Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 n.11 Pretoria Nov. 2020
http://dx.doi.org/10.7196/samj.2020.v110i11.15249
RESEARCH
COVID-19 deaths in South Africa: 99 days since South Africa's first death
V Pillay-van WykI; D BradshawII; P GroenewaldIII; I SeocharanIV; S MandaV; R A RoomaneyVI; O AwotiwonVII; T NkwenikaVIII; G GrayIX; S S ButheleziX; Z L MkhizeXI
IPhD, Co-Director; Burden of Disease Research Unit, South African Medical Research Council, Cape Town, South Africa
IIDPhil, Chief Specialist Scientist; Burden of Disease Research Unit, South African Medical Research Council, Cape Town, South Africa
IIIMB ChB, MPH, Specialist Scientist: Burden of Disease Research Unit, South African Medical Research Council, Cape Town, South Africa
IVBTech, IT Specialist; Biostatistics Unit, South African Medical Research Council, Durban, South Africa
VPhD, Director; Biostatistics Unit, South African Medical Research Council, Pretoria, South Africa
VIMPH, Senior Scientist; Burden of Disease Research Unit, South African Medical Research Council, Cape Town, South Africa
VIIMSc, Senior Scientist: Burden of Disease Research Unit, South African Medical Research Council, Cape Town, South Africa
VIIIMSc, Junior Statistician; Biostatistics Unit, South African Medical Research Council, Pretoria, South Africa
IXMB BCh, FC Paecî (SA), President and CEO; South African Medical Research Council, Cape Town, South Africa
XMB ChB, Director-General of Health; National Department of Health, Pretoria, South Africa
XIMB ChB, Minister of Health; National Department of Health, Pretoria, South Africa
ABSTRACT
BACKGROUND. Understanding the pattern of deaths from COVID-19 in South Africa (SA) is critical to identifying individuals at high risk of dying from the disease. The Minister of Health set up a daily reporting mechanism to obtain timeous details of COVID-19 deaths from the provinces to track mortality patterns.
OBJECTIVES. To provide an epidemiological analysis of the first COVID-19 deaths in SA.
METHODS. Provincial deaths data from 28 March to 3 July 2020 were cleaned, information on comorbidities was standardised, and data were aggregated into a single data set. Analysis was performed by age, sex, province, date of death and comorbidities.
RESULTS. SA reported 3 088 deaths from COVID-19, i.e. an age-standardised death rate of 64.5 (95% confidence interval (CI) 62.3 - 66.8) deaths per million population. Most deaths occurred in Western Cape (65.5%) followed by Eastern Cape (16.8%) and Gauteng (11.3%). The median age of death was 61 years (interquartile range 52 - 71). Males had a 1.5 times higher death rate compared with females. Individuals with two or more comorbidities accounted for 58.6% (95% CI 56.6 - 60.5) of deaths. Hypertension and diabetes were the most common comorbidities reported, and HIV and tuberculosis were more common in individuals aged <50 years.
CONCLUSIONS. Data collection for COVID-19 deaths in provinces must be standardised. Even though the data had limitations, these findings can be used by the SA government to manage the pandemic and identify individuals who are at high risk of dying from COVID-19.
South Africa (SA) is one of the first countries on the African continent to tackle COVID-19 head-on. Our country is different from the developed countries that have already faced the full force of the pandemic, due to its quadruple burden of disease of HIV/ AIDS and tuberculosis (TB); other communicable diseases, perinatal conditions, maternal causes and nutritional deficiencies; non-communicable diseases; and injuries.[1,2] These factors have left much uncertainty about how the pandemic would progress in SA. COVID-19 reached SA about 6 weeks after the outbreak in Europe[3,4] and on 11 March 2020 the World Health Organization (WHO) characterised COVID-19 as a pandemic.[5] The SA government, guided by the WHO and the progress of the outbreak in other parts of the world, locked down the country to give provinces time to prepare for the onslaught of COVID-19. On 27 March 2020, we heard the sad news that SA had experienced its first death from COVID-19.[6] By the end of April there had been 103 deaths,[7] which increased to 683 deaths by the end of May[8] and to over 2 657 deaths by the end of June.[9] Due to the time lag for the national cause of death data based on death notifications to be processed, the Minister of Health has set up a daily reporting mechanism to obtain timeous details of COVID-19 deaths from the provinces to track the progression of the pandemic.
Objectives
This article reports on COVID-19 deaths for SA and its nine provinces for the first 99 days since our first COVID-19 death.
Methods
The COVID-19 death reports provided to the National Department of Health (NDoH) by each province between 28 March and 3 July 2020 were used in this analysis. The data were provided in an unstandardised format from each province in either PDF documents or Microsoft Excel workbooks. The variables and clinical information provided by each province varied. In data sets with identifiers, duplicate records were identified and removed. Twenty-four duplicates were identified and removed from the data, 23 from Gauteng and one from Western Cape. It was not possible to identify whether there were any duplicate cases in Eastern Cape, as no identifiers were provided. The clinical data provided were interrogated in order to identify comorbidities, which were then recorded in a standard format for each individual (Annexure A, http://samj.org.za/public/sup/15249-l.pdf). Comorbidities were reported as a mix of full disease names and abbreviated names, sometimes including conditions that were a consequence of COVID-19 itself rather than comorbidities that the individual had prior to becoming ill with COVID-19. Variables that were common across the provinces were collated into a single data set and included date of death, age, province and comorbidities. The data set was checked for missing data (Annexure B, http://samj.org.za/public/sup/15249-2.pdf). Age was categorised into 5-year age bands, 10-year age bands and broad age bands <50 years, 50 - 69 years and >70 years.
Statistical analysis was conducted using Microsoft Excel (Microsoft Corp., USA) and Stata 15 (StataCorp, USA).
Summary statistics involved expressing continuous data as medians and interquartile ranges (IQRs) for age distribution across sex, province and comorbidities. Discrete or categorical data such as comorbidities were summarised using frequencies and percentages with the associated 95% confidence intervals (CIs) by age group sex, province and comorbidities. Associations between presence or absence of a specific comorbidity and age group, sex and province were quantified by x2tests and p-values, using a cut-off value of 0.05 for a statistically significant association. Death rates and associated 95% CIs[10] were calculated using mid-year population estimates from the Thembisa model[11] and standardised using the WHO world standard population.[12]
Results
A total of 3 088 deaths were provided from the nine provinces for the period 28 March - 3 July 2020. Table 1 shows the characteristics of individuals who were reported to have died from COVID-19. The median (IQR) age was 61 (52 - 71) years. The age was similar for both sexes. Only 20.3% of the deaths were in individuals aged <50 years, and 29.4% of those who died were aged >70 years. There were six deaths reported in children aged <10 years, accounting for 0.2% of the total deaths.

Provinces with <100 deaths were combined due to small numbers and presented as other provinces'. Western Cape reported the highest number of deaths for SA (65.5%), followed by Eastern Cape (16.8%) and Gauteng (11.3%). The six other provinces collectively reported 6.4% of the deaths for SA (Fig. 1A).
Fig. 1B shows the increase in the number of deaths over time. March and April were combined due to small numbers, and June and July were combined as deaths for July only included deaths from 1 July to 3 July. There was an almost four-fold increase in deaths between May and June-July. Fig. 1C shows the age-standardised death rate; this measure removes the effect of the age structure of the population and allows for direct comparison of death rates across provinces. Western Cape had the highest age-standardised death rate, followed by Eastern Cape and Gauteng.
The crude death rate for Western Cape was 288.9 deaths per million population (95% CI 276.3 - 301.5), that for Eastern Cape was 77.1 deaths per million population (95% CI 70.4 - 83.7) and that for Gauteng was 23.8 deaths per million population (95% CI 21.3 - 26.2). SA had a crude death rate of 52.1 deaths per million population (95% CI 50.3 - 53.9) (data not reported).
Fig. 2A shows the age pattern of deaths for the provinces. Even though the age pattern appears similar across the provinces, there were some differences, i.e. the highest number of deaths in Western Cape was in 60 - 64-year-olds, in Eastern Cape it was in 55 - 59-year-olds, and in Gauteng the majority of deaths were in 70 - 74-year-olds. Fig. 2B shows age-specific death rates; this measure accounts for the age structure and population size for each province. Age-specific death rates increased dramatically with age for Western Cape from >35 years and for Gauteng from >70 years, with Eastern Cape showing a different pattern (Fig. 2B).
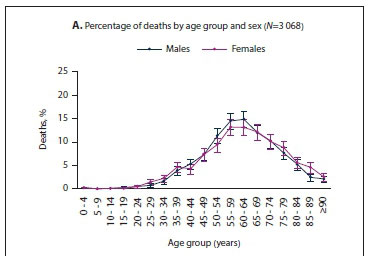
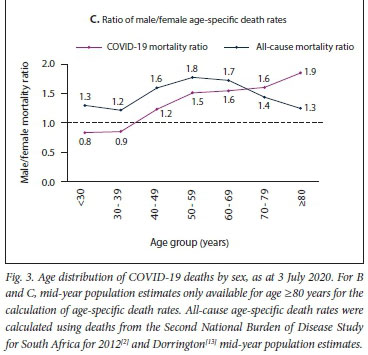
Fig. 3 shows the age distribution of deaths for males and females in SA. The highest percentage of deaths occurred in the 55 - 59- and 60 - 64-year age groups for both males and females (Fig. 3A). The percentage of deaths of individuals aged <30 years was very low for both males and females, i.e. 2.0% (61 deaths). Age-specific death rates increased as age increased for both males and females (Fig. 3B). The death rate was was higher in males compared with females in those aged >40 years (Fig. 3C). As age increased, the COVID-19 mortality ratio increased. Comparison of the COVID-19 mortality ratio with the all-cause mortality ratio for the year 2012 from the Second National Burden of Disease Study[2] for SA revealed that the COVID-19 mortality ratio was higher in individuals aged >70 years (Fig. 3C).
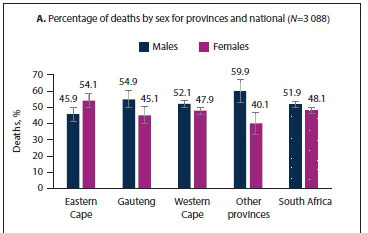
Fig. 4 shows the sex distribution of deaths for province and national. The pattern of male and female deaths across all provinces followed the national pattern, with more male deaths occurring compared with females, except for Eastern Cape, which reported more female deaths (Fig. 4A). However, once the effect of the population structure was removed, the age-standardised death rates showed that the male/female mortality ratio for Eastern Cape was similar to the national pattern (Fig. 4B and C). The age-standardised COVID-19 male/female mortality ratio was 1.5 compared with the all-cause mortality ratio of 1.4 (data not reported).
Table 2 shows the distribution of comorbidities. In a relatively high proportion of the deaths, information about comorbidities was missing or unknown (18.3% and 2.2%, respectively) (Annexure B, http://samj.org.za/public/sup/15249-2.pdf). At least one comorbidity was reported for 2 355 (95.8%) of individuals who died (Table 1). Hypertension and diabetes were by far the most common comorbidities among both males and females, occurring in more than half of the national deaths (60.5%; 95% CI 58.5 - 62.4 and 54.9%; 95% CI 52.9 - 56.9, respectively). Hypertension was significantly more common in females (66.2%; 95% CI 63.5 - 68.8) than males (55.0%; 95% CI 52.3 - 57.8), and diabetes was more common in males (56.4%; 95% CI 53.6 - 59.1) than females (53.3%; 95% CI 50.5 - 56.2). These were followed by HIV (13.9%; 95% CI 12.6 - 15.3), chronic respiratory conditions, i.e. asthma and chronic obstructive pulmonary disease (13.3%; 95% CI 12.0 - 14.7) and chronic kidney disease (13.3%; 95% CI 12.0 - 14.7). Cardiovascular diseases were reported for 6.3% (95% CI 5.4 - 7.3), obesity for 5.5% (95% CI 4.7 - 6.5) and cancers for 3.3% (95% CI 2.7 - 4.1) of the deaths. Other diseases combined were reported for 9.1% (95% CI 8.0 - 10.3) of the deaths, and eight females were pregnant (data not reported). Current TB was reported in 3.3% (95% CI 2.6 - 4.0) of the deaths and differed significantly by sex, with 4.0% (95% CI 3.0 - 5.2) in males and 2.5% (95% CI 1.8 - 3.6) in females.
There is a strong age pattern in the reported comorbidities. The ranking of the five most common comorbidities reported for all ages differed when disaggregated by broad age group. HIV and TB were much higher among individuals aged <50 years (33.2%; 95% CI 29.1 -37.6 and 7.8%; 95% CI 5.7 - 10.6, respectively), and chronic kidney disease was much higher in individuals aged >70 years (19.2%; 95% CI 16.4 - 22.3). Diabetes ranked highest among <50-year-olds (41.2%; 95% CI 36.9 - 45.7), followed by hypertension (34.2%; 95% CI 30.1 - 38.7) and HIV (33.2%; 95% CI 29.1 - 37.6). Hypertension ranked highest in both 50 - 69-year-olds (64.3%; 95% CI 61.7 - 66.9) and >70-year-olds (71.2%; 95% CI 67.7 - 74.4), followed by diabetes (62.4%; 95% CI 59.7 - 65.1 in 50 - 69-year-olds and 50.4%; 95% CI 46.7 - 54.1 in >70-year-olds).
Hypertension was the most common comorbidity reported in Western Cape (63.8%; 95% CI 61.4 - 66.2), Eastern Cape (58.6%; 95% CI 53.8 - 63.3) and Gauteng (48.1%; 95% CI 42.6 - 53.6), followed by diabetes (Table 2). The comorbidity proportions were statistically significantly different across provinces. Differences were observed in the top five comorbidities across provinces; for example, Gauteng and Eastern Cape reported cardiovascular disease in their top five comorbidities. TB was more common in Western Cape than in the other provinces (3.9%; 95% CI 3.1 - 5.0). Of the 167 deaths in which previous TB was reported, 159 were from Western Cape (data not reported). The findings on comorbidities must be interpreted with caution, as the proportions could be influenced by the low proportion of deaths with no comorbidities, which in turn could be related to the high proportion of unknowns in the data.
Fig. 5 shows the number of comorbidities per individual where comorbidity information was available. Most individuals had either one comorbidity (37.3%; 95% CI 35.4 - 39.2) or two comorbidities reported (36.3%; 95% CI 34.4 - 38.2). Only 4.2% (95% CI 3.4 - 5.0) had no comorbidities. More than half (58.6%; 95% CI 56.6 - 60.5) of the individuals who died had two or more comorbidities.

Fig. 6 shows the overall pattern of comorbidities, showing how often each condition was reported. This includes multiple comorbidities for individuals with more than one comorbidity. Hypertension and diabetes accounted for >50% of the comorbidities reported for most provinces except North West and Mpumalanga.

Table 3 reports on the top ten combinations of comorbidities in individuals. For those reported to have a comorbidity, the combination of diabetes and hypertension (19.9%; 95% CI 18.3 - 21.6) was most common, followed by hypertension only (13.4%; 95% CI 12.1 - 14.8) and diabetes only (12.7%; 95% CI 11.5 - 14.1).
Discussion
Even though SA is still in the early stages of the COVID-19 pandemic, it is important to track how the pandemic is progressing and understand the pattern of deaths as they unfold. The SA government's swift actions to set up a daily reporting of deaths to the NDoH has provided some understanding of the nature of the pandemic in SA.
Our analysis found that the various provinces in SA are at different stages of the COVID-19 pandemic, with Western Cape being at a much later stage than its counterparts, contributing to 65% of total deaths with a death rate of 324.4 deaths per million population, a rate that is five times higher than the national death rate of 64.5 deaths per million population. The number of deaths peaked at 55 - 59 years in Eastern Cape, 60 - 64 years in Western Cape and 75 - 79 years in Gauteng. Most deaths occurred in individuals aged >30 years, starting slightly younger than in other countries, which could be due to SA having a younger population; SAs median age is 27.6 years, compared with Italy with 47.3 years and Spain with 44.9 years.[14] As age increased, death rates increased. The age pattern observed for Eastern Cape is different where their number of deaths peaked at a younger age and no observable increase in death rates as age increases is difficult to explain and requires further investigation.
Our finding that males had higher death rates than females is similar to other countries.[15] However, the divergence of death rates between males and females as age increased was not observed in other countries. This could be indicative of the stage of the pandemic in SA. We found that the COVID-19 male/female mortality ratio of 1.5 for SA is similar to the all-cause male/female mortality ratio of 1.4 for our country for 2012.pl The age pattern of SAs all-cause mortality ratio was similar to that observed in other countries where the male/ female gap for death rates becomes smaller after 69 years of age. The male-female gap for COVID-19 death rates became bigger after 69 years of age. The COVID-19 male/female mortality ratio observed for the provinces was similar to the national ratio.
For individuals where information was available, at least one comorbidity was reported for 2 355/2 457 (95.8%) of the individuals who died. More than half (58.6%) of the deaths occurred in individuals with two or more comorbidities. Hypertension and diabetes were the most common comorbidities in individuals who were reported to have died from COVID-19; this finding is similar to what was found in Europe.[16] Hypertension and diabetes were the most common comorbidities in individuals across provinces, and across the broad age bands <50 years, 50 - 59 years, 60 - 69 years and >70 years, but the prevalence of these conditions increased significantly with age. Our findings that HIV and current TB were more common in <50-year-olds[17] and chronic kidney disease in >70-year-olds have been reported previously.[17,18] The top five comorbidities did differ by province. At the current stage of the COVID-19 pandemic, very few deaths have been reported in children.
From a health services perspective, our analysis showed that most individuals who died of COVID-19 presented with both hypertension and diabetes, followed by hypertension only and then diabetes only. This information is critical to identifying individuals who are at high risk of dying from COVID-19 and may indicate the importance of screening for and managing these conditions among people infected with SARS-CoV-2.
While a large proportion of South Africans have HIV, TB or both diseases, our analysis shows that these conditions were not the most common comorbidities in individuals who died from COVID-19.
However, as the number of deaths increases in the country, this pattern could change.
An analysis of Western Cape COVID-19 deaths by Boulle et al.[19]showed similar findings to our analysis for the province. They investigated 625 public sector COVID-19 deaths that occurred before 1 June 2020. Their study highlighted that HIV and TB should not be forgotten in the COVID-19 pandemic, as there was a 3.3 times increased risk of dying from COVID-19 in individuals with TB and a 2 times increased risk of dying in individuals with HIV, when adjusted for age and sex.[19] That said, their study found that the risk of dying from COVID-19 was also higher in individuals with hypertension (2.2 times) and diabetes (ranging from 6.1 to 12.9 times higher depending on the level of glucose control), when adjusted for age and sex.[19]
Study limitations
The study findings reflect the data that were provided and have limitations. Many provinces did not provide information on the dates of COVID-19 tests and the dates on which the results were received. It was therefore not possible to distinguish between a confirmed and probable COVID-19 death.[20] It was also unclear whether the data reported on comorbidities are complete, i.e. whether all the comorbidities have been reported, and whether missing data means no comorbidities in the individuals or this was just not reported. Furthermore, the impact of the inconsistent reporting of information across provinces and the completeness of reporting has not been investigated.
Bradshaw et al. [21] have shown, using deaths data from the Department of Home Affairs, that more deaths are being reported during the period of the pandemic compared with previous years, which could be attributed directly or indirectly to COVID-19. Their numbers are much higher than those reported in this study. This could be due to more individuals dying at home from COVID-19 and being missed in our analysis, or dying from other conditions at home because they are afraid to attend a health facility. The differences in the number of deaths require further investigation.
Conclusions
Our study provides important epidemiological information on the pattern of the current state of COVID-19 mortality in SA that, even though it is not perfect, can be used by the SA government to identify high-risk individuals. Differential patterns of COVID-19 deaths by sex, age, comorbidities and provinces point to the need for targeted and localised interventions. That said, individuals with hypertension and diabetes should be given careful attention during the COVID-19 pandemic across SA.
Furthermore, data collection for COVID-19 needs to be standardised across provinces, and systems to verify the completeness and accuracy of the data should be put in place to ensure better reporting of information on COVID-19 deaths. The timeliness of the availability of cause of death information needs to be addressed. Firstly, SAs NDoH should have access to the cause of death information at the time of death registration in order to monitor epidemics more accurately. Secondly, efforts should be made to expedite the flow and processing of cause of death information between the SAs National Department of Home Affairs and the national statistics office, Statistics South Africa. Doing this will have long-term benefits beyond the COVID-19 pandemic.
Declaration. None.
Acknowledgements. We acknowledge all the healthcare workers who collated the deaths data from the various provinces.
Author contributions. All authors contributed to the analysis, interpretation of findings and finalising the manuscript.
Funding. This work was funded by the South African Medical Research Council.
Conflicts of interest. None.
References
1. Hofman K, Madhi S. The unanticipated costs of COVID-19 to South Africa's quadruple disease burden. S Afr Med J 2020;110(8):698-699. https://doi.org/10.7196/SAMJ.2020.vll0i8.15125 [ Links ]
2. Pillay-van Wyk V, Msemburi W, Laubscher R, et aL Mortality trends and differentials in South Africa from 1997 to 2012. Second National Burden of Disease Study Lancet Glob Health 2016;4(9):e642-e653. https://doi.org/10.1016/S2214-109X(16)30113-9 [ Links ]
3. World Health Organization. Timeline of WHO'S response to COVID-19. https://www.who.int/news-room/detail/29-06-2020-covidtimeline (accessed 11 August 2020). [ Links ]
4. National Institute for Communicable Diseases. First case of COVID-19 announced - an update. 5 March 2020. https://www.nicd.ac.za/first-case-of-covid-19-announced-an-update/ (accessed 19 September 2020). [ Links ]
5. World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. 11 March 2020. http://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---ll-march-2020 (accessed 11 August 2020). [ Links ]
6. National Department of Health, South Africa. Minister Zweli Mkhize confirms first deaths due to Coronavirus COVID-19. 27 March 2020. https://www.gov.za/speeches/minister-zweli-mkhize-confrrms-frrst-deaths-due-coronavirus-covid-19-27-mar-2020-0000 (accessed 28 July 2020). [ Links ]
7. National Department of Health, South Africa. Minister Zweli Mkhize confirms total of 5 350 cases of Coronavirus COVID-19. 29 April 2020. https://www.gov.za/speeches/minister-zweli-mkhize-confirms-total-5-350-cases-coronavirus-covid-19-29-apr-2020-0000 (accessed 28 July 2020). [ Links ]
8. National Department of Health, South Africa. Minister Zweli Mkhize confirms total of 32 683 cases of Coronavirus COVID-19. 31 May 2020. https://www.gov.za/speeches/minister-zweli-mkhizi-confirms-total-32-683-cases-coronavirus-covid-19-31-may-2020-0000 (accessed 28 July 2020). [ Links ]
9. National Department of Health, South Africa. Minister Zweli Mkhize confirms total of 151 209 cases of Coronavirus COVID-19. 30 June 2020. https://www.gov.za/speeches/minister-zweli-mkhize-confirms-total-151-209-cases-coronavirus-covid-19-30-jun-2020-0000 (accessed 28 July 2020) [ Links ]
10. Keyfitz N. Sampling variance of standardized mortality rates. Hum Biol 1966;38(3):309-317. [ Links ]
11. Johnson LF, Dorrington RE. Thembisa version 4.2. A Model for Evaluating the Impact of HIV/AIDS in South Africa. Cape Town. University of Cape Town, 2019. https://www.thembisa.org/content/filedl/Thembisa4_2report (accessed 11 August 2020). [ Links ]
12. Ahmad OB, Boschi Pinto C, Lopez AD. Age standardization of rates. A new WHO standard. GPE Discussion Paper Series. No 31. 2001.10-12. https://www.who.int/healthinfo/paper31.pdf (accessed 11 August 2020). [ Links ]
13. Dorrington R. Alternative South African Mid-year Estimates, 2013. Cape Town. Centre for Actuarial Research, University of Cape Town, 2013. https://www.commerce.uct.ac.za/Research_Units/CARE/Monographs/Monographs/Monol3.pdf (accessed 11 August 2020). [ Links ]
14. United Nations, Department of Economic and Social Affairs. World Population Prospects 2019, online edition. 2019. https://population.un.org/wpp/ (accessed 11 August 2020). [ Links ]
15. Ng J, Bakrania K, Russell R Falkous C. COVID-19 mortality rates by age and gender. Why is the disease killing more men than women? RGA, 3 July 2020. https://Avwwrgare.com/docs/default-source/knowledge-center-articles/covid-19-case-fatality_agegender.pdf?sfvrsn=5806a7ea_2 (accessed 11 August 2020). [ Links ]
16. Gold MS, Sehayek D, Gabrielli S, et aL COVID-19 and comorbidities. A systematic review and metaanalysis. Postgrad Med 2020.1-7. https://doi.org/10.1080/00325481.2020.1786964 [ Links ]
17. Western Cape Government. Analysis in comorbidities in adult COVID-19 deaths. 2020. https://www.westerncape.gov.za/gc-news/147/54748 (accessed 28 July 2020). [ Links ]
18. Guan W-J, Liang W-H Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China. A nationwide analysis. Eur Respir J 2020;55(5):2000547. https://doi.org/10.1183/13993003.00547-2020 [ Links ]
19. Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa [November 2020, Vol. 110, No. 11, 29 August 2020]. Clin Infect Dis 2020;ciaa1198. https://doi.org/10.1093/cid/ciaall98 [ Links ]
20. Groenewald P, Awotiwon O, Hanmer L, Bradshaw D. Guideline for medical certification of death in the COVID-19 era. S Afr Med J 2020;110(8):721-723. https://doi.org/10.7196/SAMJ.2020.v110iS.15114 [ Links ]
21. Bradshaw D, Laubscher R, Dorrington R, Groenewald P, Moultrie T. Report on weekly deaths in South Africa: 1 January - 14 July 2020 (week 28). Cape Town. South African Medical Research Council 2020. https://www.samrc.ac.za/sites/default/files/files/2020-07-22/WeeklyDeaths14July2020_0.pdf (accessed 28 July 2020). [ Links ]
 Correspondence:
Correspondence:
VPillay-van Wyk
victoria.pillayvanwyk@mrc.ac.za
Accepted 18 September 2020
The article in context
South Africa (SA) has a quadruple burden of disease, which includes a high burden of HIV and tuberculosis. The impact of COVID-19 was unclear with our disease burden. We searched PubMed on 5 August 2020 for articles published from December 2019 until that date, using the following search string: (("coronavirus"[MeSH Terms] OR "coronavirus"[Title] OR "2019-nCoV"[Title] OR "2019nCoV"[Title] OR "COVID-19"[Title] OR "SARS-CoV-2"[Title]) AND (Africa"[MeSH Terms]) AND "humans"[MeSH Terms] AND ("2019/12"[Date - Publication] : "3000"[Date - Publication])). Filters used: Books and Documents, Meta-Analysis, Review, Systematic Review. We excluded clinical trials and randomised controlled trials. No language restrictions were applied. Twelve studies were identified. An additional evidence-based brief on COVID-19 and non-communicable diseases was identified; however, none of the studies reported on COVID-19 age-specific and age-standardised death rates, sex mortality ratios and distribution of comorbidities among individuals who died from COVID-19 for SA.
Our study reports the pattern of COVID-19 deaths by age, sex, province and comorbidities for SA for the period 28 March 2020 to 3 July 2020. Even though our finding of males having higher death rates than females has been seen in other countries, the male/female ratio is not as high in SA as in some countries; the continued increase in the male-female mortality ratio in individuals aged >70 years was also not observed in other countries. At the current stage of the pandemic, the COVID-19 male/female mortality ratio had a similar pattern to the all-cause male/female mortality ratio for SA, with the exception of individuals aged >70 years, who experienced an even higher COVID-19 mortality differential than the usual all-cause. Most of our deaths occurred in individuals with comorbidities, and 58% had two or more comorbidities. Hypertension and diabetes were the most common comorbidities reported.
The pattern of COVID-19 deaths in SA may be different from patterns in other countries. However, this may change as the pandemic progresses. Our findings have health systems and public health implications, as they identify individuals at high risk of dying from COVID-19, and this information can be used to manage the pandemic. Even though we observed differential patterns of COVID-19 deaths by sex, age, comorbidities and province, individuals with hypertension and diabetes are at high risk of dying from COVID-19 in SA and should be managed carefully when they test positive for COVID-19. Furthermore, more stringent social distancing measures should be promoted to reduce transmission of COVID-19 among those who have hypertension and diabetes, particularly those who have challenges with controlling their levels of blood pressure and glucose.
Our study has identified areas in the data collection process that should be improved, and challenges with the reporting of cause of death information in SA. Addressing these shortcomings will benefit the health system beyond the pandemic.