Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.9 Pretoria Set. 2021
http://dx.doi.org/10.7196/SAMJ.2021.v111i9.15564
RESEARCH
Evaluating the performance of the GeneXpert HIV-1 qualitative assay as a consecutive test for a new early infant diagnosis algorithm in South Africa
A MukendiI, II; T KufaIII, IV; T MurrayV, VI; M BurkeVII; R StrehlauVIII; K-G TechnauIX; C T TiemessenX, XI; G G ShermanXII, XIII; A H MazanderaniXIV
IBSc Hons; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIBSc Hons; Centre for HIV and STIs, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa
IIIMB BCh, MPH, PhD; Centre for HIV and STIs, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa
IVMB BCh, MPH, PhD;School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VPhD; Centre for HIV and STIs, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa
VIPhD; Paediatric HIV Diagnostics, Wits Health Consortium, Johannesburg, South Africa
VIIMB BCh; Empilweni Services and Research Unit, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; and Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIIMB BCh, MSc; Empilweni Services and Research Unit, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; and Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IXMB BCh, DCH (SA), Dip Hiv Man (SA); Empilweni Services and Research Unit, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; and Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XPhD; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XIPhD; Centre for HIV and STIs, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa
XIIMB BCh, MMed (Haem), DCH (SA), DTM&H; Centre for HIV and STIs, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa
XIIIEmpilweni Services and Research Unit, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; and Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XIVMB ChB, MMed Path (Clin Virol), FC Path (SA) Viro, PhD; Centre for HIV and STIs, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa
ABSTRACT
BACKGROUND. The proportion of HIV-exposed infants and young children infected with HIV in South Africa (SA) has declined markedly over the past decade as a result of the country's comprehensive prevention of mother-to-child transmission programme. This decrease has in turn reduced the positive predictive value (PPV) of diagnostic assays, necessitating review of early infant diagnosis (EID) algorithms to ensure improved accuracy.
OBJECTIVES. To evaluate the performance of the GeneXpert HIV-1 qualitative assay (Xpert EID) as a consecutive test for infants with an 'HIV-detected' polymerase chain reaction screening test at birth.
METHODS. We retrospectively analysed a longitudinal cohort of HIV-exposed infants on whom birth testing was performed, using whole-blood ethylenediaminetetra-acetic acid samples, from four tertiary sites in Gauteng Province between June 2014 and December 2019. Birth samples from all infants with a Cobas AmpliPrep/Cobas TaqMan HIV-1 Qualitative Test v2.0 (CAP/CTM v2.0) HIV-detected screening test, a concurrent Xpert EID test and a subsequent confirmatory CAP/CTM v2.0 test on a separate specimen were included. Performance of the Xpert EID in predicting final HIV status was determined as proportions with 95% confidence intervals (CIs). A comparison of indeterminate CAP/CTM v2.0 results, as per National Health Laboratory Service resulting practice, with discordant CAP/CTM v2.0 v. Xpert EID results was performed.
RESULTS. Of 150 infants who met the inclusion criteria, 6 (3.9%) had an Xpert EID result discordant with final HIV status: 5 (3.3%) were false negatives and 1 (0.7%) was false positive. As a consecutive test, the Xpert EID yielded a sensitivity of 96.5% (95% CI 92 - 98.9), specificity of 85.7% (95% CI 42.1 - 99.6), PPV of 99.3% (95% CI 95.7 - 99.9), negative predictive value of 54.5% (95% CI 32.5 - 74.9) and overall accuracy of 96.1% (95% CI 91.5 - 98.5). Using discordant CAP/CTM v2.0/Xpert EID results as criteria to verify indeterminate results instead of current practice would have reduced the number of indeterminate screening results by 42.1%, from 18 (12.6%) to 11 (7.2%), without increasing the false-positive rate.
CONCLUSIONS. Addition of the Xpert EID as a consecutive test for specimens with an HIV-detected PCR screening result has the potential to improve the PPV and reduce the indeterminate rate, thereby reducing diagnostic challenges and time to final status, in SA's EID programme.
South Africa (SA) has an extremely high proportion of pregnant women living with HIV.[1] The 2017 Antenatal Survey[2] reported a national prevalence of 30.7%, a rate that has remained fairly constant for the past decade.[2] This high maternal prevalence translates into ~300 000 HIV-exposed infants born per annum, all of whom require HIV diagnostic services.[3] In 2004, SA implemented its national antiretroviral treatment (ART) programme, including specific interventions for prevention of mother-to-child transmission.[4] Since then, significant improvements in maternal and infant health outcomes have been achieved. One of the more marked successes has been reduction in the early infant HIV transmission rate from >20% to <2%.[4,5] However, on account of the considerable burden of maternal disease, the absolute number of HIV-infected infants and children remains high.[6] Routine early infant diagnosis (EID) services for all HIV-exposed infants therefore remain a cornerstone of paediatric HIV care.
Importantly, as the prevalence of a disease decreases, so too does the positive predictive value (PPV) of diagnostic assays. The changes in the HIV epidemic, including an increase in the proportion of people living with HIV who have been diagnosed and initiated on ART, and the corresponding decrease in the proportion of people living with HIV who are undiagnosed, have led to an increase in the proportion of false-positive test results. In response, the World Health Organization (WHO) recommends using different consecutive serological tests (i.e. rapid diagnostic tests and/or enzyme immunoassays) for individuals who initially test reactive in order to diagnose HIV, even in high-burden settings.[7,8] However, owing to the trans-placental transfer of maternal antibodies, serological tests can only be used for diagnosis in adults and in children >18 months of age. To diagnose HIV in infants and children <18 months of age, nucleic acid tests (NATs) are recommended. However, instead of using consecutive assays to minimise false-positive results for infant diagnosis, the WHO recommends the use of an indeterminate range whereby potentially false-positive results can be differentiated from clearly positive cases. Additionally, guidelines recommend confirmatory testing on a second sample collected as early as possible after a positive or indeterminate screening test result.[9,10] A number of challenges with this diagnostic algorithm have been reported.[11] These include delays in making a final diagnosis as a result of exposure to infant antiretroviral prophylaxis and maternal treatment, both in utero and via breastfeeding, which can result in a marked reduction in viraemia and loss of HIV detection among infected infants.[12-16]
Commercially available EID assays are usually real-time polymerase chain reaction (PCR)-based NATs that report a cycle threshold (Ct) value. The Ct value refers to the number of thermal cycles required for the fluorescence signal of a test to cross the diagnostic threshold signifying an 'HIV-detected' or positive result. The Ct value is assay specific. Although the Ct value is usually inversely correlated with the amount of virus in a specimen,[17] tests yielding high Ct values are also more frequently associated with false-positive results. The WHO recommends a Ct >33 on the COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Test v2.0 (Roche Molecular Systems Inc., USA; hereafter referred to as CAP/CTM v2.0) as the optimal indeterminate range (i.e. for reporting an HIV-detected result as indeterminate instead of positive).[18] Without the use of an indeterminate range, it is estimated that >10% of infants could be incorrectly diagnosed as HIV-positive if their initial test result is not confirmed.[19]
In SA's public health sector, only the CAP/CTM v2.0 and Cobas HIV-1/HIV-2 Qualitative Test assays are currently used for EID. HIV PCR testing is therefore restricted to EID assays from a single manufacturer. Results with a CAP/CTM v2.0 Ct >33 (or equivalent) and/or a relative fluorescence intensity (RFI) <5 are reported as indeterminate as per National Health Laboratory Service (NHLS) guidelines.[4] This reporting practice has been associated with a high indeterminate reporting rate. Seventeen percent of all HIV-detected PCR results were reported as indeterminate between 2013 and 2015, equating to ~3 000 tests annually.[20,21] The indeterminate rate among birth samples is even higher, at ~24%.[13] The high indeterminate reporting rate has in turn resulted in a high burden of missed diagnostic opportunities and delays in ART initiation in HIV-infected infants and children - especially considering that approximately half of infants with an indeterminate result at birth have been found to be HIV-infected on follow-up.[22]
Objectives
The high volume of indeterminate HIV PCR results in SA highlights the need to revise EID algorithms to ensure accurate and timely diagnosis. In this study, we evaluate the performance of the GeneXpert HIV-1 Qualitative Assay (Cepheid, USA; hereafter referred to as Xpert EID) as a consecutive test for specimens with an HIV-detected screening result on the CAP/CTM v2.0. Furthermore, we evaluate a novel approach to verifying indeterminate results, whereby discordant CAP/CTM v2.0 v. Xpert EID results define an indeterminate result, and compare this method with current practice of using Ct/RFI, to determine whether the indeterminate rate can be reduced without compromising diagnostic accuracy.
Methods
Study design
Accuracy of the Xpert EID assay as a second consecutive test for HIV diagnosis in infants was evaluated retrospectively. Results from infants with an HIV-detected CAP/CTM v2.0 result at birth (i.e. positive and indeterminate results) and a simultaneous Xpert EID birth test, and confirmed HIV infection status, were included in the study. Confirmed HIV status was determined by additional testing of follow-up blood samples using the CAP/CTM v2.0. Infants with a confirmed HIV-positive status were defined as having at least two HIV-detected virological results from separate samples taken at two different time points, as per National Department of Health guidelines.[23] Infants with a confirmed negative HIV infection status were defined as having two or more negative HIV PCR results following an initial HIV-detected result on screening at birth without any subsequent HIV-detected result thereafter.
Study setting
This evaluation was performed on laboratory data collected from four academic healthcare facilities situated in high-burden HIV prevalence settings in Gauteng Province, SA, between June 2014 and December 2019. Charlotte Maxeke Johannesburg Academic Hospital, Chris Hani Baragwanath Academic Hospital, Kalafong Provincial Tertiary Hospital and Rahima Moosa Mother and Child Hospital were sites for an implementation study of HIV diagnostic point-of-care (PoC) testing whereby whole-blood samples were collected at the time of birth from HIV-exposed infants and tested concurrently using CAP/CTM v2.0 (in a centralised laboratory) and Xpert EID near PoC.
Study procedures
Laboratory data were extracted for all infants with a positive HIV PCR test at birth on the CAP/CTM v2.0, with concurrent testing performed on the Xpert EID. Confirmatory diagnostic results obtained from subsequent testing on a separate specimen were also extracted. To determine HIV status, clinic records and the NHLS Data Warehouse were searched for follow-up results. As SA does not have a unique patient identification system available at birth, the NHLS Data Warehouse was searched using patient demographic details and a validated probabilistic record-linking algorithm.[24]
Statistical analysis
Patient data, including demographic details, birth test (both CAP/ CTM v2.0 and Xpert EID) and follow-up HIV virological results (CAP/CTM v2.0) were exported from two REDCap databases (REDCap Consortium, Vanderbilt) of separate PoC implementation studies,[25] and imported into Microsoft Excel 2016 (Microsoft Corp., USA) for descriptive analysis. A two-by-two table was used to evaluate overall performance, determined as proportions with 95% confidence intervals (CIs), calculated using Stata 14.2 (StataCorp, USA), of the Xpert EID assay in predicting HIV status in infants with an HIV-detected CAP/CTM v2.0 screening test at birth. Finally, a novel approach to verifying indeterminate results was evaluated whereby discordant CAP/CTM v2.0 v. Xpert EID results were interpreted as indeterminate. This approach was compared with CAP/CTM v2.0 indeterminate results, as per NHLS resulting practice using the Ct/RFI cut-off, to describe the rate of indeterminate and false-positive results of the two approaches.
Ethical considerations
Permission to perform this study was granted by the Human Research Ethics Committee at the University of the Witwatersrand, Johannesburg (ref. nos M140639, M1711115 and M190645), and the University of Pretoria Research Ethics Committee (ref. no. 50/2018).
Results
A total of 172 HIV-exposed infants had an initial HIV-detected HIV PCR birth result on the CAP/CTM v2.0, and had a simultaneous Xpert EID test. Fig. 1 provides the study flow with inclusion and exclusion criteria. Of these infants, 150 met the inclusion criteria - an HIV-detected CAP/CTM v2.0 birth test, a simultaneous test result obtained on the Xpert EID assay (including both HIV-undetected and HIV-detected results), and a confirmed HIV status as determined by subsequent virological testing performed on the CAP/CTM v2.0 (Fig. 1). The median (interquartile range (IQR)) age at the time of birth testing was 1 (0 - 1) day. The median (IQR) age at the time of final HIV status was 1 (IQR 1 - 3) day for infants with an HIV-positive status and 20 (IQR 7 - 43) days for infants with an HIV-negative status.
Of 150 infants with an initial HIV-detected CAP/CTM v2.0 screening test, 143 (95.3%) were found to have an HIV-infected status on follow-up (Table 1), of whom 139 (97.2%) had an HIV-detected result on Xpert EID at birth. Of the 7 (4.7%) infants with an HIV-uninfected status on follow-up, 6 (85.7%) tested HIV-undetected on Xpert at birth (Table 2). In a total of 6 infants (3.9%), the Xpert EID result at birth was discordant to HIV status on follow-up: 5 (3.3%) had a false- negative and 1 (0.7%) a false-positive Xpert EID result. Birth testing performed on the latter infant at birth yielded positive test results on the CAP/CTM v2.0 and on the Xpert EID. Upon subsequent testing, results obtained from the infant on both assays were negative. Three months later, further testing was performed with an indeterminate test result received. Additional blood collected from this infant was tested on a single-copy assay with a result of <3 copies/1.5 million cells. At 510 days, treatment was halted with subsequent testing giving an HIV-negative result. The patient was followed up until 1 254 days, when HIV testing produced a negative result. As a second consecutive assay, the Xpert EID yielded a sensitivity of 96.5% (95% CI 92 - 98.9), specificity of 85.7% (95% CI 42.1 - 99.6), PPV of 99.3% (95% CI 95.7 - 99.9), negative predictive value of 54.5% (95% CI 32.5 - 74.9), and overall accuracy of 96.1% (95% CI 91.5 - 98.5).

Eighteen infants (12.6%) had an indeterminate HIV PCR screening result on CAP/CTM v2.0 at birth, as verified by the NHLS. Upon subsequent testing on the CAP/CTM v2.0, 5 infants (27.8%) were found to have an HIV-negative status. All these infants had tested HIV-undetected on Xpert EID at birth. The remaining 13 infants (72.2%) had an HIV-infected status, of whom 11 had an HIV-detected result on Xpert EID at birth. Using alternative criteria for verifying indeterminate results whereby specimens with discordant CAP/CTM v2.0 v. Xpert EID results were interpreted as indeterminate, and concordant HIV-detected
CAP/CTM v2.0 and Xpert EID results(regardless of Ct/RFI value) interpreted as HIV-detected, there would have been 11 infants (7.2%) with an indeterminate birth result, of whom 5 (45.5%) would have been found to have an HIV-positive status on follow-up testing.
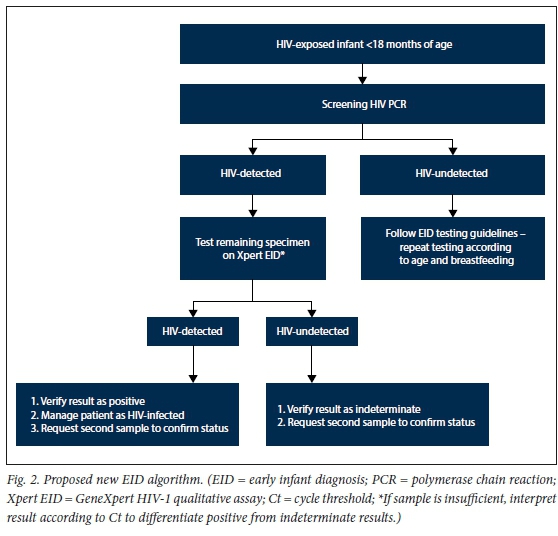
If reporting practice was informed by concordant or discordant qualitative CAP/ CTM v2.0 and Xpert EID results regardless of Ct/RFI value (Fig. 2), a drop in the indeterminate rate of 42.1% would have been achieved without increasing the rate of false-positive results.
Discussion
This study demonstrated excellent PPV and overall accuracy of the Xpert EID as a second consecutive test for specimens that screened HIV-detected on CAP/CTM v2.0 at birth. The Xpert EID result had reduced sensitivity when compared with the CAP/ CTM v2.0, and was discordant with final status in only 6 cases (3.9%), of which 5 (3.3%) were false-negatives and 1 (0.7%) was a false-positive. The addition of the Xpert EID as a confirmatory test therefore provides an opportunity to improve the diagnostic accuracy and PPV of EID algorithms, thereby reducing the indeterminate rate and number of paediatric diagnostic dilemmas in SA. This is especially important at birth, as HIV-infected infants increasingly demonstrate low-level viraemia and may test negative on subsequent samples as a result of exposure to antiretroviral prophylaxis and maternal ART. By reducing the indeterminate rate and providing prompt diagnosis, the volume of diagnostic dilemmas and time to ART initiation among infected infants will be reduced.
Because of a short analytical turnaround time of only 90 minutes, using the Xpert EID assay in the centralised EID laboratories for consecutive testing of all HIV-detected CAP/ CTM v2.0 results would result in minimal delays in result verification while potentially improving linkage to care and reducing loss to follow-up.[26,27] As the Xpert EID assay has been validated for both whole-blood ethylenediaminetetra-acetic acid (EDTA) and dried blood spot (DBS) use,[28] the fact that DBS specimens represent the predominant sample type for EID in SA should not limit its use.
Study limitations
A number of important limitations need to be considered regarding these findings. Surprisingly, only 12.6% of infants had an indeterminate birth result, a much lower proportion than previously described, even among EDTA whole-blood samples.[13] This finding may reflect the small number of infants enrolled in the study, as well as challenges with obtaining follow-up results -13% of infants were excluded from the final analysis for this reason. Furthermore, analysis was restricted to whole-blood EDTA samples collected from HIV-exposed infants at birth from four facilities in Gauteng, and tested using a single laboratory assay, the CAP/CTM v2.0. As such, findings may not represent the overall EID programme. However, as intrauterine HIV-infected infants tested at birth have lower-level viraemia than older age groups,[14] and therefore have a higher rate of indeterminate HIV PCR results, it is likely that the Xpert EID assay would yield fewer false-negative results among older infants and children. Although testing was performed using EDTA whole blood and not DBS samples, which have been found to under-quantify total nucleic acid compared with EDTA samples,[20] the use of DBS samples for the proposed new EID algorithm will not necessarily result in a higher indeterminate rate than demonstrated in this study. This is because the diagnostic sensitivity of DBS samples has been found not to be inferior to EDTA whole-blood specimens on Xpert EID,[29] and the same sample type would be used for consecutive testing.
Conclusions
The Xpert EID demonstrated excellent PPV and overall accuracy as a second consecutive test for specimens that yield an HIV-detected result on CAP/CTM v2.0. Incorporating the Xpert EID as a consecutive assay has the potential to enhance EID accuracy by bringing down the rate of indeterminate results without increasing the false-positive rate, with minimal cost and delay in turnaround time.
Declaration. None.
Acknowledgements. The authors thank Prof. Louise Kuhn, on behalf of the LEOPARD study group, for allowing them access to HIV birth testing PCR data.
Author contributions. AM, GGS and AHM conceptualised the study. AM, RS, K-GT and MB assisted with data collection. AM and TK performed data analysis. AM wrote the first draft of the manuscript. All authors reviewed the manuscript and approved the final version for submission.
Funding. The financial support provided by the Clinton Health Access Initiative (CHAI)/UNITAID, the South African Research Chairs Initiative of the Department of Science and Innovation, and the National Research Foundation of South Africa is very gratefully acknowledged.
Conflicts of interest. None.
Disclaimer. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any agency to which they are affiliated.
References
1. Human Sciences Research Council, South Africa. The Fifth South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017: HIV Impact Assessment Summary Report. July 2018. http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_ Summary_ZA_ADS_cleared_PDFA4.pdf (accessed 18 March 2020). [ Links ]
2. Woldesenbet SA, Kufa T, Lombard C, et al The 2017 National Antenatal Sentinel HIV Survey Key Findings, South Africa. National Department of Health, South Africa, July 2019. https://www.nicd.ac.za/wp-content/uploads/2019/07/Antenatal_survey-report_24July19.pdf (accessed 20 November 2020). [ Links ]
3. National Department of Health, South Africa. 2015 National Antenatal Sentinel HIV & Syphilis Survey Report. Pretoria: NDoH, 2017. [ Links ]
4. National Department of Health, South Africa. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Pretoria: NDoH, April 2015. https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (accessed 16 September 2019). [ Links ]
5. Sherman GG. Testing at birth - update from South Africa. Plenary presented at the 8th HIV Pediatric Workshop, Durban, South Africa, 15 - 16 July 2016. [ Links ]
6. Moyo F, Mazanderani AH, Barron P, et al. Introduction of routine HIV birth testing in the South African National Consolidated Guidelines. Pediatr Infect Dis J 2018;37(6):559-563. https://doi.org/10.1097/INF.0000000000001840 [ Links ]
7. World Health Organization. Consolidated Guidelines on HIV Testing Services. Geneva: WHO, 2019. https://www.who.int/publications/i/item/978-92-4-155058-1 (accessed 11 February 2020). [ Links ]
8. World Health Organization. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, confidentiality, counselling, correct results and connection. Geneva: WHO, 2015. https://apps.who.int/iris/handle/10665/179870 (accessed 11 February 2020). [ Links ]
9. Vojnov L, Penazzato M, Sherman G, et al Implementing an indeterminate range for more accurate early infant diagnosis. J Acquir Immune Defic Syndr 2019;82(3):E44-E46. https://doi.org/10.1097/QAI.0000000000002081 [ Links ]
10. World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: Interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: WHO, 2018. https://www.who.int/publications/i/item/WHO-CDS-HIV-18.51 (accessed 15 September 2019). [ Links ]
11. Mazanderani AH, Sherman GG. Evolving complexities of infant HIV diagnosis within prevention of mother-to-child transmission programs. F1000Research 2019;8:1-8. https://doi.org/10.12688/f1000research.19637.1 [ Links ]
12. Mazanderani AH, du Plessis NM, Thomas WN, et al. Loss of detectability and indeterminate results: Challenges facing HIV infant diagnosis in South Africa's expanding ART programme. S Afr Med J b2014;104(8):574-577. https://doi.org/10.7196/SAMJ.8322 [ Links ]
13. Technau KG, Mazanderani AH, Kuhn L, et al. Prevalence and outcomes of HIV-1 diagnostic challenges during universal birth testing - an urban South African observational cohort. J Int AIDS Soc 2017;20(Suppl 6):21761. https://doi.org/10.7448/IAS.207.21761 [ Links ]
14. Mazanderani AH, Moyo F, Kufa T, Sherman GG. Brief report: Declining baseline viremia and escalating discordant HIV-1 confirmatory results within South Africa's early infant diagnosis program, 2010 - 2016. J Acquir Immune Defic Syndr 2018;77(2):212-216. https://doi.org/10.1097/QAI.0000000000001581 [ Links ]
15. Strehlau R, Paximadis M, Patel F, et al. HIV diagnostic challenges in breast-fed infants of mothers on antiretroviral therapy. AIDS 2019;33(11):1751-1756. https://doi.org/10.1097/QAD.0000000000002276 [ Links ]
16. Golemba MD, Mecikovsky D, de Zarate MO, et al. Unraveling HIV-1 diagnosis in special pediatric cases. J Clin Virol 2020;104343. https://doi.org/10.1016/j.jcv.2020.104343 [ Links ]
17. Mazanderani AH, Moyo F, Kufa T, et al. Differentiating clearly positive from indeterminate results: A review of irreproducible HIV-1 PCR positive samples from South Africa's early infant diagnosis program, 2010 - 2015. Diagn Microbiol Infect Dis 2018;91(3):248-255. https://doi.org/10.1016/j.diagmicrobio.2018.02.019 [ Links ]
18. World Health Organization. The 2018 Optimal Formulary and Limited-Use List for paediatric ARVs. Geneva: World Health Organization, 2018. https://apps.who.int/iris/rest/bitstreams/1141279/retrieve (accessed 22 November 2019). [ Links ]
19. Luo R, Boeras D, Broyles LN, et al. Use of an indeterminate range in HIV early infant diagnosis: A systematic review and meta-analysis. J Acquir Immune Defic Syndr 2019;82(3):281-286. https://doi.org/10.1097/QAI.0000000000002104 [ Links ]
20. Jennings C, Harty B, Scianna SR, et al. The stability of HIV-1 nucleic acid in whole blood and improved detection of HIV-1 in alternative specimen types when compared to dried blood spot (DBS) specimens. J Virol Methods 2018;261(4):91-97. https://doi.org/10.1016/j.jviromet.2018.08.009 [ Links ]
21. Mazanderani AH, Technau KG, Hsiao NY, et al. Recommendations for the management of indeterminate HIV PCR results within South Africa's early infant diagnosis programme. S Afr J HIV Med 2016;17(1):1-5. https://doi.org/10.4102/sajhivmed.v17i1.451 [ Links ]
22. Mazanderani AH, Moyo F, Sherman GG. Missed diagnostic opportunities within South Africa's early infant diagnosis program, 2010 - 2015. PLoS ONE 2017;12(5):1-11. https://doi.org/10.1371/journalpone.017717 [ Links ]
23. National Department of Health, South Africa. Guideline for the Prevention of Mother to Child Transmission of Communicable Infections. Pretoria: NDoH, October 2019. https://www.nicd.ac.za/wp-content/uploads/2019/11/Guidelines-for-the-Prevention-of-Transmission-of-Communicable-Diseases-from-mother-to-child_28-October.pdf (accessed 3 January 2020). [ Links ]
24. Technau KG, Kuhn L, Coovadia A, et al. Improving early identification of HIV-infected neonates with birth PCR testing in a large urban hospital in Johannesburg, South Africa: Successes and challenges. J Int AIDS Soc 2017;20(1):21436. https://doi.org/10.7448/IAS.20.01/21436 [ Links ]
25. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010 [ Links ]
26. Evans D, Sineke T, Schnippel K, et al. Impact of Xpert MTB/RIF and decentralised care on linkage to care and drug-resistant tuberculosis treatment outcomes in Johannesburg, South Africa. BMC Health Serv Res 2018;18(973):1-12. https://doi.org/10.1186/s12913-018-3762-x [ Links ]
27. Jani IV, Meggi B, Mabunda N, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr 2014;67(1):1-4. https://doi.org/10.1097/QAI.0000000000000250 [ Links ]
28. World Health Organization. WHO Prequalification of In Vitro Diagnostics: Public Report. Public Report Product: Xpert® HIV-1 Qual Assay. June 2016, version 2.0. WHO reference number: PQDx 0259-070-00. http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/160613PQPublicReport_0259-0700-00_XpertQualHIV_v2.pdf (accessed 9 June 2019). [ Links ]
29. Bassett IV, Huang M, Cloete C, et al. Assessing the completeness and accuracy of South African National Laboratory CD4 and viral load data: A cross-sectional study. BMJ Open 2018;8(8):1-7. https://doi.org/10.1136/bmjopen-2018-021506 [ Links ]
 Correspondence:
Correspondence:
A Mukendi
aureliemuk@gmail.com
Accepted 6 April 2021














