Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 n.9 Pretoria Sep. 2021
http://dx.doi.org/10.7196/SAMJ.2021.v111i9.15330
RESEARCH
Clinical aspects and outcomes of patients with malaria at Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
D J FoxI; A KarstaedtII; C N MenezesIII
IBSc, MB BCh, Dip HIV Man (SA), FCP (SA), MMed (Int Med); Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCh; Department of Internal Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMD, MMed (Int Med), Dip HIV Man (SA), Cert ID (SA), PhD; Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. South Africa (SA) is currently experiencing a significant increase in malaria cases despite having shifted focus from malaria control towards malaria elimination. The clinical features of malaria are nonspecific, but their relative frequency on presentation are not well described. HIV and malaria are both independently associated with high mortality in sub-Saharan Africa. There are important interactions between HIV and malaria.
OBJECTIVES. To describe the population characteristics of patients with malaria at Chris Hani Baragwanath Academic Hospital, Johannesburg, SA, clinical and biochemical features of severity, the proportion of patients with HIV infection, management and outcomes.
METHODS. A prospective observational study was conducted whereby patients with a confirmed laboratory diagnosis of malaria were identified, approached and consented for study inclusion over the time period January 2017 - January 2018. Clinical and biochemical data were collected at the time of consent and later analysed.
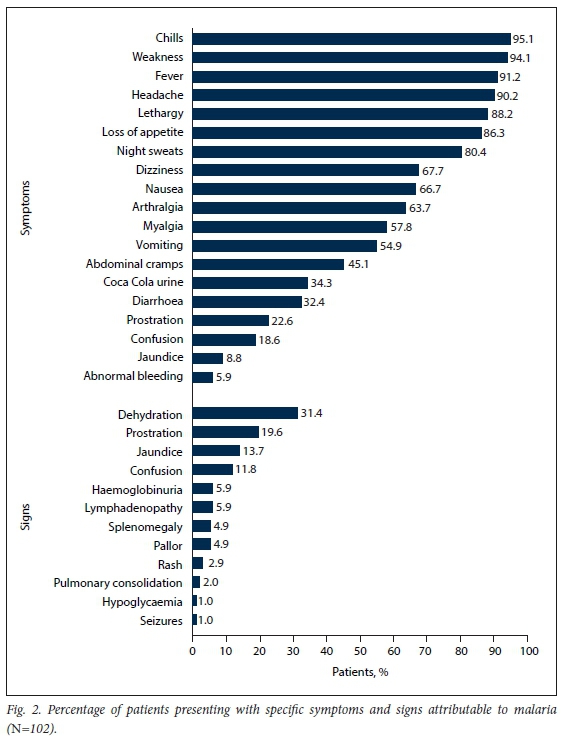
RESULTS. The mean (standard deviation) age was 35.7 (12.98) years, and 72 (70.6%) of the 102 patients were male. Peak admissions for malaria were in January, with 58 patients (56.9%) admitted during January 2017 and 2018. All malaria cases were imported, with 74.5% associated with travel to Mozambique. The majority of the patients (61.8%) were expatriates living in SA. The most common presenting symptoms were chills (95.1%), weakness (94.1%), fever (91.2%), headache (90.2%) and lethargy (88.2%). The most common clinical signs were dehydration (31.4%), prostration (19.6%) and jaundice (13.7%). Among the 40 patients (39.2%) who had severe malaria, prostration was the most common feature of severity (19.6%), 8 (7.8%) were admitted to an intensive care unit, and 6 (5.9%) required haemodialysis. The median (interquartile range) duration of hospital stay was 5 (3 -6) (range 2 - 35) days. HIV status was known in 83 patients (81.4%), of whom 32 (38.6%) were HIV-positive. Malaria prophylaxis had been taken by only 8 patients. The all-cause mortality rate was 4.9%, and mortality attributable to malaria 3.9%.
CONCLUSIONS. There was a high proportion of complicated malaria cases, particularly in January. The majority of patients were young expatriate males with a history of travel to southern Mozambique or Limpopo Province, with very few taking malaria prophylaxis. Most clinical signs and symptoms were constitutional and nonspecific. A large number of patients were found to be HIV-positive, and most were newly diagnosed. Mortality was high, at around five times the national average, and may have been an underestimate.
Malaria still imposes a significant burden of disease throughout the world. Approximately 216 million cases were reported by the World Health Organization (WHO) in 2016, together with ~445 000 deaths.[1] Sub-Saharan Africa continues to be disproportionately affected, with an estimated 80% of the disease burden and mortality.[1] In South Africa (SA), malaria is endemic in low-altitude regions of Limpopo, Mpumalanga and northern KwaZulu-Natal provinces, with an estimated 10% of the national population at risk.[2,3] Gauteng Province is not an endemic malaria area, but treats ~18% of the national disease burden.[4] Public healthcare facilities manage the majority of malaria cases in Gauteng.[4] There has been a large increase in the number of malaria cases since 2016.[5]
The vast majority of malaria cases managed in Gauteng are imported, with 71 - 97% of these cases associated with travel to or from Mozambique.[2-4] In Gauteng, the patient population with malaria has typically been young adult males.[4,6,7] Economic factors have been shown to drive population movement between Gauteng and malaria-endemic areas.[2,8] Small amounts of local malaria transmission in Gauteng do occasionally occur and result in sporadic outbreaks.[9] These cases of odyssean malaria are exceedingly rare, but are well reported by Frean et al.,[9,10] and carry a very high case mortality rate of ~13%.
The majority of malaria cases in Gauteng are caused by Plasmodium falciparum (99%), with 10 - 30% of these cases being classified as severed[4,6,7] Seasonality of malaria in Gauteng differs somewhat from the triphasic peaks described in endemic areas such as Mpumalanga.[11] The peaks of malaria in Gauteng mirror endemic areas to some extent, but data from local tertiary facilities demonstrate a large peak in January related to travel during the December festive season.[4,6,7]
For the management of malaria, the median duration of hospital stay in tertiary facilities in SA has been reported to be ~3 days, but ranges broadly (1 - 32 days).[6,12] Local data on mortality show a national case fatality average of 0.6 - 1% and a case fatality rate in Gauteng of 1 - 4°%.[4,6,7,9] Malaria mortality in north-eastern SA has been reported to be on the increase.[13]
Clinical features of acute falciparum malaria are described by the WHO but are nonspecific.[14] Acute malaria begins with malaise, which is then followed by headache, myalgias, dizziness, anorexia, abdominal pains, nausea and vomiting, and occasionally diarrhoea[14] accompanied by fevers, chills and occasionally rigors.f14] Physical signs that may be found include pallor, jaundice and splenomegaly.[14] Certain signs and symptoms are considered to indicate clinically severe/complicated malaria infection when present, and imply an increased risk of morbidity and mortality and inform on treatment strategies.[14] The features used for defining severe malaria for research purposes are listed in Table 1. The WHO provides a more bedside-friendly severe malaria definition for use in clinical practice, which is essentially the same but without the defining parameters and includes three features not listed in the research definition (convulsions, prostration and hyperlactataemia).[14]
There is a paucity of local and international data on the relative frequency of presenting clinical symptoms and signs of patients with malaria at hospitals in non-endemic regions. Cohen et al.[6] found the most common features of severity at Chris Hani Baragwanath Academic Hospital (CHBAH), Johannesburg, SA, to be renal impairment (n=23 patients; 6.8%), acidosis (n=14; 4.2%) and hepatic dysfunction (n=14; 4.2%). In a retrospective study by Francis et al.,[15] the most common symptoms reported in association with imported malaria cases seen at three hospitals in East London, UK, were fever (93.6%), vomiting (36.8%), diarrhoea (23.3%) and haemoglobinuria (1.5%). However, this data set included children, which may affect the relative frequency of presenting symptoms.[14] In another retrospective study of 100 patients with imported malaria, conducted in the USA by Akselrod et al.,[16] the commonest presenting symptoms were fever (92%), chills (78%), headache (64%), myalgias/arthralgias (53%), nausea/vomiting (35%), diarrhoea (26%), fatigue/malaise/weakness (25%), abdominal pain (18%) and altered mentation (9%). Neither of these studies reported on the frequency of clinical features such as splenomegaly.
Management of severe malaria ideally requires admission to an intensive care unit (ICU) or the highest level of care when facilities are available. Management of severe malaria may include mechanical ventilation, haemodialysis and blood transfusions.[14] The cost of these interventions can be immense, and the local demands on limited resources should be monitored. Previous work at CHBAH showed 6% of 336 patients being admitted to an ICU and 6.3% receiving haemodialysis.[6] Admission to other higher levels of care and the use of blood products have not been well described in local publications.
Artesunate has begun to replace quinine as first-line treatment for severe malaria because of its proven mortality benefit and a superior safety profile.[17,18] Historically, the most commonly used antimalarial regimens for the treatment of malaria in Gauteng were quinine based (79 - 97%). No local data have been published on the frequency of artesunate use at tertiary facilities. Malaria prophylaxis is recommended in national guidelines for South Africans travelling to malaria-endemic areas, although the public healthcare system does not currently offer this service.[19,20]
SA has one of the highest rates of HIV infection in the world.[21] A number of interactions between HIV and malaria have been described. A decline in CD4 count has been associated with an increased risk of clinical malaria in endemic countries.[22-24] There are conflicting data on whether HIV is a risk factor for severe malaria, but HIV appears to be a risk factor for severe malaria in patients who are non-immune.[6] Furthermore, a lower CD4 count appears to be associated with an increased risk of severe malaria[6]Malaria also has an effect on HIV viral load, with an up to seven-fold increase that can be maintained for 8 weeks post infection.[25-28] The CD4 count also appears to decline post malaria infection, but returns to baseline.[27] It has also been suggested that some of the reduction in morbidity and mortality with the use of co-trimoxazole can be attributed to reduced rates of malaria in endemic countries.[29,30] In a previous study at CHBAH, the proportion of patients with malaria who had HIV infection was 33%.[6]
Objectives
SA is currently facing a malaria epidemic and increasing mortality due to malaria in endemic areas. Despite not being a malaria-endemic area, Gauteng has also seen an increase in malaria numbers and mortality. The objectives of this study were therefore to define population, clinical and laboratory characteristics, ascertain the proportion of patients with HIV, and define the clinical management and outcomes of patients with malaria at CHBAH during this epidemic.
Methods
Study design and setting. A prospective observational study was conducted at CHBAH over the period January 2017 - January 2018. CHBAH is a large tertiary public referral hospital situated in the south of Johannesburg, Gauteng, that provides healthcare services to the people of Soweto and the surrounding areas. Ethics approval was obtained from the University of the Witwatersrand Human Research Ethics Committee prior to commencement of the study (ref. no. M1611137).
Study participants. All patients >18 years of age presenting to CHBAH with a laboratory-confirmed diagnosis and clinically compatible features of malaria over the defined time period were considered eligible to be entered into the study and were approached for possible inclusion with the use of an information sheet. Patients were only included in the study if adequate informed consent could be obtained from the patient or next of kin. Patients who had been discharged or had died prior to being identified for possible inclusion were excluded from the study.
Definitions. Case definitions were similar to those used in a previous study conducted at CHBAH,[3] to allow for comparison. A diagnosis of malaria was defined as a positive rapid diagnostic test (RDT) for malaria and/or a positive Giemsa stain on a thick or thin blood smear, together with a clinical picture in keeping with acute malaria infection. The RDT used at the time of study recruitment was the ICT (immunochromatographic test) malaria P. falciparum antigen kit (ICT Diagnostics, SA). In cases where the RDT was positive and the blood smear was negative, patients were included if they had not received treatment for malaria during the preceding month. Semi-immunity was defined as the patient having lived in an endemic malaria area for at least 5 years during childhood.[3] The WHO criteria for both clinical and research purposes, i.e. including the research definition criteria and the additional three criteria used in the clinical definition, were used to define severe malaria and were included on the data collection sheet (Tables 1 and 4)[9]
Patient identification and consent. Patients were identified via positive specimen results recorded by the CHBAH National Health Laboratory Service or by clinical staff working at the hospital. Once identified, patients were approached and informed consent was obtained, with the assistance of translators when necessary. Where patients were unable to consent owing to delirium or altered level of consciousness, consent was obtained on a separate form from their next of kin. All patients who survived to discharge gave informed consent themselves.
Data collection. Data were collected from January 2017 to the end of January 2018. Once patients were entered into the study, their data were collected on standardised data collection sheets. Patient and family interviews were used to ascertain travel history and other relevant history. Clinical records, limited clinical examination, review of laboratory records and follow-up over the course of hospital stay were used to ascertain clinical and laboratory characteristics and inform on supportive and specific treatments. Data collected included demographics, travel history, details of residence, hospital stay, symptoms and clinical features of malaria, presence of severe malaria, HIV status and treatment, laboratory results, management and outcome data. Data once recorded were entered into and stored on an Excel database (Office 365; Microsoft, USA) for later analysis.
Sample size. Based on previous research done at CHBAH,[6]1 in order to obtain a representative sample with at least 10% of participants meeting the criteria for severe malaria so as to be adequately powered to make comparisons between groups with severe and non-severe malaria, the target sample size was n=140 based on the formula: n = Z2P (1 - P)/e2.
Data analysis. Categorical variables were described using frequencies and percentages. Differences in normally distributed continuous variables between patients by HIV status and immune status were assessed using Student's f-test. Wilcoxon's rank sum test was used to compare medians for non-normally distributed variables. For categorical variables, χ2 analysis was used; however, where n<5 in any of the cells, Fisher's exact test was used. Binary logistic regression analysis was used to assess risk factors for severe malaria. All analyses were conducted in Stata version 15 (StataCorp, USA). Significance was set at 5%.
Results
A total of 122 potential participants were approached for possible inclusion during the prescribed recruitment period. Fourteen patients were excluded: 4 owing to early discharge, 7 because they were <18 years of age and 2 because informed consent could not be obtained due to language barriers, while 1 patient refused consent. Of the 108 participants enrolled, 5 were excluded because of incomplete data collection sheets and 1 because he had received treatment for malaria 2 weeks prior to his current admission. This left a final sample size of 102. The proportion of patients with severe malaria greatly exceeded 10% despite the target of 140 participants not being reached owing to time constraints imposed by the study design.
Population characteristics
The mean (standard deviation (SD)) age of the study participants was 35.7 (12.98) years. All but 1 were of black African descent and 72 (70.6%) were male. None of the 30 female patients was pregnant. Of the 102 patients, 64 (62.7%) were born outside SA. The most common country of birth was Mozambique (84.4%), followed by Ethiopia, Malawi and Zimbabwe (each 4,7%) and Paraguay (n=1). Fifty-nine patients (57.8%) had spent at least 5 years growing up in malaria-endemic areas and were considered to be semi-immune. Among the patients who were semi-immune, the median (interquartile range (IQR)) duration of residency in SA was 10.5 (5.0 - 24.0) years.
All the patients had travelled to (96.1%) or were visitors from (3.9%) a malaria-endemic area. There were no cases of odyssean malaria. Malaria infection was most frequently acquired in Mozambique (74.5%), followed by Limpopo Province in SA (17.6%), Zimbabwe (3.9%) and Malawi (3.9%). Three patients reported having travelled through more than one endemic malaria country during their travels. Sixty-three percent of patients reported travelling to an endemic malaria area on more than four previous occasions, and of these 66.7% were born outside SA.
Peak malaria period
The number of patients admitted with malaria infection was highest in January 2017 and January 2018 (n=29 each) and February 2017 (n=14) (Fig. 1). There was a low number of baseline cases seen throughout the rest of the year, except in the winter months (June -August) and early spring (September).
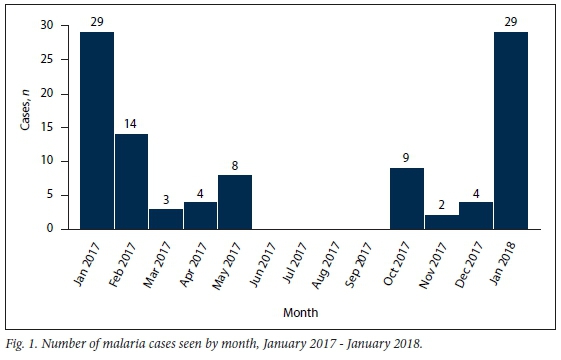
P falciparum was identified in 101 patients (99.1%) and P ovale in 1. Of the 102 patients, 100 had RDTs and 99/100 (99.0%) tested positive. The only negative RDT with a positive smear, as expected, was seen in the patient with P. ovale infection. Twelve patients (11.8%) were RDT-positive but smear-negative. In 2 patients the diagnosis of malaria was made on a smear without an RDT.
Clinical and laboratory features
The mean (SD) time from returning from travelling to presentation at a healthcare facility was 11.39 (7.33) days. The mean (SD) time from symptom onset to presentation at CHBAH was 5.08 (3) days. The frequency of clinical signs and symptoms is presented in Fig. 2.
From admission laboratory results, the median (IQR) white cell count was 5.6 (4.5 -7.3) x 109/L, the mean (SD) haemoglobin concentration 12.4 (2.4) g/dL and the median (IQR) platelet count 64.5 (36.5 - 92.8) x 109/L. The platelet count was <150 x 109/L in 92 (90.2%) of the patients on admission. On presentation, the median (IQR) blood urea was 6.6 (4.6 - 9.7) mmol/L, the median (IQR) serum creatinine 96 (79 - 120) μιηοΙ/L and the median (IQR) total serum bilirubin 28 (18 - 47) μιηοΙ/L. Of 46 patients who had a follow-up platelet count done on day 3 of admission, 39 (84.8%) had counts <150 x 109/L. Four patients, 1 of whom had cerebral malaria, underwent lumbar punctures, all of which were normal. In the 12 patients who had blood cultures on admission, all were negative.
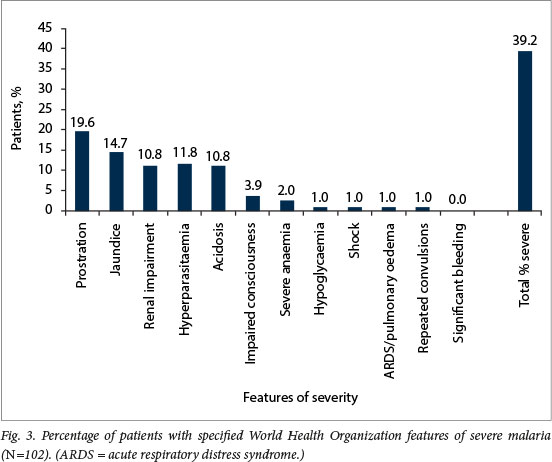
Forty patients (39.2%) were assessed as having severe malaria based on at least one feature of severity. The most common features of severity were prostration/severe weakness in 20 (19.6%) patients, jaundice in 15 (14.7%), renal impairment in 11 (10.8%) and acidosis in 11 (10.8%) (Fig. 3). Prostration was the only feature of severity in 6 patients, and parasitaemia >4% the only feature of severity in 2. A single feature of severity was present in 22 patients (21.6%), 2-4 features of severity in 14 (13.7%) and >5 features of severity in 4 (3.9%). Cerebral malaria was present in 4/5 (80.0%) of patients who died. Of 11 patients with renal impairment, 4 (36.4%) had acidosis as a feature of severity as well. Neither HIV infection (adjusted odds ratio (aOR) 1.40; 95% confidence interval (Cl) 0.43 4.52 ") nor non-immunity to malaria (aOR 1.57; Cl 0.51 4.79 ") were found to be risk factors for severe malaria on multivariate analysis (Table 2). There were no significant differences in the features of severity between immune and semi-immune patients (Table 3).
Proportion of patients with HIV infection
HIV infection was the commonest comor-bidity, followed by asthma in 3 patients and heart failure in 2. Of the 83 patients (81.3%) whose HIV status was known, 32 (31.4% of the total cohort) were HIV-positive. Of the 12 with previously known status, 11 were on antiretroviral treatment, of whom 7/8 (87.5%) whose HIV viral load was known were virologically suppressed (viral load <400 copies/mL). None was taking co-trimoxazole prophylaxis. The other 20 patients were newly diagnosed with HIV during their current admission. Most patients with HIV infection were asymptomatic for HIV. The WHO classification was used to categorise the 20 newly diagnosed HIVpositive patients: 13 (65.0%) were WHO clinical stage 1, 5 (25.0%) were clinical stage 2, 1 (5.0%) was clinical stage 3 based on unexplained weight loss >10% and persistent oral candidiasis, and 1 (5.0%) was clinical stage 4 based on the diagnosis of pneumocystis pneumonia. The CD4 count was known in 19/32 patients with HIV infection (59.4%).
The mean (SD) CD4 count was 261.3 (144) cells/μL. In secondary analysis, there were a few significant differences between the HIVpositive and negative groups (Table 4). The HIV-positive patients were more likely to have acidosis as a feature of severity (n=7 v. n=3; p=0.042), to have a longer duration of stay (5 days v. 4 days; p=0.03) and to receive haemodialysis (n=6 v. n=0; p=0.007). There was no significant difference in severe malaria between the two groups.
Management
The median (IQR) length of hospital stay was 5 (3 6 ") (range 2 35 ") days. Nineteen patients were admitted to a higher level of care, 17 of whom had severe malaria. Eight patients (7.8%) were admitted to the ICU, with a mean (SD) length of stay of 5 (2.42) days. Five patients were admitted to high care, with a mean (SD) length of stay of 3 (1.83) days, and 6 patients (2 of whom did not have features of severe malaria) were admitted to a short-stay ward, with a mean (SD) length of stay of 2 (0.75) days.
Thirty-eight patients with malaria received parenteral therapy, 19 (50.0%) intravenous quinine and 19 (50.0%) intravenous artesunate (Table 5). Of those patients who received intravenous quinine, 13 (68.4%) received the correct loading dose. Of the 102 patients, 95 (93.1%) received artemether-lumefantrine combination therapy, with 91.6% of them having taken the correct dosing schedule. Eight patients received artemether-lumefantrine therapy for >72 hours. Doxycycline was administered to 7 patients, always in combination with quinine. Clindamycin was administered to 2 patients after treatment with artesunate. Of the 12 patients who received artesunate at a higher level of care (high care/ICU), 4 required mechanical ventilation, 6 required dialysis and 6 received blood transfusions.
Eight patients (7.8%) reported having taken some form of malaria chemo-prophylaxis. The drug most used for chemo-prophylaxis was mefloquine (n=4 patients). One patient had taken doxycycline, and in 3 patients the chemoprophylactic agent could not be reliably identified. Of the 102 patients, 23 (22.5%) reported previously being treated for one or more episodes of malaria infection after travelling to malaria-endemic areas. Of these 23 patients, only 3 reported taking malaria prophylaxis during their most recent travels.
Outcomes
The all-cause mortality during the study period was 5/102 (4.9%); 4 patients (3.9%) died of malaria, and 1 patient who was successfully treated for malaria died later due to nosocomial sepsis. For 2 patients who died, the date of symptom onset was available; they had presented at 6 and 9 days after symptoms began. Of the patients who died, 3 had 5 features of severity and 2 had 3 features of severity. All patients who died received artesunate as first-line therapy and were managed in the ICU.
Discussion
There were many severe malaria cases (39.2%) and a relatively high mortality rate (4.9%) over the study period. Most malaria cases attended to were young black African male travellers who had visited their country of birth during the December festive period. These groups of travellers, that are increasingly representative of malaria infections in non-endemic areas around the world, are being referred to as travellers visiting friends and relatives (VFRs).[31] A large proportion of the study group were born and grew up in malaria-endemic areas, and whether semi-immunity to malaria is protective against severe malaria in our setting is suggested but remains unclear. The use of artesunate, particularly in the sickest groups of patients managed in high care/ICU, appears to have been more widely adopted.
The present study supports data from previous studies conducted in Gauteng that demonstrated a large seasonal peak in January associated with travel, mainly to Mozambique, during the festive period[4,6,7] All malaria infections were contracted in sub-Saharan Africa. The patient characteristics were also similar in that the majority were young black African male expatriates born in Mozambique who were resident in SA. The group was also similar to the group described by Cohen et al.[6]at the same facility 17 years ago, in that a significant proportion of the patients were semi-immune, having grown up in malaria-endemic areas. The semi-immune group tended to have less prostration and lower parasitaemia, but there was no statistically significant difference in the number with severe malaria. There is currently no reliable clinical biomarker to assess immunity, but it is likely that immunity wanes the longer time is spent away from endemic areas.[31] The median duration of residency for patients who grew up in malaria-endemic areas was -10 years. It is likely that the economic promises Gauteng offers will result in continued numbers of malaria cases related to travellers VFRs.
There is a paucity of local and international data on the relative frequencies of signs and symptoms of malaria in patients who present to hospital in non-endemic areas for care. The frequency of a few clinical findings reported by two international studies was similar but had some notable differences, which may be explained by higher rates of non-falciparum malaria in those studies. A higher percentage of patients reported headache, nausea and vomiting, and abdominal pain/cramps. Where overlapping features were reported, a comparison can be seen in Table 6.[6,15,16]' The frequencies of splenomegaly and pallor were notably low at 4.9% each. The most common clinical features are nonspecific and therefore dearly demonstrate the importance of a high clinical index of suspicion for malaria during peak periods. A detailed travel history and an awareness of areas with endemic transmission, both within and outside of SA, are essential to the early recognition and diagnosis of malaria. Early recognition is especially important because delayed diagnosis and institution of therapy are associated with adverse outcomes in malaria. The importance of early recognition can be validated by the high case fatality rates seen with odyssean malaria, where there is often delayed diagnosis owing to lack of clinical suspicion.[9] Lastly, it is important to note that although none of the patients in this study contracted malaria in Gauteng, the possibility of odyssean malaria does exist without a travel history, and it should be considered in unexplained acute febrile illness.
Our study population demonstrated a large proportion (39.2% by the WHO clinical definition and 33.3% by the WHO research definition) of patients with severe malaria. This proportion was higher than in previous studies conducted in Gauteng, which demonstrated severe malaria in 10 - 30% of patients presenting for care.[4,6,7] This high proportion may be partly accounted for by the fact that only patients admitted from casualty to the medical wards were included in the study, whereas in some studies patients seen in and discharged from casualty departments were also included[4,6] However, it is worth noting that in Cohen et al.,[6] 17% of patients were excluded because they were discharged before data could be collected. In the present study the commonest feature of severity was prostration, followed by jaundice, renal impairment and acidosis. It is worth considering that prostration in this study may be overestimated owing to self-reporting bias. In Cohen et al.,[6] the commonest features of severity were renal impairment, acidosis and hepatic dysfunction/jaundice (prostration was not listed as a feature of severity, as the definitions were updated by the WHO in 2014). Discounting prostration data from this study supports the previous findings on features in Cohen et al.[6] In concordance with Cohen et al., acidosis was more commonly a feature of severity in HIV-positive patients (p<0.05).
On multivariate analysis, we found that a higher white blood cell count (aOR 1.41; 95% CI 1.07 - 1.86) and probably a higher parasite level (aOR 1.31; 95% CI 1.08 - 1.59) were associated with an increased risk of severe malaria. HIV infection and non-immunity were not shown to be risk factors for the development of severe malaria in the present study, despite Cohen et al [6]demonstrating an association between the risk of severe malaria and HIV in non-immune patients. The association between HIV infection and risk of severe malaria was not a primary objective of the study, and it was not powered to detect this outcome. The lack of association between HIV infection and severe malaria in our patient population may be explained by waning immunity related to duration of residency outside malaria-endemic areas.[31] Despite not being demonstrated as a risk factor on multivariate analysis, a higher parasite level was observed in the non-immune v. the semi-immune patients (p<0.02).
Around a third of the patients in the present study were found to be HIV-positive, 62.5% (n=20/32) of whom were newly diagnosed, highlighting the importance of overlap between the two conditions in our setting. This figure was similar to the 33% of patients found to be HIV-positive by Cohen et alX6HIV testing was not performed in just under one-fifth of the patients. It is important that all patients presenting with an acute febrile illness (including malaria) and a travel history be offered voluntary counselling and testing. Severe malaria appeared more frequent in the non-immune group compared with the semi-immune group, but the p-value was not statistically significant (p=0.089). Comparison of the HIV-positive and negative groups demonstrated that in our sample, HIV-positive patients with malaria were more likely to present with acidosis as a feature of severity (p<0.05), were more likely to have a more prolonged hospital stay (median 6 v. 4 days; p=0.003) and were more likely to require haemodialysis (p=0.007). Data have shown that malaria infections increase as a result of falling CD4 counts,[24] but in our sample there was no significant difference in CD4 count between immune and semi-immune patients with HIV co-infection (p=0.232). The vast majority of patients were asymptomatic for HIV infection, in keeping with Cohen et al.[6]
Despite evidence of a mortality benefit as well as an improved safety profile (reduced risk of hypoglycaemia and QT prolongation) with the use of artesunate compared with quinine for the management of severe malaria, only 17/40 (42.5%) of our patients with severe malaria received artesunate-based regimens.[17,18,32] Mortality in the group of patients who received artesunate was significantly higher than in the non-artesunate group (p=0.009). This higher mortality is probably because, while artesunate was available at CHBAH through section 21 application during the study period, it seems to have been used more frequently in the subset of patients with severe malaria who were more ill, required admission to high care or the ICU and had a higher baseline risk of mortality, rather than indicating that artesunate use was causal in the higher mortality rate. This higher use of artesunate in the ICU and high care is probably protocol driven and may be related to the amount of work involved in section 21 application after hours and in busy general wards. Despite the limited use of artesunate in all patients with severe malaria, it appears from the data that clinician-driven clinical decisions to treat some cases of severe malaria with quinine-based regimens did not appear to result in adverse outcomes in this group of patients. Artesunate was not available when the earlier studies were conducted in Gauteng.[6,7] It appears from the data that the use of artemether-lumefantrine in both uncomplicated malaria and after initial treatment in complicated malaria has been admirably adopted, as >95% of patients received this antimalarial.
Only one previous study looked at the number of patients in Gauteng presenting with malaria who had taken malaria prophylaxis. Dube et al.[7] reported that 4 patients (2%) had taken some form of malaria prophylaxis. The number of patients who had taken malaria prophylaxis in the present study was low despite many of them having had previous episode/s of malaria, similar to reports from centres elsewhere in the world (Table 6).[15,16] The SA National Department of Health has provided extensive guidelines on malaria prophylaxis, updated as recently as January 2019.[19] Unfortunately the public healthcare sector in SA does not offer malaria prophylaxis to travellers as part of the current extended drug list.[20]
The case fatality rate in the present study can be said to be 3.9% (n=4). All these patients died in the ICU and received treatment with artesunate. The case fatality rate was nearly four times the national average and twice the rate reported in previous studies in Gauteng tertiary facilities (1 - 2%),[6,7] but was similar to a larger study in Gauteng where the case fatality rate was 4%.[4] The mortality rate far exceeded those in the studies conducted in Washington, DC, and London.[15,16] This increased mortality rate may be due to delayed presentation related to healthcare access disparities. The time from return to presentation was >4 days greater in our study population compared with Washington and London (Table 6).
Study strengths and limitations
Strengths of the study include the prospective design and the description of clinical characteristics that have not previously been extensively described, as well as comparison with international work. Limitations included the relatively short data collection period, which limited sample size and prevented our reaching the target sample size, and the study being conducted at a single-centre referral hospital in the public sector. There may have been lack of inclusion of a number of patients with very severe malaria who died shortly after admission, or of patients who were not referred or were discharged from casualty, inferring some degree of selection bias. Some of the comparative statistics had small sample sizes. Some patients were excluded owing to inability to consent because of language barriers, possibly resulting in exclusion bias. Some of the questionnaire relied on patient recall of symptomatology, which may have resulted in recall bias. There are possible confounders due to the overlap of variables assessed that may be affected by both HIV and malaria, such as renal dysfunction and anaemia. Another limitation was that not all data were available for analysis, such as HIV results, CD4 counts and HIV viral loads.
Conclusions
Malaria continues to pose a significant problem in southern Africa. CHBAH continues to manage many severe malaria cases shortly after the December festive season that are imported mainly from southern Mozambique and Limpopo Province. The presenting signs and symptoms in the present study were nonspecific and highlight the need for a high index of suspicion during peak malaria months. Many patients were HIV-positive and were newly diagnosed on admission. Despite its proven mortality benefit and improved safety profile, there appeared to be limited use of artesunate outside high-care and ICU environments, probably as a result of the section 21 application process. The case fatality rate was higher than previously reported and was possibly underestimated. The next step to eliminating malaria in SA requires a national multicentre study to better delineate the characteristics of ongoing malaria risk in the different healthcare areas and sectors of SA.
Declaration. The research for this study was done in partial fulfilment of the requirements for DJF's MMed (Internal Medicine) degree at the University of the Witwatersrand.
Acknowledgements. We thank the National Health Laboratory Service laboratory at CHBAH for assistance with identifying cases, and the nursing staff at CHBAH for assistance with translations.
Author contributions. DJF wrote the article. AK and CNM were DJF's MMed supervisors.
Funding. The University of the Witwatersrand provided funding for publication.
Conflicts of interest. None.
References
1. World Health Organization. World malaria report 2017. 29 November 2017. https://www.who.int/malaria/publications/world-malaria-report-2017/en/ (accessed 9 December 2019). [ Links ]
2. Maharaj R, Raman J, Morris N, et aL Epidemiology of malaria in South Africa: From control to elimination. S Afr Med J 2013;103(10):779-783. https://doi/10.7196/SAMJ.7441 [ Links ]
3. Maharaj R, Morris N, Seocharan I, et al. The feasibility of malaria elimination in South Africa. Malar J 2012;11:423. https://doi.org/10.1186/1475-2875-11-423 [ Links ]
4. Weber IB, Baker L, Mnyaluza J, et al The burden of imported malaria in Gauteng province. S Afr Med J 2010;100(5):300-303. [ Links ]
5. World Health Organization. World malaria report 2018. 19 November 2018. https://www.who.int/publications/i/item/9789241565653 (accessed 11 December 2019). [ Links ]
6. Cohen C, Karstaedt A, Frean J, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis 2005;41(11):1631-1637. https://doi.org/10.1086/498023 [ Links ]
7. Dube S, Ismail N, Hoosen AA. A retrospective review of malaria cases seen in a non-endemic area of South Africa. Travel Med Infect Dis 2008;6(5):296-300. https://doi.org/10.1016/j.tmaid.2008.06.010 [ Links ]
8. Landau LB, Gindrey V. Gauteng 2055 Trend Paper: Population & Migration. University of the Witwatersrand Forced Migration Studies Programme, 27 October 2008. http://www.migration.org.za/wp-content/uploads/2017/08/Poverty-Trend-Analysis-for-Gauteng-Province-2009-2055.pdf (accessed 18 December 2019). [ Links ]
9. Frean J, Brooke B, Thomas J, et al. Odyssean malaria outbreaks in Gauteng province, South Africa, 2007 - 2013. S Afr Med J 2014;104(5):335-338. https://doi.org/10.7196/SAMJ.7684 [ Links ]
10. Frean J. Communicable Diseases Communique 2018;17(January):1-11. https://www.nicd.ac.za/wp-content/uploads/2017/03/NICD_Communicable_Diseases_Communique_January_2018.pdf (accessed 11 August 2021). [ Links ]
11. Silal SP, Barnes KI, Kok G, et al. Exploring the seasonality of reported treated malaria cases in Mpumalanga, South Africa. PLoS ONE 2013;8(10):1-9. https://doi.org/10.1371/journal.pone.0076640 [ Links ]
12. Opie J, Freeks R, du Pisani LA. The burden of imported malaria in Cape Town, South Africa. S Afr Med J 2014;104(5):347-349. https://doi/10.7196/SAMJ.7904 [ Links ]
13. Byass P, Collinson MA, Kabudula C, et al. The long road to elimination: Malaria mortality in a South African population cohort over 21 years. Glob Health Epidemiol Genomics 2017;2:E11. https://doi.org/10.1017/gheg.2017.7 [ Links ]
14. World Health Organization. Severe malaria. Trop Med Int Health 2014;19(s1):7-131. https://doi.org/10.1111/tmi.12313_2 [ Links ]
15. Francis BC, Gonzalo X, Duggineni S, et al. Epidemiology and clinical features of imported malaria in East London. J Travel Med 2016;23(6):1-6. https://doi.org/10.1093/jtm/taw060 [ Links ]
16. Akselrod H, Swierzbinski MJ, Zheng Z, et al. Characteristics and severity of disease among 100 cases of imported malaria seen at a U.S. University Hospital, 2000 - 2017. Am J Trop Med Hyg 2018;99(6):1511-1517. https://doi.org/10.4269/ajtmh.18-0608 [ Links ]
17. Newton PN, Angus BJ, Chierakul W, et al. Randomised comparison of artesunate and quinine in the treatment of severe falciparum malaria. Clin Infect Dis 2003;37(1):7-16. https://doi.org/10.1086/375059 [ Links ]
18. Sinclair D, Donegan S, Isba R, et al. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev 2012, Issue 6. Art. No.: CD005967. https://doi.org/10.1002/14651858.CD005967.pub4 [ Links ]
19. National Department of Health, South Africa. National Guidelines for the Prevention of Malaria, South Africa: 2018. Updated January 2019. https://www.nicd.ac.za/wp-content/uploads/2019/03/National-Guidelines-for-prevention-of-Malaria_updated-08012019-1.pdf (accessed 23 December 2019). [ Links ]
20. National Department of Health, South Africa. Standard Treatment Guidelines and Essential Medicines List for South Africa: Hospital level (Adults). 5th ed., 2019. https://www.sapc.za.org/Media/Default/Documents/STG%20hospital%20level%20adult%202019_v2.0.pdf (accessed 10 December 2019). [ Links ]
21. World Health Organization, UNICEF, UNAIDS. Global update on HIV treatment 2013: Results, impact and opportunities. June 2013. https://www.unaids.org/sites/default/files/media_asset/20130630_treatment_report_en_0.pdf (accessed 10 December 2019). [ Links ]
22. Francesconi P, Fabiani M, Dente MG, et al HIV, malaria parasites, and acute febrile episodes in Ugandan adults: A case-control study. AIDS 2001;15(18):2445-2450. https://doi.org/10.1097/00002030-200112070-00013 [ Links ]
23. Laufer MK, van Oosterhout JJG, Thesing PC, et al Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis 2006;193(6):872-878. https://doi.org/10.1086/500245 [ Links ]
24. French N, Nakiyingi J, Lugada E, et al Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS 2001;15(7):899-906. https://doi.org/10.1097/00002030-200105040-00010 [ Links ]
25. Hoffman IF, Jere CS, Taylor TE, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS 1999;13(4):487-494. https://doi.org/10.1097/00002030-199903110-00007 [ Links ]
26. Alemu A, Shiferaw Y, Addis Z, et al. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors 2013;6(1):1. https://doi.org/10.1186/1756-3305-6-18 [ Links ]
27. Bentwich Z, Maartens G, Torten D, et al Concurrent infections and HIV pathogenesis. AIDS 2000;14(14):2071-2081. https://doi.org/10.1097/00002030-200009290-00002 [ Links ]
28. Kublin JG, Patnaik P, Jere CS, et al. Effect of Plasmodium fcalcipcarum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: A prospective cohort study. Lancet 2005;365(9455):233-240. https://doi.org/10.1016/s0140-6736(05)17743-5 [ Links ]
29. Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004;364(9443):1428-1434. https://doi.org/10.1016/s0140-6736(04)17225-5 [ Links ]
30. Mermin J, Ekwaru JP, Liechty CA, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: A prospective cohort study. Lancet 2006;367(9518):1256-1261. https://doi.org/10.1016/s0140-6736(06)68541-3 [ Links ]
31. Mischlinger J, Ronnberg C, Álvarez-Martínez MJ, et al. Imported malaria in countries where malaria is not endemic: a comparison of semi-immune and nonimmune travelers. Clin Microbiol Rev 2020;33(2):1-34. https://doi.org/10.1128/cmr.00104-19 [ Links ]
32. Dondorp AM, Fanello CI, Hendriksen IC, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open-label, randomised trial. Lancet 2010;376(9753):1647-1657. https://doi.org/10.1016/s0140-6736(10)61924-1 [ Links ]
 Correspondence:
Correspondence:
D J Fox
djoshfox@gmail.com
Accepted 12 April 2021.














