17 of whom had grade 2 disabilities (G2Ds), as shown in Table 5.
Association with HIV
Of the patients, 23 (28.7%) tested negative for HIV and 8 (10.0%) tested positive, while in 49 (61.3%) HIV testing was not done. Of the 8 patients with a positive HIV result, 6 had a CD4 count >200 cells/ μL and only 1 had a CD4 count <200 cells/μL. CD4 count testing was not done on the rest of the patients. Of the HIV-positive patients, 2 had ENL, compared with 8 who were HIV-negative. Fisher's exact test indicated that there was no association between HIV status and leprosy reaction (p=0.584).
Treatment outcomes
Of the patients, 52 had completed treatment and 26 (50.0%) were cured. Cure was not documented for the other half. A total of 21 (26.2%) defaulted, 3 (3.8%) experienced relapse and 4 (5.0%) were transferred (Table 6).
Discussion
This study reports on leprosy patterns at CHBAH. A male predominance was noted, which is consistent with global evidence[18-10,35] and may be due to socioeconomic differences in favour of men, making healthcare access easier for them. The mean age of presentation was in the middle-age group, which is consistent with the findings of other similar studies.[7-9] This group represents the workforce, who are often breadwinners in families and therefore of great concern. Very few patients were children (<15 years old), in line with the findings of other studies.[10,12,35]
Around half of the patients were SA-born, with leprosy most common in patients from Gauteng and KZN. This finding is of concern, as it may indicate clusters of active infection transmission in these locations. The high proportion of patients originating from Gauteng may be a result of easier access to CHBAH. The presence of a similarly high proportion from KZN, which surpasses that of neighbouring provinces, is concerning as it may indicate the presence of higher numbers of patients with active infection in the province. The immigrant population comprised about half of the total number of our patients.
The study found that more than two-thirds of patients had no known history of contact with an index patient[36] Richardus et al.[13]found a lower proportion (one-third) of new leprosy patients with no contact history in low-endemic Thailand compared with high-endemic Bangladesh. Our study was done in SA, which is a low-endemic area; however, our proportion of patients who had no history of contact was higher.
An increasing trend from 1999 to 2001 was followed by a decline from 2001, which stabilised in 2004 with very little change in numbers thereafter. The reason for the increasing number of leprosy patients during the earlier period is unclear, but it may represent a period of increased influx of immigrants from leprosy-endemic neighbouring countries. The declining trend noted from 2001 is similar to patterns described worldwide. [37,38]
We identified a predominance of MB patients in our study (88.8%), with LL forming the majority of cases in this group. The WHO reported that in the Democratic Republic of the Congo and Ethiopia, 60 80% -of new patients detected in 2016 had MB forms.[2] A similar pattern of predominance of MB forms has been recorded in other similar studies.[7,8,12] The predominance of MB forms in our population may be due to poor immunity against leprosy.
In the present study, 26.2% of patients had type 2 reactions, while 11.2% had a type 1 reaction at some point during their follow-up period. Previous studies have found varying prevalence rates of type 1 reactions, ranging from 19% to 30%.[9221D The lepromatous group (LL and BL) predominated in our study, so it is not surprising that type 2 reactions were common.
Nearly half (46.2%) of our patients had G2Ds, 5.4% of whom were children. Varying proportions of patients with G2Ds have been reported. In a global leprosy update in 2016, the WHO reported that 14.5% of new leprosy patients had G2Ds in the African region compared with 4.6% in the south east Asian region.[2] Lower proportions of patients with G2Ds have been reported in similar studies.[7,10] Delay in diagnosis and default in treatment are among the high risk factors for G2Ds.[7,39] The high percentage of G2Ds in the present study may indicate late presentation or delayed diagnosis in our region. Furthermore, the percentage of children presenting with G2Ds was higher in our study than in comparable reports.[2,7]
Almost a third of our patients had had an HIV test. Leprosy-HIV co-infection was found in 10.0%, and the majority of these had CD4 counts >200 cells/μL..Case reports have shown that HIV did not appear to increase leprosy susceptibility, but co-infected patients may have an increased number of reactions.[24] Among our HIV-positive patients, a few had ENL and none had a type 1 reaction. This is in contrast with reports of frequent reactions in co-infected patients; [24,25] however, our numbers were small.
In the present study, 65.0% of patients completed treatment. Half of these were cured, and in the remainder cure was not documented. Our cure rate was 32.5%, which is lower than the clinical cure rate of 64 100% - reported in other studies.[7,28] The loss of 32.5% of patients soon after treatment completion meant that cured patients in this group were not recorded, contributing to our low recorded cure rate. Cure rates have been reported to continue to improve up to 18 months after completion of treatment.[40] In our study, the follow-up period was 12 months after completion of therapy. Extending the follow-up period could therefore have favourably altered our cure rate. Our relapse rate was 3.8%, and all these patients had MB leprosy. Reported relapse rates range from 0% to 20%.[29-32] Dacso et al.[29] and Ali et al.[30] showed lower relapse rates of 0.8% and 0.84%, respectively, in MB patients. Our relapse rates are comparable to rates reported in the literature.
The present study highlighted the presence of active areas of leprosy transmission, mainly from unknown and possible undiagnosed contacts. These mainly result in high bacillary MB forms associated with continued infection spread. In addition, imported infections were found. Delayed diagnosis is also a focus, as a significant number of patients presented with G2Ds. Poor access to specialist dermatology services is a reality in parts of our country, which may be responsible for the delay in diagnosis.
Active patient detection strategies need to be intensified to facilitate early diagnosis and prompt treatment, particularly in KZN. Leprosy screening programmes for immigrants, especially from endemic areas, at country entry ports should also be considered.
Study limitations
In terms of limitations of the present study, bias in reporting could not be excluded, as this was a retrospective analysis. Selection bias is also possible, as it was a single-centre study conducted in a tertiary hospital. Our sample size was small, and the follow-up period was limited to 1 year after treatment completion.
Conclusions
Our analysis of available records shows that despite decreasing numbers of leprosy patients in our centre, there are still clusters of transmission where MB forms predominate. Furthermore, the high rate of G2Ds in new patients, including children, indicates late presentation, which is an obstacle to reducing the disease burden further. The constant emergence of leprosy infections highlights shortfalls in our efforts to achieve the leprosy-free region that was envisioned by the WHO for 2020. Our low cure rates and significant loss of patients to follow-up pose difficulties in achieving a leprosy-free region. The need for further efforts aimed at improving early diagnosis and community awareness education campaigns cannot be over-emphasised.
Declaration. The research for this study was done in partial fulfilment of the requirements for LJN's MMed (Dermatology) degree at the University of the Witwatersrand.
Acknowledgements. None.
Author contributions. LJN designed the study, collected data and prepared the manuscript. CNM and JMLT supervised the study.
Funding. None.
Conflicts of interest. None.
References
1. Beldarrain-Chaple E. Historical overview of leprosy control in Cuba. MEDIC C Rev 2017;19( 1):23-30. [ Links ]
2. World Health Organization. Global leprosy update, 2016: Accelerating reduction of disease burden. Wkly Epidemiol Rec 2017;92(35):501-519. https://www.who.int/publications/i/item/who-wer9235 (accessed 3 May 2018). [ Links ]
3. White C, Franco-Paredes C. Leprosy in the 21st century. Clin Microbiol Rev 2015;28(1):80-94. https://doi.org/10.1128/cmr.00079-13 [ Links ]
4. World Health Organization. Global leprosy situation, 2010. Wkly Epidemiol Rec 2010;85(35) :337-348. https://www.who.int/publications-detail-redirect/who-wer8535 (accessed 10 April 2018). [ Links ]
5. World Health Organization. Global Leprosy Strategy 2016 - 2020: Accelerating towards a leprosy-free world 2016. https://apps.who.int/iris/bitstream/handle/10665/208824/9789290225096_en.pdf (accessed 3 May 2018). [ Links ]
6. Fischer M. Leprosy - an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges 2017;15(8):801-827. https://doi.org/10.1111/ddg.13301 [ Links ]
7. Al Awaidy ST. Progress towards a leprosy-free country: The experience of Oman. PLoS Negl Trop Dis 2017;11( 1 l):e0006028. https://doi.org/10.137l/journalpntd.0006028 [ Links ]
8. Mansori K, Ayubi E, Nasehi M, Mansouri Hanis S, Amiri B, Khazaei S. Epidemiology of leprosy in Iran from 2005 to 2015. Tanaffos 2017;16(2): 144-148. [ Links ]
9. Chhabra N, Grover C, Singal A, Bhattacharya SN, Kaur R Leprosy scenario at a tertiary level hospital in Delhi: A 5-year retrospective study. Indian J Dermatol 2015;60( 1):55-59. https://doi.org/10.4103/0019-5154.147793 [ Links ]
10. Muthuvel T, Isaakidis P, Shewade HD, Kattuppara L, Singh R Govindarajulu S. Leprosy trends at a tertiary care hospital in Mumbai, India, from 2008 to 2015. Glob Health Action 2016;9( 1):32962. https://doi.org/10.3402/gha.v9.32962 [ Links ]
11. Price VG. Factors preventing early case detection for women affected by leprosy: A review of the literature. Glob Health Action 2017; 10(Suppl 2):1360550. https://doi.org/10.1080/16549716.2017.1360550 [ Links ]
12. Nazario AP, Ferreira J, Schuler-Facdni L, Fiegenbaum M, Artígalas O, Vianna FSL. Leprosy in southern Brazil: A twenty-year epidemiological profile. Rev Soc Bras de Med Trop 2017;50(2):251-255. https://doi.org/10.1590/0037-8682-0229-2016 [ Links ]
13. Richardus JH, Meima A, van Marrewijk CJ, Croft RP, Smith TC. Close contacts with leprosy in newly diagnosed leprosy patients in a high and low endemic area: Comparison between Bangladesh and Thailand. Int J Lepr Other Mycobact Dis 2005;73(4):249-257. [ Links ]
14. Puchner KP, Parisi S, Schwienhorst-Stich EM, Kasang C, Salah M, Tanyous E. Trends and patterns in leprosy in nine states of the Republic of the Sudan 7 years after the introduction of routine contact screening Trans R Soc Trop Med Hyg 2017;111(8):354-359. https://doi.org/10.1093/trstmh/trx063 [ Links ]
15. Rodrigues IA jr, Gresta LT, Noviello MdeLM, Cartelle CT, Lyon S, Arantes RME. Leprosy classification methods: A comparative study in a referral center in Brazil. Int J Infect Dis 2016;45:118-122. https://doi.org/10.1016/j.ijid.2016.02.018 [ Links ]
16. Ridley DS, Jopling WH. Classification of leprosy according to immunity: A five-group system. Int J Lepr Other MycobacDis 1966;34(3) :255-273. [ Links ]
17. Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ 1974;51(5):451-465. [ Links ]
18. Walker SL, Lockwood DN. Leprosy. Clin Dermatol 2007;25(2):165-172. https://doiorg/10.1016/j.clindermatol.2006.05.012 [ Links ]
19. Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: Clinical aspects and diagnostic techniques. J Am Acad Dermatol 2020;83(1):1-14. https://doi.org/10.1016/j.jaad.2019.12.080 [ Links ]
20. James W, Berger T, Elston D. Andrews' Disease of the Skin. 10th ed Philadelphia: Saunders Elsevier, 2006. [ Links ]
21. Antunes DE, Ferreira GP, Nicchio MV, et al Number of leprosy reactions during treatment: Clinical correlations and laboratory diagnosis. Rev Soc Bras de Med Trop 2016;49(6):741-745. https://doi.org/10.1590/0037-8682-0440-2015 [ Links ]
22. Kahawita IP, Walker SL, Lockwood DNJ. Leprosy type 1 reactions and erythema nodosum leprosum. An Bras Dermatol 2008;83(1):75-82. [ Links ]
23. Schulz E. Leprosy and cutaneous tuberculosis: South African perspectives. S Afr J Cont Med Educ 1984;2(9):39-51. [ Links ]
24. Galtrey CM, Modarres H, Jaunmuktane Z, et al Leprosy in a patient infected with HIV. Pract Neurol 2017;17(2):135-139. https://doi.org/10.1136/practneurol-2016-001519 [ Links ]
25. John TJ, Muliyil J. Care after cure in leprosy. Lancet 2001;357(9252):313. https://doi.org/10.1016/s0140-6736(05)71761-x [ Links ]
26. Maymone MBC, Venkatesh S, Laughter M, et al. Leprosy: Treatment and management of complications. J Am Acad Dermatol 2020;83(1):17-30. https://doi.org/10.1016/j.jaad.2019.10.138 [ Links ]
27. Alves CRP, Ribeiro MM, Melo EM, Araujo MG. Teaching of leprosy: Current challenges. An Bras Dermatol 2014;89(3):454-459. https://doi.org/10.1590/abd1806-4841.20142444 [ Links ]
28. Kaur P, Singh G. Fixed duration MDT in leprosy and clinical cure. Indian J Dermatol Venereol Leprol 1996;62(1):33-35. [ Links ]
29. Dacso MM, Jacobson RR, Scollard DM, Stryjewska BM, Prestigiacomo JF. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J 2011;104(10):689-694. https://doi.org/10.1097/srrj.0b013e31822d6014 [ Links ]
30. Ali MK, Thorat DM, Subramanian M, Parthasarathy G, Selvaraj U, Prabhakar V. A study on trend of relapse in leprosy and factors influencing relapse. Indian J Lepr 2005;77(2):105-115. [ Links ]
31. Kaimal S, Thappa DM Relapse in leprosy. Indian J Dermatol Venereol Leprol 2009;75(2):126-135. [ Links ]
32. Guerrero-Guerrero MI, Muvdi-Arenas S, Leon-Franco CI. Relapses in multibacillary leprosy patients: A retrospective cohort of11 years in Colombia. Lepr Rev 2012;83(3):247-260. [ Links ]
33. Chris Hani Baragwanath Hospital. The Chris Hani Baragwanath Hospital. https://wwwchrishanibaragwanathhospital.co.za/ (accessed 24 May 2018). [ Links ]
34. Phaff C, van den Broek J, MacArthur A jr, Ndeve A, Stuip Y. Characteristics and treatment outcomes of leprosy patients detected during a leprosy elimination campaign in Mozambique compared with routinely detected patients. Lepr Rev 2003;74(3):229-239. [ Links ]
35. World Health Organization. Global leprosy update, 2015: Time for action, accountability and inclusion. Wky Epidemiol Rec 2015;91(35):405-420. https://www.who.int/publications/i/item/who-wer9135 (accessed 22 February 2018). [ Links ]
36. Blok DJ, de Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: Are we on track? Parasit Vectors 2015;8:548. https://doi.org/10.1186/s13071-015-1143-4 [ Links ]
37. World Health Organization. Global leprosy update, 2017: Reducing the disease burden due to leprosy. Wky Epidemiol Rec 2018;93(35):445-456. https://www.who.int/publications/i/item/who-wer9335 (accessed 25 August 2019). [ Links ]
38. Rao PN, Suneetha S. Current situation of leprosy in India and its future implications. Indian Dermatol Online J 2018;9(2):83-89. https://doi.org/10.4103/idoj.IDOJ_282_17 [ Links ]
39. Odriozola EP, Quintana AM, Gonzalez V, et al. Towards leprosy elimination by 2020: Forecasts of epidemiological indicators of leprosy in Corrientes, a province of northeastern Argentina that is a pioneer in leprosy elimination. Mem Inst Oswaldo Cruz 2017;112(6):419-427. https://doi.org/10.1590/0074-02760160490 [ Links ]
40. Kumar A, Girdhar A, Girdhar BK. A randomised controlled trial to compare cure and relapse rate of paucibacillary multidrug therapy with monthly rifampicin, ofloxacin, and minocycline among paucibacillary leprosy patients in Agra District, India. Indian J Dermatol Venereol Leprol 2015;81(4):356-362. https://doi.org/10.4103/0378-6323.159929 [ Links ]
 Correspondence:
Correspondence:
L J Nkehli
jabszako@yahoo.co.uk
Accepted 19 April 2021.














