Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.111 no.2 Pretoria feb. 2021
http://dx.doi.org/10.7196/samj.2021.v111i2.14905
RESEARCH
Causative pathogens and antibiotic resistance in community-acquired urinary tract infections in central South Africa
J L FourieI; F M CiaassenII; J J MyburghIII
IMB ChB, MMed (Urol); Department of Urology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIMB ChB, MMed (Urol), PhD; Department of Urology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIIMB ChB, MMed (Urol), FCUrol (SA); Department of Urology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
BACKGROUND: Urinary tract infections (UTIs) are very common in community practice. Both the South African (SA) antibiotic stewardship programme (2015) and the Essential Medicines List for SA (2018) recommend ciprofloxacin as first-line treatment for community-acquired urinary tract infections (CAUTIs). The pathogens responsible for CAUTIs and their susceptibility profiles need to be documented, which is important for developing and updating treatment protocols.
OBJECTIVES: To determine the causative pathogens of CAUTIs in the greater Bloemfontein area, central SA, and to review their susceptibilities to commonly prescribed antibiotics.
METHODS: Urine samples sent for microscopy and culture between 2011 and 2015 by the three largest primary healthcare facilities in Bloemfontein were analysed retrospectively. Specimens with a significant count (>105 CFU/mL) of a single uropathogen were included. These results were obtained from the National Health Laboratory Service central data warehouse after the required consent. Data regarding age, gender, pathogen cultured and antimicrobial susceptibilities were captured. All calculations were carried out with statistical analysis software SPSS 17.0 (SPSS Inc., USA).
RESULTS: A total of 712 samples met the inclusion criteria. Women accounted for 481 (67.6%) of the infections. The prevalence of UTIs per age group was as follows: 1 month - 25 years (n=146; 20.51%); 26 - 50 years (n=324; 45.5%); and 51 - 75 years (n=199; 27.9%). The distribution of pathogens did not differ between age groups. Escherichia coli was the most prevalent uropathogen cultured from 410 (57.6%) specimens, followed by Klebsiella spp. from 97 (13.6%) and Enterococcus spp. from 71 (10.0%) specimens. E. coli showed resistance rates of 77.1% to amoxicillin, 15.6% to amoxicillin-clavulanate, 18.5% to ciprofloxacin, 4% to nitrofurantoin and 11% to trimethoprim-sulfamethoxazole (TMP-SMX). The distribution of uropathogens was different for men and women, with a lower prevalence of E. coli in men (p=0.045).
CONCLUSIONS: As expected, E. coli comprised most of the isolates, with a higher than expected number of Klebsiella isolates cultured. The susceptibility of E. coli to commonly prescribed oral antibiotics has decreased in the research setting, which mirrors a global trend. This study provides data showing that TMP-SMX and nitrofurantoin can be used safely as alternatives to first-line ciprofloxacin in CAUTIs in central SA.
Urinary tract infections (UTIs) are very common in community practice and have a major impact on morbidity, quality of life and healthcare costs. It is estimated that -50% of women will have at least one UTI episode in their lifetime, while 20 - 40% will have recurrent episodes.[1] Community-acquired urinary tract infections (CAUTIs) occur predominantly in women of child-bearing age, and less often in older men. Escherichia coli is the most common uropathogen in CAUTIs, and is cultured in 75 - 95% of cases.[2] These infections are often treated with empirical broad-spectrum antibiotics, as results from urine susceptibility testing are not always readily available, or may take 3-5 days to become available. The choice of empirical antibiotics should be based on the spectrum of organisms in a particular area and data reflecting their susceptibility to available drugs.[3]
The World Health Organization (WHO) has declared antibiotic resistance to be a serious threat to public health, because of increased mortality, prolonged hospital stay, loss of prophylaxis and increased costs due to resistant organisms.[4] This global increase in the emergence of antimicrobial resistance is linked directly to the injudicious use of antibiotics by the medical and agricultural fraternities, as well as few new drugs being discovered over the past couple of decades.[5] Internationally, the increase in fluoroquinolone resistance in particular has reached alarming levels, including 20% reported for North America and northern Europe, while approaching 50% in the Mediterranean area and parts of Asia.[6] Another major concern regarding the use of fluoroquinolones is their propensity to cause collateral damage, such as adverse ecological effects, and their potential for serious neurological side-effects.[7,8]
Recent studies in South Africa (SA) mirror the increase in resistance to commonly prescribed antibiotics for CAUTIs.[2,9] This establishes a need to continuously monitor the profiles of uropathogens that cause CAUTIs and their susceptibilities to empirically used antibiotics. Data on CAUTIs in central SA are limited and relate to small sample sizes.[9] The aim of this study was to determine the causative uropathogens responsible for CAUTIs in the greater Bloemfontein area in central SA, and to review their susceptibility to commonly prescribed antibiotics. Information obtained from this study has the potential to improve treatment protocols, reduce selection of resistant organisms and decrease costs.
Methods
Setting
We reviewed the findings of urine samples sent for microscopy, culture and sensitivity (MC&S) testing by three large primary healthcare facilities in Bloemfontein, i.e. National District Hospital casualty and outpatient clinic, Mangaung University Community Clinic and Pelonomi Regional Hospital casualty department. These facilities provide primary care for most of Bloemfontein and the greater Mangaung and are located -10 km from each other. Urine samples from National District Hospital were analysed by the National Health Laboratory Service (NHLS) at Universitas Academic Complex, while the remainder were analysed at Pelonomi NHLS.
Study design and data collection
A retrospective, descriptive study was conducted. We obtained laboratory results of urine samples sent by the three clinics from 2011 to 2015. Only samples that had yielded a single pathogenic organism count of >105CFU/mL were included. Variables captured in an Excel (Microsoft, USA) spreadsheet were the patients' age and gender, pathogen cultured and its susceptibilities to amoxicillin, amoxicillin-clavulanate, ceftriaxone, ciprofloxacin, ertapenem, genta-micin, amikacin, nitrofurantoin and trimethoprim-sulfamethoxazole (TMP-SMX).
Culture and susceptibility testing
Calibrated loops were used to inoculate uncentrifuged urine onto growth media. Inoculated plates were incubated aerobically for 18 - 24 hours at 35 - 37°C, after which these were inspected for growth. Cultured isolates were then tested for antimicrobial susceptibility by the Kirby-Bauer disc diffusion method, using Mueller-Hinton agar plates.[10]
Statistical analysis
Results were summarised as percentages and medians and presented as tables and graphs. Students f-test and the χ- test were used to compare baseline characteristics of patients and antibiotic resistance. A p-value <0.05 was regarded as a statistically significant difference. All calculations were done with SPSS 17.0 (SPSS Inc., USA).
Ethical approval
Ethical approval was obtained from the Health Sciences Research Ethics Committee (HSREC), University of the Free State (ref. no. UFS-HSD 2018/0803/2711). Written permission was granted by the academic committee of the research office of the NHLS, after which the data were made available for analysis by the central data warehouse. No personal data were captured and only the researcher and his supervisor had access to the results. The study was executed in accordance with the 2013 World Medical Association Declaration of Helsinki.
Results
A total of 712 specimens that met the inclusion criteria were analysed, of which 481 (67.6%) were obtained from female patients. The median age of the women was 39 years (range 45 days - 91 years) compared with 47.1 years for men (range 30 days -86 years) (p=0.0001).
Causative pathogens
The bacterial aetiologies comprised 8 groups of species with Gram-negative bacteria predominating, as expected (Fig. 1). E. coli and Klebsiella pneumoniae accounted for the most prevalent pathogens in both sexes, while Pseudomonas was the least prevalent, with 4 isolates in each of the sexes. The group labelled other' contained atypical and less common organisms, possibly representing contaminants or complicated infections. These included Acinetob acter, Streptococcus, Morganella, Citrobacter, Alcaligenes, Stenotrophomonas, Serratia and Providencia species.
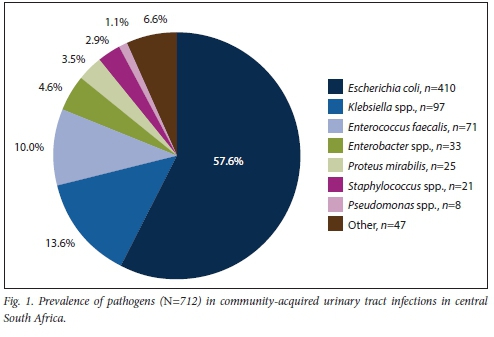
The distribution of pathogens was different for men and women, with a lower prevalence of E. coli in men (p=0.045) (Table 1). There was no significant difference in the distribution of pathogens per age group (Table 2).
Antibiotic resistance
Sensitivity to a wide range of antibiotics was reported, but only antibiotics commonly used were included in the study. The antimicrobial resistance profiles of predominant pathogens are summarised in Table 3.The prevalence of resistance of E. coli to common oral antibiotics was the highest for amoxicillin (76.8%), followed by ciprofloxacin (18.5%) and amoxicillin-clavulanate (15.6%).
The lowest rates were reported for nitrofurantoin (4%) and co-trimoxazole (11%). There were no significant differences in susceptibilities to oral antibiotics for men and women (Table 4).
Among the isolates, Klebsiella showed very high resistance to all oral antibiotics, especially the aminopenicillins. Proteus mirabilis proved to be quite susceptible to most agents, with the exception of nitrofurantoin. During the study period (2011 - 2015), the NHLS did not routinely test for susceptibility to fosfomycin.
Discussion
We described the most common pathogens that cause CAUTIs in the greater Bloemfontein area in central SA, as well as their respective susceptibilities to commonly prescribed antibiotics. UTIs occurred most commonly in women of child-bearing age and older men, a trend that could be explained by perineal colonisation and urinary stasis.[11] The Gram-negative bacteria E. coli and Klebsiella spp. were the most prevalent organisms causing infections in our study population, corresponding with findings of previous studies in our region[2] and internationally.
In comparison with a Gauteng-based study by Lewis et al.,[2]the prevalence of E. coli (n=410; 57.6%) in our study was lower than their reported 75 - 95% as the cause of CAUTIs, while Klebsiella spp. (n=97; 13.6%) were isolated at a higher rate than the 5 - 10% reported previously.[2] This finding was concerning, because Klebsiella spp. are often resistant to many of the available oral antibiotics (Table 3). Some of these organisms have the potential to produce extended-spectrum ß-lactamases (ESBLs), which enable them to inactivate ß-lactam antibiotics, rendering the drug ineffective.[11] The production of ESBLs is an important cause of treatment failure, and can have serious consequences for infection control.[13] An increase in the prevalence of these organisms in the community is concerning.
We noticed a difference in the distribution of pathogens between male and female patients in our region, with E. coli being more prevalent in women. This was also the finding in a recent large trial in The Netherlands, which investigated gender-stratified differences in UTIs.[14] Previous studies also described differences in antimicrobial susceptibility between gender-associated organisms,[15] but no difference was observed in our population.
This study points out a high prevalence of resistance to oral antibiotics. Resistance of E. coli to amoxicillin (76.8%), ciprofloxacin (18.5%) and amoxicillin-clavulanate (15.6%) is alarmingly high. These results compare well with those of previous studies in SA, with the exception of resistance to ciprofloxacin and TMP-SMX.[2,9] Our reported E. coli resistance rate of 18.5% for ciprofloxacin is much higher than the 6% reported by Lewis et al.[2]and the 11% of Bosch et al[9]A notable finding in our study was that E. coli resistance to TMP-SMX was much lower (11%) than the 40 - 54% reported by previous studies in our region.[2,9]. Previously, it was believed that the high resistance to TMP-SMX could be explained by the widespread use of this agent in the prophylaxis against Pneumocystis jirovecii in patients with AIDS.[9] The decrease in resistance to TMP-SMX could potentially be explained by the improved roll-out of antiretroviral drugs in the fight against HIV and tuberculosis, resulting in fewer prescriptions of this antibiotic. However, we could not find any evidence to support this theory.
Protocols for treating CAUTIs vary worldwide owing to the geographical variance in resistance patterns of pathogens causing infections. Internationally, guidelines for North America and Europe are similar. These guidelines recommend nitrofurantoin, fosfomycin and pivmecillinam (where available) for the treatment of uncomplicated CAUTIs. TMP-SMX is advised only if resistance in the area is known to be <20%, or if the infecting pathogen is known to be susceptible, ß-lactam agents should be administered only if other agents cannot be used. The guidelines advocate against the use of amoxicillin due to its poor efficacy and high prevalence of resistance. Despite still being efficacious in many cases, fluoroquinolones are not recommended, and have been removed from most protocols owing to increasing resistance and their propensity to cause collateral damage.[7,16]
According to the SA antibiotic stewardship programme (2015),[17] fluoroquinolones are still advised for the empirical treatment of uncomplicated CAUTIs. The most commonly used protocols for the empirical treatment of everyday community-acquired conditions are available in the Essential Medicines List, which is revised annually by the national Department of Health.[18] The empirical treatment of CAUTIs, according to the Essential Medicines List, consists of fluoroquinolones as first-choice antibiotic. Nitrofurantoin is advised in cases of pregnancy or other contraindications to fluoroquinolones. Amoxicillin-clavulanate is recommended for children weighing <35 kg.[18]
This study provides evidence of increasing resistance to commonly used oral antibiotics in our region, mirroring the international trend[6] Despite the increase in resistance shown to fluoroquinolones, its propensity for collateral damage and its potentially serious side-effects, it is still the first choice of empirical antibiotic in all protocols at community level. It is the authors' opinion that the formulation of protocols for treating CAUTIs in our region should be revisited. Alternatives that could be considered as first-line treatment are agents with lower resistance rates, such as nitrofurantoin and TMP-SMX, as shown by our findings. Fosfomycin is a popular choice for treating uncomplicated UTIs, due to its low resistance rates and easy dosing schedule.[7] However, we can not comment on its use owing to a lack of data.
The prevalence of highly resistant organisms, such as Pseudomonas and Acinetobacter baumanii, in CAUTIs in our region was beyond the scope of our study, but was noticed in a few samples. We also noted an increase in the prevalence of Klebsiella spp. These organisms usually cause nosocomial infections and their presence in the community causes concern. This finding may be explained by previous hospitalisations or antibiotic use by patients, and could be an area for future research.
Study limitations and strengths
The retrospective nature of this study and the absence of clinical history made the interpretation of the results difficult. Another limiting factor was that some of the positive samples had not been tested for susceptibility to all available oral antibiotics. The strength of our study is its large sample size from primary healthcare facilities only, which excludes hospitalised patients, and represents CAUTIs in our region.
Conclusions
Gram-negative bacteria E. coli and Klebsiella are the most prevalent pathogens causing UTIs in our community. Resistance of pathogens to TMP-SMX and nitrofurantoin is low. Treatment protocols in our region should be revised, as fluoroquinolones still form the cornerstone of empirical therapy. We recommend that nitrofurantoin and TMP-SMX are used empirically in our community for men and women who present with uncomplicated UTIs.
Declaration. The research for this study was done in partial fulfilment of the requirements for JLF's MMed (Urol) degree at the University of the Free State, Bloemfontein, SA.
Acknowledgements. We thank the Department of Medical Microbiology NHLS and Faculty of Health Sciences, University of the Free State for support, and Dr Daleen Struwig, medical writer/editor, for technical and editorial preparation of the manuscript.
Author contributions. JLF designed the study and wrote the manuscript; FMC provided guidance and did the statistical analysis; and JJM provided input and support.
Funding. All costs involved were covered by the Department of Urology Faculty of Health Sciences, University of the Free State.
Conflicts of interest. None.
References
1. Dash M, Padhi S, Mohanty I, Panda P, Parida B. Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India. J Fam Commun Med 2013;20(1):20-26. https://doi.org/10.4103/2230-8229.108180 [ Links ]
2. Lewis DA, Gumede LY, van der Hoven LA, et aL Antimicrobial susceptibility of organisms causing community-acquired urinary tract infections in Gauteng Province, South Africa. S Afr Med J 2013;103(6):377-381. https://doi.org/10.7196/SAMJ.6722 [ Links ]
3. Nzalie RN, Gonsu HK, Koulla-Shiro S. Bacterial etiology and antibiotic resistance profile of community-acquired urinary tract infections in a Cameroonian city. Int J Microbiol 2016;2016:3240268. https://doi.org/10.1155/2016/3240268 [ Links ]
4. World Health Organization. Antimicrobial resistance. Global health report on surveillance. 2014. https://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/ (accessed 29 April 2020) [ Links ]
5. Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance. Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol 2014;22(8):438-445. https://doi.org/10.1016/j.tim.2014.04.007 [ Links ]
6. Lee DS, Lee SJ, Choe HS. Community-acquired urinary tract infection by Escherichia coli in the era of antibiotic resistance. Biomed Res Int 2018;2018. https://doi.org/10.1155/2018/7656752 [ Links ]
7. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician 2011;84(5):519-526. [ Links ]
8. Food and Drug Administration. FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. 2018. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm612995.htm (accessed 29 April 2020). [ Links ]
9. Bosch FJ, van Vuuren C, Joubert G. Antimicrobial resistance patterns in outpatient urinary tract infections - the constant need to revise prescribing habits. S Afr Med J 2011;101(5):328-331. https://doi.org/10.7196/SAMJ.4346 [ Links ]
10. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard. 11th ed. Wayne, Pa. CLSI, 2012 [ Links ]
11. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections. Epidemiology mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13(5):269-284. https://doi.org/10.1038/nrmicro3432 [ Links ]
12. Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in JNMC Hospital Aligarh, India. Ann Clin Microbiol Antimicrob 2007;6(4). https://doi.org/10.1186/1476-0711-6-4 [ Links ]
13. Pitout JDD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterob acte riaceae. An emerging public-health concern. Lancet Infect Dis 2008;8(3):159-166. https://doi.org/10.1016/S1473-3099(08)70041-0 [ Links ]
14. Den Heijer CDJ, Penders J, Donker GA, Bruggeman CA, Stobberingh EE. The importance of gender-stratified antibiotic resistance surveillance of unselected uropathogens. A Dutch nationwide extramural surveillance study. PLoS ONE 2013;8(3):e60497. https://doi.org/10.1371/journal.pone.0060497 [ Links ]
15. Bean DC, Krähe D, Wareham DW. Antimicrobial resistance in community and nosocomial Escheria colt urinary tract isolates, London 2005 - 2006. Ann Clin Microbiol Antimicrob 2008;7(1). https://doi.org/10.1186/1476-0711-7-13 [ Links ]
16. Pickard RP, Bartoletti R, Bjerkiund-Johansen T, et al. EAU guidelines on urological infections. European Association of Urology. 2016. https://uroweb.org/wp-content/uploads/EAU-Guidelines-Urological-Infections-2016-1.pdf (accessed 29 April 2020). [ Links ]
17. Wasserman S, Boyles T, Mendelson, M. A pocket guide to antibiotic prescribing for adults in South Africa. South African antibiotic stewardship programme. 2015. https://www.fidssa.co.za/Content/Documents/SAASP_Antibiotic_Guidelines_2015.pdf (accessed 26 November 2020). [ Links ]
18. National Department of Health. Standard Treatment Guidelines and Essential Medicines List for South Africa. Hospital Level (Adult). 4th ed. Pretoria. NDoH, 2015. http://www.health.gov.za/index.php/standard-treatment-guidelines-and-essential-medicines-list (accessed 29 April 2020). [ Links ]
 Correspondence:
Correspondence:
J L Fourie
wcksfourie@gmail.com
Accepted 22 June 2020














