Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.2 Pretoria Fev. 2021
http://dx.doi.org/10.7196/samj.2020.v111i2.14522
RESEARCH
The adjunctive use of Carbimazole during radioactive iodine treatment reduces the cure rate of Graves' disease
F DocratI; T MokoenaII; V O L KarusseitIII; A O AnkrahIV
IMB ChB, FCS, MMed; Department of Surgery, Faculty of Health Sciences, University of Pretoria, South Africa
IIMB ChB, DPhil, FRCS; Department of Surgery, Faculty of Health Sciences, University of Pretoria, South Africa
IIIMB ChB, MMed, FCS; Department of Surgery, Faculty of Health Sciences, University of Pretoria, South Africa
IVMB ChB, FCNP, MMed; Department of Nuclear Medicine, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
BACKGROUND: Radioactive iodine (RAI) is widely used in the treatment of hyperthyroidism. Adjunctive antithyroid drugs (ATDs) are commonly prescribed to treat the hyperthyroid state before the RAI has taken effect. However, there is no consensus on the use of or timing of adjunctive ATD treatment with RAI.
OBJECTIVES: To determine the influence of the ATD carbimazole on the cure rate of RAI treatment for Graves' disease.
METHODS: A retrospective chart review was conducted in the Department of Nuclear Medicine of the Steve Biko Academic Hospital in Pretoria. The cure rate of patients treated with RAI for Graves' disease was analysed. The effect of adjunctive carbimazole treatment with regard to its use and timing with RAI dosing was analysed. The cure rate was determined in patients treated with carbimazole either before RAI or before and after RAI administration. Cure rate was defined by the biochemical thyroid function status (thyroxine (T4), thyroid-stimulating hormone (TSH)) as euthyroid or hypothyroid from 3 months and sustained at 12 months. The need for a second dose of RAI was recorded.
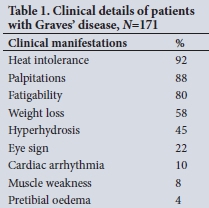
RESULTS: RAI treatment was administered to 171 patients with Graves' disease. The cure rate was higher in patients receiving a higher dose of RAI. The overall cure rate increased progressively from 3 months and was 91% at 12 months. The cure rate in 97 patients not receiving carbimazole was 98%. The cure rate of the 27 patients on carbimazole treatment given before RAI administration was 81%, and 73% in the 37 patients in whom it was resumed after RAI administration. The overall cure rate was lower in patients who received carbimazole (p<0.001), but especially in patients in whom carbimazole was continued after RAI administration (p<0.001).
CONCLUSIONS: Adjunctive carbimazole treatment decreased the RAI cure rate of Graves' disease significantly.
Hyperthyroidism is associated with significant morbidity and mortality, especially from cardiovascular complications.[1] It occurs due to excessive production or release of thyroid hormone by the thyroid gland. The common causes of excessive hormone production are Graves' disease, multinodular goitre and functional adenoma.
Three modalities are available for treatment of hyperthyroidism. Radio-iodine (RAI) ablation is most commonly used where it is available.[1-3] The other modalities are antithyroid drugs (ATDs) and surgery. RAI treatment was introduced in 1941 and has become a preferred modality of treatment of hyperthyroidism because of its low cost and low complication rate.[4,5] The perception of optimal RAI use differs significantly among physicians.[2]
Several factors have been considered as influencing the outcome of RAI treatment, such as the RAI dose, patient age and sex, the cause of hyperthyroidism and the use of ATD drugs.[3,6] There has been much debate about the concomitant use of ATDs during RAI treatment.[7,8] ATDs are used as adjunctive treatment of hyperthyroidism, especially because there is a significant lag period in the hyperthyroid patient before RAI's suppressive effects are manifested, and RAI therapy may lead to a transient rise of circulating thyroid hormone as a result of radiation thyroiditis. The optimal use and timing of ATD treatment during RAI has not been determined. The question arises whether ATDs are a necessary or even counterproductive adjunctive treatment in RAI therapy.
The aim of this study was to determine the cure rate in hyperthyroid patients with Graves' disease treated with RAI and to assess the influence of associated use of ATD treatment.
Methods
A retrospective study was undertaken of RAI treatment of patients with Graves' disease at Steve Biko Academic (SBAH) and Kalafong hospitals. These tertiary hospitals serve the northern areas of Gauteng Province and Mpumalanga Province and are part of the training platform of the University of Pretoria. The study population comprised all patients treated for Graves' disease with RAI at the Department of Nuclear Medicine of SBAH during the period 2005 -2015. Patients were identified from the records of the Department of Nuclear Medicine and the data on RAI treatment were retrieved. Demographic, clinical and follow-up details were obtained from the records from the referral clinics within these two hospitals. Biochemical data in files (triiodothyronine (T3), thyroxine (T4) and thyroid-stimulating hormone (TSH)) were correlated with laboratory data from the National Health Laboratory Services (NHLS). All patients underwent an iodine-123 (123I) or technetium-99m (Tc 99m) uptake scan which was also used for planning RAI treatment. The diagnosis was confirmed by the presence of anti-TSH receptor antibodies.
The usual practice at the Department of Nuclear Medicine in treating Graves' disease is to administer varying doses of RAI at the discretion of the treating nuclear medicine doctor, depending on the size of the thyroid gland and the degree of pretreatment iodine uptake. Therapeutic RAI was given as a single oral dose of [3]I. Follow-up of the patients included biochemical thyroid function tests (TFTs), repeated at 3, 6, 9 and 12 months. Therapy with carbimazole was at the discretion of the clinical or nuclear medicine treating doctor.
Carbimazole treatment was discontinued 7 - 10 days before RAI administration. Resumption of carbimazole treatment was mostly delayed for about 2 - 3 weeks after RAI administration, with reliance on beta-adrenergic blockade in the interim to control symptoms of hyperthyroidism if necessary.
The outcome from 3 months was assessed as euthyroid, hypothyroid or residual hyperthyroid. This was based on reduced serum T4 and increased TSH values. Treatment was regarded as successful at 6 months and sustained at12 months if the patient was biochemically euthyroid or hypothyroid. If treatment was deemed to be unsuccessful at 3 months, a second dose of RAI was administered.
Permission to conduct the study was granted by the Research Ethics Committee of the Faculty of Health Sciences of the University of Pretoria (ref. no. 337/2016)
Data analysis
Cure rate at 6 months was calculated as a proportion. Observed data were mainly nominal/ordinal in nature and were summarised using frequency, percentage, cross-tables and 95% confidence intervals. Factors associated with outcome were assessed using odds ratios for univariate analysis and adjusted odds ratio from multivariate logistic regression analysis. The Stata statistical software 12.1 (StataCorp., USA) was utilised for statistical analysis and a p-value of <0.05 was regarded as significant.
Results
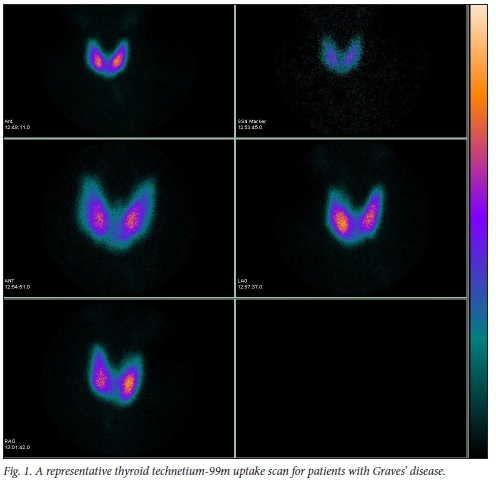
One hundred and seventy-one patients with Graves' disease were treated with RAI (Table 1). The mean (standard deviation (SD)) age was 43.2 (13.1) years (range 8 - 79). There was a strong female predominance of 144 of 171 patients (84%). The majority of males (16/27; 60%) were 40 years of age or younger, whereas the majority of females (96/144; 67%) were 40 years of age or older. Patients presented with typical symptoms and signs of hyperthyroidism. All patients showed a diffuse increase uptake on 123I or Tc 99m uptake scan (see Fig. 1 for a representative scan).
The mean (SD) therapeutic ablation dose of 131I was 15.4 (2.3) mCi. This ranged between 5 and 25 mCi, and was given as a single oral dose.
The sex and age of the patients was found to have no significant influence on the cure rate (p=0.73). The only effect of the patients' age on the cure rate was in patients older than 60 years, who were more likely to be cured by a single dose of RAI (p=0.027).
Patients receiving a higher initial RAI dose were more likely to be cured (Table 2). The overall cure rate after initial RAI dose was 90% (153/171). However, when divided doses were assessed (low <15 mCi and high >15 mCi), there was a significant association between the RAI dose and the cure rate (p=0.05), with more patients cured on the higher RAI dose.
Complete ATD treatment data were available for 169 patients. The requirement for a second RAI dose correlated with the use of carbimazole (Spearman's rank correlation coefficient with Bonferroni adjustment r=0.38; p<0.001). Fourteen patients received a second dose of RAI, all of whom had received carbimazole. Seventy-two patients (43%) received carbimazole treatment. The use of carbimazole adversely affected the cure rate (Table 3). Patients who received carbimazole treatment at any time had a lower cure rate (81%) than those who did not receive it (p<0.05). Patients who received carbimazole both before and after RAI administration had an even lower cure rate of 73% (27/37). This is significantly lower than the overall cure rate of 90% (p<0.001). Patients who received carbimazole before RAI administration only had a cure rate of 96%, which is similar to the cure rate in patients who did not receive carbimazole (95/97; 98%). TFTs (TSH and T4) are shown in Table 4.
Discussion
In this study, 90% of patients were cured of hyperthyroidism by RAI therapy. This is similar to recently published studies. Sundaresh et a/.'91 reported a successful outcome in 92% of patients. However, cure rates as low as 77% have been reported.'10-Patients who received a lower dose of RAI had a poorer outcome and consequently required a second dose. This corresponds to the universal finding of previous studies in which higher cure rates are achieved with a higher initial dose of RAI.'11-14-
The cure rate in this study was significantly influenced by use of carbimazole. A poorer outcome was found in patients who received carbimazole before and/or after RAI administration, but not
in those receiving Carbimazole before RAI treatment only. Many clinicians prefer to render their patients euthyroid with ATDs before RAI in order to stabilise the disease and to minimise the risk of exacerbation of hyperthyroidism.[11] Older patients are more susceptible to complications of hyperthyroidism and may require control with ATDs during RAI treatment.[1] However, ATDs may confer a so-called radioprotective effect, making RAI less effective and possibly resulting in RAI treatment failure.[12,13]
On the other hand, some clinicians continue ATD treatment after administration of RAI. This is done to control ongoing symptoms of hyperthyroidism in vulnerable patients, and because there is a lag period of 6 - 8 weeks before involution of thyrocytes due to radioactivity.[4
In almost all studies, outcome of RAI is poorer if associated with ATD treatment, irrespective of the timing.[12,13] In a meta-analysis of randomised trials, Walter et al.[15] reported that there was a risk of treatment failure with ATD treatment both before (risk ratio 1.48) and after (risk ratio 1.32) RAI administration. The adjunctive use of ATDs is, nevertheless, widespread. In our patients the highest cure rate (98%) was in the group who did not receive carbimazole. Poorer outcome was seen in patients who received carbimazole at any time, but especially when it was continued after RAI administration (75% cure rate). This is in contrast to the outcome in patients receiving carbimazole before RAI administration only (96% cure rate). This result is similar to that obtained in a prospective study performed by Andrade et al.[14] in which pretreatment with methimazole had no effect on the cure rate of Graves' disease 1 year after treatment with RAI.
Only a few studies have investigated the effect of ATD use after RAI, and the findings have been mixed. Bonnema et al.[16] found that the resumption of methimazole after RAI administration did not affect the outcome of RAI. Several older studies found that the use of ATDs after RAI reduced the therapeutic effect of RAI.[17-19]
Limitations of the study include its retrospective nature; there was no uniform or standardised treatment and data collection. Patients were managed severally and simultaneously by both clinicians and nuclear medicine specialists, leading to some differences in adjunctive treatment. Furthermore, the requisite data were obtained from different treatment sites with different diligence in data storage; this means some data should be interpreted with caution.
Conclusions
RAI was found to be a highly effective modality of treatment for Graves' disease but may be affected by the use of Carbimazole. This study confirms that carbimazole reduces the RAI cure rate of Graves' disease, especially if used after administration of the RAI dose. Our study therefore recommends that carbimazole should be discontinued after RAI administration if it was used beforehand, and if its use is indicated for symptom control after RAI, it should be postponed for at least 2 weeks and reliance placed on the use of beta-adrenergic blockers during this time.
Declaration. None.
Acknowledgements. None.
Author contributions. FD: protocol, data collection, writing, final approval; TM: concept, protocol, writing, final approval; VOLK: data critiquing, writing, final approval; AOA: protocol, data collection, final approval.
Funding. None.
Conflicts of interest. None.
References
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016;26(10):1343-1421. https://doi.org/10.1089/thy.2016.0229 [ Links ]
2. Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med 2011;364(6):542-550. https://doi.org/10.1056/NEJMct1007101 [ Links ]
3. Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 2012;33(6):920-980. https://doi.org/10.1210/er.2012-1030 [ Links ]
4. Lee SL. Radioactive iodine therapy. Curr Opin Endocrinol Diabetes Obes 2012;19(5):420-428. https://doi.org/10.1210/er.2012-1030 [ Links ]
5. Donovan PJ, McLeod DS, Little R, Gordon L. Cost-utility analysis comparing radioactive iodine, anti-thyroid drugs and total thyroidectomy for primary treatment of Graves' disease. Eur J Endocrinol 2016;175(6):595-603. https://doi.org/10.1530/EJE-16-0527 [ Links ]
6. Allahabadia A, Daykin J, Sheppard MC, Gough SC, Franklyn JA. Radioiodine treatment of hyperthyroidism - prognostic factors for outcome. J Clin Endocrinol Metab 2001;86(8):3611-3617. https://doi.org/10.1210/jcem.86.8.7781 [ Links ]
7. Perros P. Anti-thyroid drug treatment before radioiodine in patients with Graves' disease: Soother or menace? Clin Endocrinol 2000;53(1):1-2. https://doi.org/10.1046/j.1365-2265.2000.01020.x [ Links ]
8. Bogazzi F, Martino E, Bartalena L. Antithyroid drug treatment prior to radioiodine therapy for Graves' disease: Yes or no? J Endocrinol Invest 2003;26(2):174-176. https://doi.org/10.1007/BF03345148 [ Links ]
9. Sundaresh V, Brito JP, Thapa P, Bahn RS, Stan MN. Comparative effectiveness of treatment choices for Graves' hyperthyroidism: A historical cohort study. Thyroid 2017;27(4):497-505. https://doi.org/10.1089/thy.2016.0343 [ Links ]
10. Onimode YA, Ankrah A, Kayode SA. Outcome of radioiodine therapy in a West African population. World J Nuclear Med 2016;15:24-29. https://doi.org/10.4103/1450-1147.167585 [ Links ]
11. Cooper DS. Antithyroid drugs and radioiodine therapy: A grain of (iodised) salt. Ann Intern Med 1994;121(8):612-614. https://doi.org/10.7326/0003-4819-121-8-199410150-00009 [ Links ]
12. Schneider DF, Sonderman PE, Jones MF, et al. Failure of radioactive iodine in the treatment of hyperthyroidism. Ann Surg Oncol 2014;21(13):4174-4180. https://doi.org/10.1245%2Fs10434-014-3858-4 [ Links ]
13. Alexander EK, Larsen PR. High dose 131I therapy for the treatment of hyperthyroidism caused by Graves' disease. J Clin Endocrin Metabol 2002;87(3):1073-1077. https://doi.org/10.1210/jcem.87.3.8333 [ Links ]
14. Andrade VA, Gross JL, Maia AL. The effect ofmethimazole pretreatment on the efficacy of radioactive iodine therapy in Graves' hyperthyroidism: One-year follow-up of a prospective, randomised study. J Clin Endocrinol Metab 2001;86(8):3488-3493. https://doi.org/10.1210/jcem.86.8.7707 [ Links ]
15. Walter MA, Briel M, Christ-Crain M, et al. Effects of antithyroid drugs on radioiodine treatment: Systematic review and meta-analysis of randomised controlled trials. BMJ 2007;334(7592):514. https://doi.org/10.1136%2Fbmj.39114.670150.BE [ Links ]
16. Bonnema SJ, Bennedbaek FN, Gram J, Veje A, Marving J, Hegedüs L. Resumption of methimazole after 131I therapy of hyperthyroid diseases: Effect on thyroid function and volume evaluated by a randomised clinical trial. Eur J Endocrinol 2003;49(6):485-492. https://doi.org/10.1530/eje.0.1490485 [ Links ]
17. Marcocci C, Gianchecchi D, Masini I, et al. A reappraisal of the role of methimazole and other factors on the efficacy and outcome of radioiodine therapy of Graves' hyperthyroidism. J Endocr Investigation 1990;13(6):513-520. https://doi.org/10.1007/BF03348615 [ Links ]
18. Nakazato N, Yoshida K, Mori K, et al. Antithyroid drugs inhibit radioiodine-induced increases in thyroid autoantibodies in hyperthyroid Graves' disease. Thyroid 1999;9(8):775-779. https://doi.org/10.1089/thy.1999.9.775 [ Links ]
19. Velkeniers B, Vanhaelst L, Cytryn R, Jonckheer MH. Treatment of hyperthyroidism with radioiodine: Adjunctive therapy with antithyroid drugs reconsidered. Lancet 1988;331(8595):1127-1129. https://doi.org/10.1016/S0140-6736(88)91950-2 [ Links ]
 Correspondence:
Correspondence:
T Mokoena
taole.mokoena@up.ac.za
Accepted 8 October 2020














