Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 no.12 Pretoria Dez. 2020
http://dx.doi.org/10.7196/samj.2020.v110i12.14695
RESEARCH
A retrospective review of the adverse effects of biological therapy and reasons for its discontinuation in a resource-limited setting
J W RoodI; R du ToitII
IMB ChB, Dip HIV Man (SA); Department of Internal Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
IIMB ChB, MMed (Int), Cert Rheumatology (SA); Division of Rheumatology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital Cape Town, South Africa
ABSTRACT
BACKGROUND: Biological disease-modifying antirheumatic drug therapies have become the gold standard of treatment for refractory rheumatic conditions in well-resourced countries. There is a significant risk of infection and reactivation of latent infections, in particular tuberculosis, with the use of biological therapies. Their safety and reasons for discontinuation in a resource-limited environment are still unclear
OBJECTIVES: The primary objective was to describe the nature and frequency of adverse events as well as the main reason for discontinuation of biological treatment
METHODS: We conducted a retrospective, descriptive folder review of all patients started on biological therapy for rheumatic conditions from November 2011 to December 2016
RESULTS: A total of 31 patients were included. The rheumatic diseases included in the study were ankylosing spondylitis (AS) (35%), rheumatoid arthritis (RA) (19%), systemic lupus erythematosus (16%), juvenile idiopathic arthritis (13%), vasculitides (10%) and psoriatic arthritis (7%). Adverse events occurred in 26 patients (84%). Serious adverse events occurred in 14 patients (45%) with recurrent uveitis being the most common, occurring in 5 patients (16%). One patient developed pulmonary tuberculosis (PTB). Discontinuation or switching of biological therapy occurred in 13 patients (42%), with the main reasons being serious adverse events in 7 patients (23%) and treatment failure in 6 (19%). The median (interquartile range (IQR)) Bath Ankylosing Spondylitis Disease Activity Index score improved from 6.4 (5 -7.4) to 2.8 (0.9 - 5.0), a statistically significant difference of-3.5 (p=0.001) (95% confidence interval (CI) -5.3 --1.7) over a median (IQR) of 20 (9 - 30) months in the AS group. The median (IQR) Clinical Disease Activity Index score improved from 39 (34.5 - 43) to 21 (18.7 - 25.5), a statistically significant difference of -17.4 (p=0.044) (95% CI -34.1 - -0.7) over a median (IQR) of 39 (21 - 50) months in the RA group
CONCLUSIONS: Recurrent uveitis occurred in almost half of the patients with AS and was also the main reason for discontinuation of biological therapy. We did not document an increased risk of PTB. Disease activity scores showed significant improvement. The study is limited by the small number of patients on biological therapy, a reflection of the impact of severe resource constraints
Background
Rheumatological disorders such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and spondyloarthropathies (SpAs) (including psoriatic arthritis (PsA) and ankylosing spondylitis (AS)) place a considerable disease burden upon individuals and upon society in general. The severity and chronicity of these diseases have far-reaching implications for the economy, and for patients themselves because of loss of capacity to work due to irreversible joint damage, reduced life expectancy, and acceleration of other disease processes including cardiovascular disease and lymphoma.[1-4]
Current therapy
The mainstay of treatment for these conditions includes a variety of immunosuppressive medications, aiming to control the inflammatory processes that drive progressive damage of various systems, including the musculoskeletal and other organ systems. For RA, early initiation of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), of which methotrexate (MTX) remains the cornerstone, is essential. However, the majority (66%) of patients with RA will fail to achieve full disease remission on MTX monotherapy, an alternative csDMARD (87%) or csDMARD
combination therapy (64%).[5,6] Hodkinson et al.[7]showed that in indigent South Africans, fewer than one-third of patients with early RA had low disease activity at 12 months.[7] Also, the therapeutic options available for conditions such as AS are limited compared with other rheumatic diseases, with no oral csDMARDs shown to have disease-modifying effects, on axial involvement in particular.[8,9] In view of these limitations, there was a clear need for alternative therapeutic options in rheumatology.
Biological therapy
Biological disease-modifying antirheumatic drugs (bDMARDs) have ultimately become the gold standard of treatment for refractory rheumatic conditions in well-resourced countries for patients who fail csDMARDs. The bDMARDs are genetically engineered drugs manufactured or synthesised in vitro from molecules such as proteins, genes and antibodies present in living organisms. These agents are then used to target specific components of the inflammatory cascade and thereby alter the body's response to inflammation.[10] Most widely used of these biological agents are the class of the tumour necrosis factor inhibitors (TNFi). The TNFi have provided remarkable improvement for patients with refractory RA, but a
prospective study done in South Africa (SA) by Pettipher et al.[11] reported that fewer than one-third of patients remained on TNFi, mainly owing to infections and inadequate response to treatment thereby highlighting the need for alternative bDMARDs.[11]
The cost of these therapies varies depending on the specific rheumatological condition, the dose required and the weight of the patient. Estimated costs in the public sector range between ZAR50 000 per annum for etanercept (Enbrel) to ZAR120 000 per annum for adalimumab ( Humira)[12] - a significant expense compared with MTX, which costs ZAR2 - 3 per 2.5 mg tablet (annual cost at maximum dose ZAR1 560), or sulphasalazine, which costs ZAR4 - 6 per 500 mg tablet (annual cost at maximun dose ZAR1 872).[13] To interpret the cost-utility measures of costly therapies in terms of their societal acceptance, they are often compared with other expensive but widely accepted (and reimbursed) therapies. Cost-utility ratios of TNFi in RA models based on original progression data range from -ETJR3 000 to EUR48 000 per quality-adjusted life-year (QALY), and in AS models from EUR15 000 to EUR50 000 per QALY. These results are within the range of other accepted or mandated interventions such as intensive glycaemic control for diabetes mellitus, antiretroviral therapy in HIV, haemodialysis, or colon cancer screening.[14,15] Most studies evaluating QALYs are done in the setting of high-income countries. In low- and middle-income countries, the value of labour is low and the majority of workers are manual labourers. In this setting, bDMARD therapy aims to protect against job losses and may provide unconvincing cost-effective returns. However, gains in quality of life for the patient, and their family and community, may be considerable.[12]
Biological treatment use in a resource-limited setting
Tygerberg Academic Hospital (TBH) in Cape Town, SA, renders a tertiary service to a population of -3.6 million people. Rheumatology is a predominant outpatient-based service, with 4 500 patient visits annually. This number includes 1 200 patients with RA and 200 patients with an SpA, including AS and PsA.
The South African Rheumatism and Arthritis Association (SARAA) and the European League Against Rheumatism (EULAR) provide guidelines for the use of csDMARDs and bDMARDs based on disease activity criteria and failure of first-line oral therapy (determined by the underlying disease treated).[9,16-19]
Currently, the SA essential medicines list does not include bDMARDs for rheumatological use, while the tertiary and quaternary provincial code list (as of 2019) includes rituximab for refractory RA.[13] At TBH there are numerous patients who meet the SARAA criteria for bDMARDs, but owing to severe resource constraints the use of biological therapy is reserved for those who have the worst refractory disease.
A combined multidisciplinary decision is then made based on accumulated information (disease activity and therapy, socioeconomic factors and employment status) to select the most suitable patients for biological therapy.
Although SARAA guidelines are followed, access to bDMARDs in SA in the public sector is dependent on criteria individually developed by various provinces as well as other factors including access to and type of medical insurance.
Risk of biological therapy
Tumour necrosis factor (TNF) plays an essential role in the inflammatory cascade, which has a protective function against infections. Inhibition of TNF has been associated with the reactivation of latent infections, specifically tuberculosis (TB). The risk of TB reactivation ranges from 5- to 56-fold in varying reports.[20-22] Certain TNFi (monoclonal antibodies) appear to have higher associations with TB reactivation than other biological therapies such as the soluble TNF receptor (etanercept), while the class of anti-CD20 antibodies (rituximab) appears to have the safest profile.[23]
SA is a TB-endemic country, among the top 8 countries in the world with regard to new TB cases in 2019. According to the World Health Organization (WHO) global report, 60 million lives were saved through effective diagnosis and treatment from 2000 to 2019, and globally the incidence of TB is falling by 2% per year Ending the TB epidemic by 2030 is one of the health targets of the WHO Sustainable Development Goals.[24] The role of bDMARDs in resource-limited settings such as SA is still unknown: 'There is a clear need for biologic registries in developing countries to better understand their safety and role in a resource limited environment.'[25]
Objectives
The primary objective of the study was to describe the nature and frequency of adverse events related to the use of biological treatment as well as the main reason for discontinuation of treatment. Secondary objectives included assessing the efficacy of current TB prophylaxis protocols, and of the selection criteria for biological therapy with regard to retaining or regaining employment.
Methods
We conducted a retrospective, descriptive folder review. All patients started on biological therapy at TBH for rheumatic conditions from November 2011 (when bDMARDs were first accessed by the rheumatology department) to December 2016 were considered for inclusion.
Inclusion criteria. All adult patients treated with bDMARDs for rheumatic disorders.
Exclusion criteria. Patients treated with bDMARDs for indications other than rheumatic disorders.
Patient records were retrieved and manually assessed by the first author (JWR). The specific data required were recorded and entered into Excel spreadsheet, version 2007 (Microsoft, USA), for evaluation.
Adverse events were defined as 'undesired harmful effects resulting from medication', and serious adverse events were defined as 'any untoward medical occurrence that at any dose: results in death, is life threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability, or requires permanent discontinuation of therapy'
Statistical analysis
Collected data were sent to the Stellenbosch University biostatistics department for basic statistical analysis. Adverse events, both major and minor, were calculated as absolute events during the study period, stratified according to biological therapy, and displayed as frequency plots.
Box-and-whisker plots were used to display improvement in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Clinical Disease Activity Index (CDAI) scores in the AS and RA groups, respectively. Even though the sample size was small, the improvements in BASDAI and CDAI scores were parametrically distributed, so the f-test was used to determine statistical significance (p-values <0.05 were considered significant).
Ethical considerations
The study was approved by the Health Research Ethics Committee of Stellenbosch University (ref. no. S16/10/191). Research was conducted according to the ethical guidelines and principles of the International Declaration of Helsinki, the South African Guidelines for Good Clinical Practice and the South African Medical Research Council ethical guidelines.
All data collected were treated as confidential, and the privacy of the subjects was protected. Data for analysis, including any publications, will remain anonymous.
Results
A total of 31 patients were included in the study period. Apart from discontinuation due to death (1 patient), biological therapy was discontinued in 2 patients (7%). None were lost to follow-up.
The spectrum of rheumatological diseases included AS (n=l1), RA (n=6), PsA (n=2), vasculitides (n=3) and juvenile idiopathic arthritis (JIA) (n=4). Biological therapy was also used in 5 refractory cases of SLE: lupus myocarditis (n=3), neurolupus (n=l) and idiopathic thrombocytopenic purpura (n=l).
Indications for biological use in the vasculitides were Takayasu's arteritis, granulomatosis with polyangiitis (GPA) and severe rheumatoid vasculitis.
Non-TNF biologicals included rituximab (SLE and vasculitis, n=7) and abatacept (JIA, n=2). Etanercept was used preferentially in 15 out of 19 patients with RA/SpA because of its cost-effectiveness and relative safety profile with regard to acquiring TB, compared with monoclonal TNFi.
Adverse events
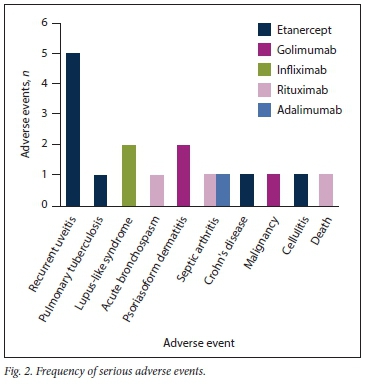
Adverse events occurred in 26 patients (84%), with some patients experiencing more than one adverse event (Figs 1 and 2).

Non-serious adverse events
Seventeen non-serious adverse events occurred in 12 patients (39%) (Fig. 1). Skin manifestations (including sebaceous cyst, pityriasis versicolor, eczema, chickenpox and folliculitis) and upper respiratory tract infections were the most frequent non-serious adverse events.
Serious adverse events
Seventeen serious adverse events occurred in 14 patients (45%) (Fig. 2). Recurrent uveitis (>2 episodes) was the most common serious adverse event, occurring in 5 patients (16%). This occurred exclusively among patients treated for AS (5/11 patients; 45%) (Table 1). In 4 patients recurrent uveitis occurred on etanercept, with a median (interquartile range) duration of 35 (28 - 40) months before the first episode of uveitis. In our study, the incidence rate of uveitis events was 27.3 per 100 person-years.
Two patients developed a lupus-like syndrome on infliximab. One of them developed a photosensitive rash, with antinuclear antibody titres of 1:1 280 and anti-double-strand DNA >200 IU/mL. No other features of SLE were present, and the patient did not meet the clinical criteria for SLE. The second patient developed an autoimmune pericarditis after infliximab treatment.
Two patients developed septic arthritis. The first developed right prosthetic hip septic arthritis while on adalimumab. The second was an SLE patient who received rituximab for resistant myocarditis and neurolupus in October 2014. She also had lupus nephritis (class IV) and haemolytic anaemia. She developed Streptococcus pneumoniae septic arthritis in 2016 in her left hip, which was damaged as a result of avascular necrosis secondary to chronic steroid use.
One patient developed sputum-positive, rifampicin-sensitive pulmonary tuberculosis (PTB). He was subsequently switched to rituximab (for seropositive RA) after completion of TB treatment, with sustained low disease activity. He had no household TB contact, and risk factors were those of RA and recently started etanercept. This patient did not have evidence of latent tuberculous bacterial infection (LTBI) (purified protein derivative <5 mm and a normal chest radiograph) when starting etanercept. Although he received isoniazid (INH) prophylaxis, this was unfortunately interrupted 4 months prior to his being diagnosed with sputum-positive PTB. He had completed 8 months of etanercept treatment by the time the PTB was diagnosed.
One patient died during the study. He was suffering from refractory GPA and died despite a second course of rituximab. The cause of death was a severe flare of GPA after the patient had been in remission following rituximab treatment 5 years previously. The rituximab was used as rescue therapy for resistant disease and is unlikely to have contributed to his death.
The patient who suffered severe bronchospasm during a rituximab infusion for RA was a known asthmatic and was subsequently placed on nebulisation.
The patient who developed carcinoid syndrome secondary to a neuroendocrine tumour developed symptoms after etanercept was started. As far as we are aware, there are no known associations between etanercept and neuroendocrine tumours.
Response to treatment
For the AS and RA groups, the median BASDAI and CDAI scores, respectively, are reflected in Figs 3-6.




For AS, a clinically significant response is defined as 'improvement of at least 50% or 2 units (on a 0 - 10 scale) of the BASDAI' after 6 -12 weeks of treatment.[26] This was achieved in 8 out of 11 patients.
For RA, lack of efficacy was defined as a failure to achieve improvement in a CDAI score of 10 - 20 after 3 months or failure to achieve a low disease activity CDAI score <10 during the later course of treatment. This was achieved in 3 out of 6 patients. (CDAI scores were used instead of SDAI scores and based on SARAA recommendations.[27])
The SLE patients treated with rituximab for off-label indications such as resistant autoimmune myocarditis, resistant neurolupus and refractory ITP had varied responses. Two out of 3 patients with myocarditis had clinical and echocardiographic improvement, while the third patient's condition remained unchanged. One patient was treated for neuropsychiatrie symptoms that resolved after treatment. One RA patient was treated for vasculitis that responded well to rituximab with complete resolution of skin lesions.
A single patient with resistant Takayasu's arteritis was treated with infliximab with improvement in disease activity clinically and as evident by positron emission tomography/computed tomography follow-up.
Discontinuation or switching to alternative biological therapy
Discontinuation or switching of biological therapy occurred in 13/31 patients (42%). The main reason for discontinuation or switching was serious adverse events. Treatment failure occurred in a total of 6 out of 13 patients (46%), 3 patients each from the RA and AS groups. Only 1 patient had treatment failure related to a second biological agent by the time the study period ended.
Chemoprophylaxis and vaccinations
None of the patients who received continuous TB prophylaxis or treatment for LTBI developed TB disease. A single patient developed PTB after discontinuation of INH prophylaxis. Patients on rituximab and abatacept did not require chemoprophylaxis.
Employment
Most patients remained employed or continued their schooling during our study period, with 1 patient dying and 2 patients gaining employment. Two patients reached pensionable age and were no longer eligible for employment; 1 had previously been employed.
Discussion
A total of only 31 patients received biological therapy for a variety of rheumatological conditions. This includes 23 out of >1 500 patients with inflammatory arthritis seen at the outpatient clinic, reflecting the current resource constraints and limited access to biological agents rather than clinical indications for bDMARDs. At the time of the study, access to bDMARDs for RA, SpA and PsA was limited to 15 patients according to provincial guidelines. Additional patients gained access through individual motivations to the hospital pharmaceutical and therapeutics committee. AS was the most common condition for which biological therapy was prescribed. This probably reflects a selection bias. With a limited total number of patients who can gain access to biological therapy, and the limited therapeutic options available for AS, these patients were selected preferentially above those with conditions such as RA, for which a wider range of non-biological csDMARD therapies is available.
Recurrent uveitis was the main serious adverse event in our study occurring in 5/11 (45%) of the AS group, an incidence of 27.3 uveitis cases per 100 person-years. This finding is in keeping with the increased incidence in the literature.[28-30] In our cohort, uveitis did not occur with any of the other conditions. Of the 5 patients who developed uveitis, 4 were using etanercept.
Uveitis is the most common extra-articular manifestation of AS. The National Ankylosing Spondylitis Society of the UK reports that uveitis occurs in 30 - 40% of patients with AS.[31] The association of an increased risk of uveitis with etanercept compared with other TNFi is starting to become less controversial. In a recent article published in the Annals of Rheumatic Disease (2017),[29] a Swedish registry study reported a 2 - 4-fold increased risk of anterior uveitis associated with etanercept compared with infliximab or adalimumab within 2 years of initiating treatment in AS patients. Lim et al.,[30] in a registry-based study, also showed a significant drug-specific relationship of uveitis associated with etanercept in comparison with infliximab and adalimumab after excluding patients with systemic diseases such as AS, JIA, PsA and Crohn's disease associated with uveitis. In view of the above, our unit will preferentially be using infliximab or adalimumab instead of eternacept for AS patients with a history of uveitis.
A single patient on etanercept developed PTB, as mentioned above. He was not on continuous INH prophylaxis at the time of his PTB diagnosis, which may have contributed to his PTB disease in a high TB-endemic area. No other patient in the study developed TB disease. Although the number of patients has been small, the chemoprophylaxis protocol implemented at TBH as previously stated seems to be effective in preventing TB.
A recent comparison between the South African Biologies Registry (SABIO) and reported data from international registries found a 10-fold higher incidence of TB among SA patients receiving biological therapy. The significantly higher incidence is probably due to the high TB prevalence in SA, with the added risk of biological therapy. Strong consideration should therefore be given to available alternatives such as rituximab or abatacept for patients with RA. For AS, only TNFi are currently available in the state sector, with ustekinumab (Stelara) being a safer alternative available in the private sector. Unpublished data from SABIO did not reveal a significant difference between etanercept and non-TNFi in terms of TB risk.[32]
Infusion reactions to rituximab, such as cough, dyspnoea and bronchospasm, are known complications of which the practitioner needs to be aware,[33,34] and bronchospasm occurred in one patient in our study.
We found a statistically significant decrease in disease activity scores of the various arthritides. The associated slowing of disease progression ultimately limits disability in the long term, which has a beneficial effect on employment status and future prospects.[35,36] The efficacy of the second biological therapies in the AS group was not as pronounced as that of the first biological therapies, which may be explained by the fact that a significant proportion of patients were switched to a second biological drug because of adverse events while already achieving an improvement in disease activity scores after the first biological therapy. A sustained response rather than treatment failure is therefore reflected in the figures. The other potential contributing factor might be the shorter time exposed to the second biological therapy (median (IQR) 9 (8 - 12) months) compared with the first (20 (9 - 30) months). It should be noted that this study was not powered to assess efficacy of biological therapies.
Gaining access to biological therapy for a selected group of patients in the state sector led to the setting out of a number of goals by the Division of Rheumatology at TBH. One of these was to reduce disability and limit the impact of the disease by regaining and retaining employment. The SA unemployment rate reached a 15-year high of 29% in 2019,[37] adding to the various factors influencing employment status in patients with a disabling disease such as RA or AS. Selecting patients who are motivated to continue working or regain employment by means of a thorough occupational therapy and social worker assessment seems to be effective. This effectiveness is evident by the fact that most patients were able to retain employment, two patients gained employment and two patients reached pensionable age.
Study limitations
This study was done retrospectively and relied on the accuracy of reports on adverse events and clinical data. The small number of patients included in the study is a reflection of the impact of severe resource constraints. It does, however, limit the statistical strength of some of the results reported. Selection bias with regard to specific conditions treated as well as the type of biological therapy used was explained earlier in the article.
Conclusions
We report on a selected group of patients who received biological therapy for various rheumatological conditions. The majority of patients showed a significant clinical response to treatment.
Recurrent uveitis occurred in almost half of the patients with ankylosing spondylitis and was also the main reason for discontinuation of biological therapy. We did not document an increased risk of PTB in our patients. The screening as well as the chemoprophylaxis protocol implemented at TBH appears to be effective in preventing reactivation of or infection with Mycobacterium tuberculosis; however, considering the small numbers and the fact that this was a single-centre study, conclusions are limited. To determine the true risk of TB associated with biological therapy and the efficacy of current TB prophylaxis in a high-risk community will require multi-centre registry verification, comparing the data with a csDMARD rheumatological control group.
Declaration. The research for this study was done for partial fulfilment of the requirements for JWR's MMed (Internal Medicine) degree at Stellenbosch University.
Acknowledgements. None.
Author contributions. JWR: first author (writing and editing), data collection, analysis and interpretation. RdT: second author (writing and editing), expert opinion and review.
Funding. None.
Conflicts of interest. None.
References
1. Klein A, Polliack A, Gafter-Gvili A. Rheumatoid arthritis and lymphoma. Incidence, pathogenesis, biology, and outcome. Hematol Oncol 2018;36(5):733-739. https://doi.org/10.1002/hon.2525 [ Links ]
2. Buleu F, Sirbu E, Caraba A, et al Heart involvement in inflammatory rheumatic diseases. A systematic literature review. Medicina 2019;55(6):249. https://doi.org/10.3390/medicina55060249 [ Links ]
3. Reiss AB, Silverman A, Khallan M, et al. Accelerated atherosclerosis in rheumatoid arthritis. Mechanisms and treatment. Curr Pharm Des 2019;25(9):969-986. https://doi.org/10.2174/1381612825666190430113212 [ Links ]
4. Krüger K. Nüblein H. Kardiovaskuláre Komorbiditáten bei rheumatoider Arthritis. Z Rheumatol 2019;78:221-227. https://doi.org/10.1007/s00393-018-0584-5 [ Links ]
5. Van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YR et al. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis 2007;66(10):1356-1362. https://doi.org/10.1136/ard.2006.066662 [ Links ]
6. Mittal N, Mittal R, Sharma A, et al. Treatment failure with disease-modifying antirheumatic drugs in rheumatoid arthritis patients. Singapore Med J 2012;53(8):532-536. [ Links ]
7. Hodkinson B, Musenge E, Ally M, et al. Response to traditional disease-modifying anti-rheumatic drugs in indigent South Africans with early rheumatoid arthritis. Clin Rheumatol 2012;31:613-619. https://doi.org/10.1007/sl0067-011-1900-5 [ Links ]
8. Van der Horst-Bruinsma IE, Clegg DO, Dijkmans BA. Treatment of ankylosing spondylitis with disease modifying antirheumatic drugs. Clin Exp Rheumatol 2002;20(Suppl 28):S67-S70. [ Links ]
9. Van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondylarthritis. Ann Rheum Dis 2017;76(6):978-991. https://doi.org/10.1136/annrheumdis-2016-210770 [ Links ]
10. Hadam J, Aoun E, Clarke K, et al. Managing risks of TNF inhibitors. An update for the internist. Cleve Clin J Med 2014;81(2):115-127. https://doi.org/10.3949/ccjm.81a.l2121 [ Links ]
11. Pettipher C, Rudolph R Musenge E, et al. A prospective study of anti-tumor necrosis factor therapy in South African rheumatoid arthritis patients. Int J Rheum Dis 2016;19(6):594-599. https://doi.org/10.1111/1756-185X12299 [ Links ]
12. Hodkinson B, Blockman M. Strategies and ethics to ensure equitable access to biological medicines in the treatment of autoimmune inflammatory diseases. Curr Allergy Clin Immunol 2018;31(4):240-244. https://doi.org/10.1111%2Fj.1365-2249.2010.04139.x [ Links ]
13. Department of Health, South Africa. Standard Treatment Guidelines and Essential Medicines List March 2020. http://www.health.gov.za/index.php/component/phocadownload/category/197 (accessed 27 April 2020). [ Links ]
14. Wong JB. Cost-effectiveness of anti-tumor necrosis factor agents. Clin Exp Rheumatol 2004;22(Suppl 35):S65-S70. [ Links ]
15. Merkesdal S, Zeidler H. TNF-blocking therapy in rheumatoid arthritis and ankylosing spondylitis. Why is cost-effectiveness a major issue? Curr Rheumatol 2005;7:254-258. https://doi.org/10.1007/S11926-005-0032-9 [ Links ]
16. South African Rheumatism and Arthritis Association. Guidelines for management of rheumatoid arthritis 2016. https://www.saraa.co.za/Positions (accessed 27 April 2020). [ Links ]
17. Smolen JS, Landewe RBM, Bijlsma IWT, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. 2019 update. Ann Rheum Dis 2020;79:685-699. https://doi.org/10.1136/annrheumdis-2019-216655 [ Links ]
18. Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies. 2015 update. Ann Rheum Dis 2016;75(3):499-510. https://doi.org/10.1136/annrheumdis-2015-208337 [ Links ]
19. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78(6):736-745. https://doi.org/10.1136/annrheumdis-2019-215089 [ Links ]
20. Jain A, Singh JA. Harms of TNF inhibitors in rheumatic diseases. A focused review of the literature. Immunotherapy 2013;5(3):265-299. https://doi.org/10.2217/imt.l3.10 [ Links ]
21. Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy. Arthritis Rheum 2009;60(8):2540. https://doi.org/10.1002/art.24632 [ Links ]
22. Lee SK, Kim SY, Kim EY, et al. Mycobacterial infections in patients treated with tumor necrosis factor antagonists in South Korea. Lung 2013;191(5):565-571. https://doi.org/10.1007/s00408-013-9481-5 [ Links ]
23. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001;345(15):1098-1104. https://doi.org/10.1056/NEJMoa011110 [ Links ]
24. World Health Organisation. Tuberculosis key facts. 14 October 2020. https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed 30 October 2020). [ Links ]
25. Tikly M, Hodkinson B, Dheda K. Biologic therapy for rheumatoid arthritis in developing countries -a place for non-TNF inhibitors as first-line treatment? Rheumatology (Oxford) 2015;54(2):208-209. https://doi.org/10.1093/rheumatology/keu040 [ Links ]
26. South African Rheumatism and Arthritis Association. Guidelines for the use of anti-tumour necrosis factor agents in the management of patients suffering from ankylosing spondylitis 2016. https://www.saraa.co.za/Positions/ATNFBlockers (accessed 27 April 2020). [ Links ]
27. South African Rheumatism and Arthritis Association. Guidelines for the use of anti-tumour necrosis factor inhibitors 2016. https://www.saraa.co.za/Positions/TNFBlockers (accessed 27 April 2020). [ Links ]
28. Cobo-Ibanez T, del Carmen Ordonez M, Munoz-Fernandez S, et ai. Do TNF-blockers reduce or induce uveitis? Rheumatology (Oxford) 2008;47(5):731-732. https://doi.org/10.1093/rheumatology/ken091 [ Links ]
29. Lie E, Lindstrom U, Zverkova-Sandstrom T, et ai. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis. Results from the Swedish biologies register. Ann Rheum Dis 2017;76(9):1515-1521. https://doi.org/10.1136/annrheumdis-2016-210931 [ Links ]
30. Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum 2007;56(10):3248-3252. https://doi.org/10.1002/art.22918 [ Links ]
31. National Ankylosing Spondylitis Society. Resources for health professionals. 2018. https://nass.co.uk/homepage/health-professionals/resources-for-health-professionals/ (accessed October 2018). [ Links ]
32. Pettipher C, Benitha R. Tuberculosis in biologic users for rheumatic diseases-. Results from the South African Biologies Registry (SABIO). Ann Rheum Dis 2020;79(2):.292-299. https://doi.org/10.1136/annrheumdis-2019-216128 [ Links ]
33. Bouidouyre MA, Cohen P, Guilievin L. Severe bronchospasm associated with rituximab for refractory Churg-Strauss syndrome. Ann Rheum Dis 2009;68(4):606. https://doi.org/10.1136/ard.2008.093773 [ Links ]
34. Kamei K, Ito S, Iijima K. Severe respiratory adverse events associated with rituximab infusion. Pediati Nephrol 2010;25(6):1193. https://doi.org/10.1007/s00467-009-1408-2 [ Links ]
35. Berner C, Haider S, Grabovac I, et al Work ability and employment in rheumatoid arthritis. A cross-sectional study on the role of muscle strength and lower extremity function. Int J Rheumatol 2018;2018:3756207. https://doi.org/10.1155/2018/3756207 [ Links ]
36. Raterman HG, Hoving JL, Nurmohamed MT, et al. Work ability. A new outcome measure in rheumatoid arthritis? Scand J Rheumatol 2010;39(2):127-131. https://doi.org/10.3109/03009740903447044 [ Links ]
37. Statistics South Africa. Unemployment rises slightly in third quarter of 2019. 29 October 2019. http://www.statssa.gov.za/?p=12689 (accessed 27 April 2020). [ Links ]
 Correspondence:
Correspondence:
J W Rood
jacquesrood@gmail.com
Accepted 1 June 2020














