Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 n.8 Pretoria Aug. 2020
http://dx.doi.org/10.7196/SAMJ.2020.v110i6.14472
REVIEW
A narrative review on spinal deformities in people with cerebral palsy: Measurement, norm values, incidence, risk factors and treatment
E BritzI; N G LangerakII; R P LambertsIII, IV
IMD, FC Orth; Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIPhD; Neuroscience Institute and the Division of Neurosurgery, Department of Surgery, Faculty of Health Sciences, University of Cape Town, South Africa
IIIPhD, FECSS; Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IVInstitute of Sport and Exercise Medicine, Division of Orthopaedic Surgery, Department of Surgical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
Spinal deformities are common in people with cerebral palsy (CP), and there is a concern of an increase during the adult ageing period. There is especially a worry about the increase of scoliosis, thoracic hyperkyphosis, lumbar hyperlordosis, spondylolysis and spondylolisthesis incidence, though supporting literature is lacking. Therefore, the aim of this narrative review is to provide a scientific overview of how spinal curvatures should be measured, what the norm values are and the incidence in people with CP, as well as a description of the risk factors and the treatment regimens for these spinal abnormalities. This review can be used as a guideline relevant for a range of clinicians, including orthopaedic and neurosurgeons, radiologists, physiotherapists, and biokineticists, as well as academics.
The earliest description of cerebral palsy (CP) was made by William J. Little in the mid-1800s. In a series of lectures, entitled 'Deformities of the human frame', Little included a description of cerebral paralysis. Although his main focus emphasised musculoskeletal complications, such as joint contractures and deformities as a result of chronic spasticity and paralysis, he specifically noted that the spasticity and paralysis was as a result of brain damage during infancy which resulted from preterm birth or perinatal asphyxia.[1] CP was therefore initially referred to as Little's disease[2]
William Osler (1889) and Sigmund Freud (1893) both further contributed to the field of CP.[3,4] In his article 'Cerebral Palsies of Children' Osler documented 151 cases which he classified as 'cerebral palsies' based on neuroanatomical pathology distribution into three main groups: infantile hemiplegia, bilateral spastic hemiplegia (i.e. spastic diplegia) and spastic paraplegia.[3]
Freud had contrasting ideas to both Little's and Osler's work, and suggested classifying CP using clinical findings only. He recognised that the pathological findings resulted from both the initial lesion as well as the repair process and, in addition, he noted differences in clinical manifestations in patients with similar neuropathology. Freud further suggested that rather than perinatal asphyxia being the cause of CP, the aetiology of the brain damage present in CP could be multifactorial. He identified three major groups of causal factors: (i) maternal and idiopathic congenital; (ii) perinatal; and (iii) postnatal factors.[4] It is worth noting that Freud's ideas and work still form part of our modern-day definition of CP.[2]
The primary condition of CP is non-progressive over time in the neurological sense.[2] However, secondary conditions of CP develop over time as a result of the primary conditions.[5] Manifestations can be grouped into primary and secondary manifestations. Primary manifestations include abnormal tone, loss of motor control, impaired balance, spasticity, hypotonia and dyskinesia. Secondary manifestations are growth and spasticity related and include contractures (initially dynamic and progress to static over time), upper extremity deformities, hip subluxations and dislocations, foot deformities, gait disorders and fractures, and spinal deformities.[6]
Spinal deformities are more commonly seen in people with CP and range from a scoliosis to increased thoracic kyphosis, increased lumbar lordosis, spondylolysis and spondylolisthesis.[7] The preferred method of measuring a spinal curvature, is with an X-ray in standing position (if possible). The curvature is described in relation to the body's anatomical planes: coronal (frontal), sagittal (lateral) and horizontal (axial or transverse).[8] The most common spinal abnormalities are a scoliosis, a hyperkyphosis, a hyperlordosis and a spondylolysis and/or spondylolisthesis.
Objectives
To provide a scientific overview of how spinal curvatures should be measured, what the norm values are and the incidence in people with CP, as well as a description of the risk factors and the treatment regimens for these spinal abnormalities.
Methods
A narrative review of the literature was conducted on six databases including PubMed, Cochrane Library, Proquest, ScienceDirect and Scopus. Specific search strategies were used for each database, using MeSH terms and/or single concepts. The following key search terms were used: 'cerebral palsy' AND 'spinal deformities'; 'cerebral palsy' AND 'spinal curvatures'; 'cerebral palsy' and 'spine'. All articles were screened for quality and appropriateness, and important references were checked and, where appropriate, included in the narrative review.
Results
Scoliosis
Scoliosis is defined as a spinal curvature in the coronal (frontal) plane.[9] Scoliosis is typically accompanied by a variable degree of spinal column rotation; scoliosis is therefore a 3D deformity.[9] The classic scoliosis curve pattern associated with CP is a long, C-shaped curve, often kyphoscoliotic or, less commonly, lordoscoliotic.[10]
Measurement
Cobb's angle measurement is the standard method used in determining scoliosis curve (Fig. 1). This is done on a frontal X-ray by identifying the upper and lower end vertebrae which are maximally tilted at the top and the bottom of the curve. A line is drawn along the cephalad end plate of the top vertebra and along the caudal end plate of the bottom vertebra. The angle where these lines cross is measured as the Cobb's angle. Besides the Cobb's angle, the curve direction (convexity left or right side) and location (apical vertebra; vertebra that is the most deviated and rotated from the midline), resulting in the curve pattern, are reported.
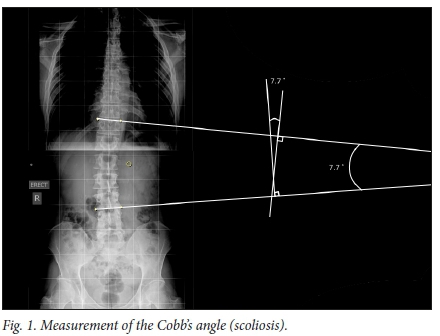
Vertebral rotation can be assessed by reviewing how far the convex pedicle has moved from the convex side of the vertebral body. This can be measured by different means, including using a special protractor (Pedriolle's torsiometer), or applying Nash and Moe's method which assesses how far the centre of the convex pedicle has moved in relation to the overall width of the vertebral body. Mehta's rib vertebra angle is another measurement option which is particularly applicable to the infantile curve.'81 The spinal balance in both the coronal and sagittal planes by means of the C7 plumb line are also assessed.[8]
Norms
Scoliosis is defined by the Scoliosis Research Society as a lateral curvature of the spine more than 10° as measured by means of the Cobb's method on a standing X-ray.[11] Values differ in literature, but generally 10° - 30°, 30° - 40° and >40° are regarded as a 'mild', 'moderate' or 'severe' scoliosis.[12,131]
Incidence
Scoliosis is by far the most common spinal deformity found in individuals with CP. The prevalence of idiopathic scoliosis in the general population is 1 - 2%. The overall prevalence of scoliosis in CP is 20%, but can vary greatly, with ranges from 14 to 91% noted in literature.[10,12,14,15] These wide ranges can be explained by the study population variations, including variations in age, CP types, neurological dysfunction severity, physical disability severity and also radiological methods used (Table 1).[10,12,16].
For example, Persson-Bunke et al.[17]reported an incidence of scoliosis (>10°) in 15% of their study-cohort of children with CP. However, when specifically examined per CP subtype, only one child with spastic hemiplegia had an increased curve, while this was reported in 14% (n=35/244) of children diagnosed with spastic diplegia. Other studies also reported an incidence of below 15% (5 - 14%) in the subgroup of children with spastic CP.[16-18] On the other hand, Madigan and Wallace,'191 who investigated 272 persons with CP (age not indicated, 20% independent ambulators) living in an institute, reported an overall incidence of 64%, with people classified as 'spastic' having the highest incidence of 69% (n=141/204) and the lowest percentage (39%) was reported in individuals with dyskinetic CP (n=9/23) (Table 1).[17]
It has been reported that in the general population scoliosis curves progress with age. Collins and Ponseti'201 conducted a long-term follow-up study with 134 ambulant adults with idiopathic scoliosis (aged 32 - 64 years). Twenty-four years after the baseline study, 69% of the cohort showed a more than 5° increase and 12% more than 25° progression in scoliosis curve. This study was followed up 10 years later by Weinstein et al. '211 who reported that 37 and 12% of the cohort showed more than 5° and 25° increase, respectively.
Progression in scoliosis curve has also been shown in adults with CP, although no such long-term follow-up studies are reported (Table 2).[14,15,22,23] Majd et al.'[15]reported a deterioration of the scoliosis curve in 18% of their cohort during an 8-year follow-up period. More specifically, they showed a progression of scoliosis with 4.4° per year (41.1° - 80.6°). Thometz and Simon[23] conducted a 16-year follow-up study and reported a progression of 1.4° per year with a baseline curve of >50°. On the other hand, Saito et al. [22]determined in their 17-year follow-up study that 85% of their cohort progressed from >40° to >60°. However, it must be emphasised that these results are all based on adults with CP who are severely spastic, mentally retarded and recruited from special centres.
Risk factors
It has been reported that the incidence of scoliosis in individuals with CP is related to a variety of factors. The chance of developing scoliosis increases with age.[17,18,24] Saito et al.[22] reported that overall 73% of their study cohort progressed after 22 years of age. Another risk for progression is the curvature at skeletal maturity.[17,22,24] Functional level is another risk factor, with an increase in incidence of scoliosis in non-ambulant individuals, or classified with higher Gross Motor Function Classification System (GMFCS) level, or lower GMFCS scores.'13,17,19- For example, Person-Bunke et al.[17] reported that there is a 50% risk of developing moderate or severe scoliosis by 18 years of age where classified with GMFCS levels IV or V. The subtype of CP has also been considered a risk factor, where Madigan and Wallace'19-and Bertoncelli et al.[13]reported a high incidence of scoliosis in participants with spasticity and quadriplegia, while other research groups did not determine a relationship with the type of CP (e.g. spastic, dyskinetic) and the incidence of scoliosis.
Selective dorsal rhizotomy (SDR) has been associated with an increased incidence of scoliosis, but despite it being common, it is rarely progressive. Spiegel et al.[25]reported a 17% incidence with a curve magnitude average of 16°, while Johnson et al.[26]reported a 24% scoliosis incidence. With a higher incidence, Steinbok et al.[27] reported 61% of their study cohort had scoliosis, 6% of whom had scoliosis of more than 35°. Golan et al.[28]reported 45% of their study cohort had a scoliosis, though only 5% had a curve magnitude more than 25°. Langerak et al.[29]reported a 57% incidence of scoliosis, of whom 50% had a curve of less than 30° and only 7% had a curve of 35°.
Treatment
The decision to offer any form of medical intervention must be based on an understanding of the natural history of a condition, that is, the outcome of the condition if left untreated. Only if the condition's status is currently unacceptable, or has an expected unacceptable outcome, and if an intervention is both likely to improve the patient's status and is tolerable to the patient, can said medical interventions be justified. Understanding the pathology, prevalence, natural history and outcomes of medical and surgical management are of major importance in our management of patients with CP.[12]
Treatment options include non-surgical and surgical options and are often complicated by other medical comorbidities. All patients should be managed with a multidisciplinary approach and specific attention should be paid during assessment to the individual's nutritional status, respiratory function, sitting and standing posture, gait, neurological function and gastrointestinal evaluation. This should be accompanied by a thorough musculoskeletal examination. [10]
Non-surgical management options include observation, custom seating, bracing and botulinum toxin injections. Indications for conservative management include a non-progressive scoliosis curve of less than 50° and as initial management options for children under 10 years, with the goal to delay until the child is older. Custom seating orthosis appears to be helpful with seating, but it is unclear whether it plays any role in either preventing or slowing down the natural course of the disease. Bracing has been shown to be beneficial in improving sitting balance and could help improve the omit of breathing, but in large it also does not appear to play a major role in preventing, or slowing down, curve progression.!101 Renshaw et al. [30] reported success, defined as less than 5° progression, in 22% of their patients managed with bracing. Terjesen et al.[3l]demonstrated that age and whether initial correction was obtained in the orthosis were the only variables that affected the progression rate.
Curve progression can result in pain, difficulty sitting and, in the more extreme cases, cardiopulmonary compromise. Progressive curve deformity of 40° to 50° in a growing patient and curve progression to more than 50° after skeletal maturity are indications for surgical intervention.[32]
Surgical management of the scoliosis aims to achieve a solid spinal fusion, with a resultant corrected, well balanced spine and level pelvis. The identifying of the patient's and his/her family's goals, as well as a risk-benefit analysis, is needed before surgery is considered. There is not much evidence that spinal fusion significantly improves function, but subjective surveys indicate that most healthcare workers assessed the patients to be more comfortable, with improved sitting balance and cosmesis.[10]
Unlike scoliosis in children, which is typically pain free, adult scoliosis can be associated with pain. This pain is often related to the thoracolumbar soft tissue strain on the convex side and facet joint degeneration on the concave side, typically treated with bolus physiotherapy and non-steroidal anti-inflammatory drugs (NSAIDs). Curve progression to more than 50° often requires surgical spinal interventions.[51
Kyphosis
The normal spine has cervical and lumbar lordotic curves, with a thoracic kyphotic curve in between (Fig. 2). This allows for equal distribution of forces across the spinal column and is referred to as a normal or positive spinal balance. Disruption of this balance will result in secondary deformities, including changes in the pelvis and lower limbs.[33]
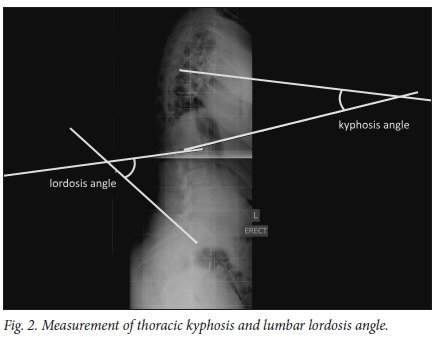
The term kyphosis describes the sagittal convexity or dorsal curvature of the normal thoracic spine. Kyphosis is often interchangeably used with hyperkyphosis, which refers to excessive curvature of the thoracic spine outside the normal range.[34,35]
Measurement
Different methods are available to calculate the degree of kyphosis. However, radiography, using Cobb's angle, is the most commonly applied method to assess sagittal spinal curves. This means that the curve is determined from the cephalad end plate of T2 (or T3 if T2 is not visible) to the caudal end plate of T12.[35] In addition to the Cobb's angle, the overall sagittal spinal balance can be determined. A plumb line can be drawn from vertebra prominence. The line should come straight down the natal cleft. If the plumb line lies to the convex side of the natal cleft, the spine is considered decompensated.[35]
It is preferable to take the X-ray in standing position,[361 though variable ways have been described in the literature. For example, Boseker et al.[37] suggested taking a standing X-ray with the arms forward, resting on a ladder or bar as the position of choice, while others reported that the patient had to stand with arms flexed and placed loosely on a support. [38]
Norms
There have been numerous studies done to define the normal range of thoracic angle, but controversy regarding the curve magnitude continues to exist (Table 3).[37,39] Stagnara et al.[401 stated that such a wide range of normal values existed that average values could not be used as normative values. Considering the varying ranges in studies, this seems to still be the case to date. Propst-Proctor and Bleck[41] reported a normal value range of 40° - 50°. Based on literature, including Bernardt and Bridwell,[381 the most commonly accepted normal range values for kyphosis is 20° - 50°. Angles of more than 50° are regarded as hyperkyphosis, while thoracic curves of 80° or thoracolumbar curves of 60° - 70° are considered severe.[42,43]
Incidence
Very little literature specifically notes the incidence and natural history of hyperkyphosis in patients with CP. It appears more commonly associated with the scoliosis curve as a kyphoscoliosis, and isolated kyphotic deformity is rare.[10,421 Madigan and Wallace[u reported a 7% incidence of hyperkyphosis in their study which reviewed 272 institutionalised individuals with CP and Lipton et al.[43]reported a 4.4% incidence of isolated hyperkyphosis in paediatric patients with CP with spinal deformities which were managed surgically. Tsirikos[42] reported that a long thoracolumbar kyphosis, resulting in a collapsing spine with positive sagittal balance, occurs relatively commonly in association with scoliosis in individuals with quadriplegic CP.
Most of the published literature regarding kyphotic deformities in CP is on spinal deformities following SDR. A multidisciplinary team from Health Quality Ontario did a literature review of short- and long-term outcomes of SDR in people with CP. They found nine studies that investigated kyphosis and lordosis following SDR, with most studies describing a hyperkyphotic curve prevalence from 2 - 17%, with only one study[27] reporting abnormal kyphosis in 41%, with 32% having worsened by 15° or more over a mean follow-up period of 4.3 years.[44,27]
Risk factors
A relationship between increased age and increased thoracic kyphosis has been reported. Increased progression has also been noted with ageing of patients with higher GMFCS levels (IV and V).[45] In addition, as previously mentioned, kyphosis is more often found concomitantly with scoliosis (kyphoscoliosis) than as an isolated deformity.[32]
Treatment
Very little literature specifically addresses the management of kyphosis in individuals with CP, mostly because it rarely occurs in isolation and, furthermore, it rarely results in significant clinical morbidity. However, significant kyphotic deformity can affect sitting ability and head control due to global sagittal spinopelvic imbalance.[7,42] Lipton et al. [43] reported a case series of 24 patients with CP with severe sagittal plane deformities which they managed surgically: 14 of these patients had a hyperkyphotic curve, two had both hyperkyphotic and hyperlordotic curves. Their indications for surgery included loss of sitting ability or balance, loss of bowel or bladder function, back pain and superior mesenteric artery syndrome unresponsive to medical treatment. They concluded that patients with CP and a severe sagittal plane deformity (>70°) could be managed surgically.
Lordosis
Lumbar lordosis describes the sagittal concavity or ventral (inward) curvature of the normal lumbar spine (Fig. 2).[35] As is the case with kyphosis, lordosis is often interchangeably used to describe hyperlordosis, which refers to an excessive lumbar lordosis outside the normal range.
Measurement
Radiography using Cobb's angle is the most commonly applied method used to assess the spinal curves for lordosis. How the X-ray is taken and the level from which the Cobb's angle is measured is variable, as mentioned for kyphosis. The most common practice is to do a standing X-ray with the patient's arms supported on a bar in front of him/her and then measuring the Cobb's angle between the superior end plate of the first lumbar vertebra and the superior end plate of the sacrum.[46-
Norms
Normal ranges for lordosis have been investigated in several studies and, as in the case of kyphosis, a wide range has been reported (Table 4).[37] Propst-Proctor and Bleck[41] reported a range of 50° - 60°. Based on literature, including the work of Bernardt and Bridwell,[38] the most commonly accepted normal range values for lordosis are 20°- 60°, while angles more than 60° are regarded as hyperlordosis.
Incidence
As with kyphosis, limited literature has been published regarding lordosis incidence and natural history in patients with CP. It rarely occurs as an isolated condition and is more commonly associated with scoliosis (lordoscoliosis), but is less common than kyphoscoliosis deformities.[42] Harada et al.[47]reviewed 84 patients with spastic diplegia ranging from 3 to 39 years of age. They reported an average lumbar lordosis curve of 54°, which is considered the upper limit of normal. Lipton et al.[43] reported an incidence of 2.6% hyperlordosis of more than 70°, and an incidence of 0.6% in both hyperkyphotic and hyperlordotic deformities. Vialle et al.[48] reported, in their study of 23 patients with spastic quadriplegia, all had hyperlordosis with curves ranging from 79° to 132°. Most of the published data are on spinal deformities after spinal interventions, such as SDR.
Risk factors
Harada et al.[47] and Steinbok et al.[27] reported a correlation between hyperlordosis and increasing age. SDR has also been associated with an increased prevalence of hyperlordosis. Studies following SDR found lordosis angles that increased over baseline ranging from 1° to 35° and the prevalence of post-operative hyperlordosis with a curve of more than 55° ranged from 21 to 50%.[44] Furthermore, young age (2 - 5 years) at time of SDR had the highest prevalence of an increase in lordosis.[29] Golan et al.[28] noted an increase in lordosis by 3.6° for every year after SDR, Johnson et al.[26] reported an increased incidence and Langerak et al.[29] noted an increased incidence of 40% in their study.
Treatment
Hyperlordosis is not generally associated with significant comorbidity and therefore rarely requires any specific treatment.[3] Hyperlordotic curves of 70°, or more, are more likely to result in bowel or bladder dysfunction, back and lower limb pain or worsening balance.[43]
Song et al. [49] reported on a case of a 14-year-old patient with severe spastic quadriplegic CP, who received a multiple-staged surgical intervention to improve the child's care and alleviate functional difficulties such as sitting posture and gastrointestinal dysfunction. In their case series of patients with CP with severe sagittal plane deformities which were managed surgically, Lipton et al.'431reported on 8 patients with a hyperkyphotic curve and 2 patients with both hyperkypotic and hyperlordotic curves. Their indications for surgery included: loss of sitting ability or balance, loss of bowel or bladder function, back pain and superior mesenteric artery syndrome unresponsive to medical treatment. They concluded that patients with CP and a severe sagittal plane deformity (>70°) could be successfully managed surgically (with posterior spinal fusion and unit rod instrumentation).
Spondylolysis and spondylolisthesis
Spondylolysis refers to a defect in, or dissolution of, the pars articularis of a vertebra (Fig. 3). The extent of the defect in the pars articularis can vary and can be viewed as a continuum ranging from stress reaction to non-union fracture with spondylolisthesis.'501 Diagnoses are made with the help of imaging, but due to its oblique orientation, imaging of the pars articularis is difficult. Some controversies surround the required radiographic workup of spondylolysis which, if all 6 views are taken, includes AP, lateral, collimated lateral, 30° AP-up and left and right oblique views. Studies have shown that the collimated lateral view is the single best view, with 84% detection, to diagnose spondylolysis.[51]
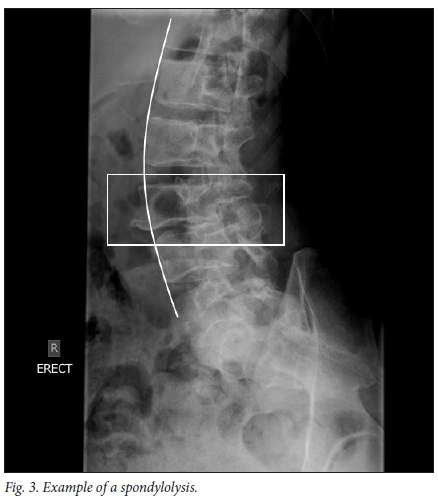
However, Auerbach et al.[52]and McTimoney et al.[53] reported that in about 20% of cases spondylolysis was only diagnosed on oblique views which are now regarded as standard practise when reviewing for spondylolysis. Oblique lumbar X-rays demonstrate the 'Scotty dog', where the superior articular recess is the ear, the pedicle the eye, the transverse process the nose, the pars articular the neck, the lamina the body and the inferior articular process the front limb. Spondylolysis is represented by a broken neck, or collar, around the dog's neck (Fig. 3). Beck et al.[54] questioned the inclusion of oblique views in standard practise in view of the additional radiation and cost. They reported no difference between 2 and 4 view studies.
Newer technologies such as bone scans, CT, single-photon emission CT (SPECT) and MRI are now often used in conjunction with radiography to help diagnose spondylolysis.[50]
Measurement
Spondylolisthesis refers to the slipping of a vertebra in relation to another vertebra. Five types of spondylolisthesis are described: dysplastic, isthmic, traumatic, degenerative and pathologic. The isthmic type is encountered in patients with CP and develops because of a pars articularis lesion. Three subclasses exist: A) due to pars articularis stress fracture; B) due to pars articularis elongation and C) due to pars articularis acute fracture.
The Meyerding classification is used to ascertain the severity, based on the percentage of translation.'551 The classification exists of 5 grades ranging from Grade I (translation up to 25%) to Grade II (translation 25-50%), Grade III (translation 51-75%), Grade IV (translation 76 - 100%) and Grade V (translation >100%).[55]
Spondylolisthesis can also be classified as stable (<50% translation) or unstable (>50% translation).[55] Radiographs include a lateral view to assess slip angle and grade, and flexion and extension views to assess for instability.[55]
Incidence
Friederickson et al.[56] reported spondylolysis in 4.4% of people at 6 years of age, which increased to 6% in general population able-bodied adults, 74% of these individuals also had spondylolisthesis. However, this study was done with X-rays (AP, oblique and lateral) taken in a supine position, which has been shown to underestimate the true incidence. In their study on the natural history of spondylolysis with a 45 year follow-up evaluation, Beutler et al. [57] also noted about 6% occurrence of spondylolysis in the general population.
Harada et al.[47] reported a 21% incidence of spondylolysis and a 4% incidence of spondylolisthesis in patients with CP and spastic diplegia. The estimated prevalence of spondylolysis of L5 in patients with CP have been reported as 21 - 30% in weight bearing adults, which is almost four times that of the general population.[5] Hennrikus et al. [58] reviewed 50 ambulatory individuals with CP and reported a 2% incidence of spondylolisthesis (Grade I, asymptomatic). They suggested that in asymptomatic, ambulatory patients with CP, routine screening for spondylolisthesis was not recommended.[5,7]
Risk factors
Age seems to be a risk factor for the development of spondylolysis. Harada et al.[47]reported no cases of spondylolysis were found in patients younger than 9 years of age, and noted an increased incidence with increased age. Further contributing factors are thought to be dystonic movements in the lumbosacral spine, specifically into extension and axial rotation, increased lumbar lordosis and increased age. Increased lordosis results in increased compressive and shear force on the posterior spinal elements. The pars articularis is particularly vulnerable to these increased mechanical stresses, and therefore at higher risk of fatigue fracture with weight bearing and ambulation, thus explaining the higher incidence found in ambulating spastic diplegic patients. [5] Rosenberg et al. [59] reported that no spondylolysis or spondylolisthesis was found radiologically in patients who had always been non-ambulatory. Furthermore, increased lumbar hyperlordosis leads to greater compression and shearing forces, which could result in spondylolisthesis. Lordosis of more than 50° has been shown to be associated with increases spondylolisthesis of L5 from 21° - 290.[7]·
SDR has been implicated in progressive spinal deformities. Spiegel et al.[25] found spondylolisthesis in 12% of their cohort, all except one was a Grade I spondylolisthesis. Johnson et al.[26]and Golan et al.[28] reported an 18 and 19% incidence of spondylolisthesis, respectively. Langerak et al.[29]reported an increased incidence of spondylolysis from 9%, after SDR, to 18%, at short-term follow-up, which further increased to 37% at long-term follow-up. In keeping with literature, spondylolysis was less commonly found in patients classified as GMFCS level III compared to patients classified as GMFCS levels I and II. The study found no increase in the incidence of spondylolisthesis.[29]
Treatment
Methods that may be helpful in the prevention of spondylolysis and spondylolisthesis include efforts to minimise significant anterior pelvic tilt in weight bearing children. In the presence of spondylolysis or spondylolisthesis, basic conservative management is usually sufficient and surgical management is only indicated in cases of failed conservative management and worsening neurology. Surgical treatment options include segmental fusion.[5]
Summary and conclusion
Overall the incidence of spinal deformities is higher in people with CP. The risk of developing spinal deformities tends to increase with aging, but little is known about how fast spinal deformities progress in people with CP and specifically during the adult aging period.[60] Risk of spinal deformities seems to be higher in people with CP who are classified as GMFCS level IV and V compared to GMFCS levels I, II and III. The minimally clinical important difference or MCID for changes in spinal curvatures is 10°,[27,28,61] which implies that only changes greater than 10° should be interpreted as a clinically meaningful change. Changes smaller than 10° can be explained by the posture of the patient while taking the X-ray,[62,63] or by the associated measurement error of determining spinal curvature angles.[61]
Declaration. None.
Acknowledgements. None.
Author contributions. EB contributed to the conception and design of the work, data acquisition, analyses, interpretation and drafting of the manuscript. NGL contributed to analyses and interpretation of the data, and critical revision of the manuscript. RPL contributed to analyses and interpretation of the data and critical revision of the manuscript. All authors approved the final version of this manuscript and accept responsibility for the accuracy and integrity of this work.
Funding. None.
Conflict of interest. None.
References
1. Little W. Lectures on the deformity of the human frame. Lancet 1843;(1):318-320. [ Links ]
2. Morris C, Baxter P, Rosenbaum P, et al. The definition and classification of cerebral palsy. Dev Med Child Neurol 2007;49(109):1-44. https://doi.org/10.1111/j.1469-8749.2007.00001.x [ Links ]
3. Osler W. The cerebral palsies of childhood. London: Lewis, 1889. [ Links ]
4. Freud S. Les diplegies cerebrales infantiles. Rev Neurolgieque 1893;(1):177-183. [ Links ]
5. Murphy KP. The adult with cerebral palsy. Orthop Clin North Am 2010;41:598-605. https://doi.org/10.1016/j.ocl.2010.06.007 [ Links ]
6. Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Prim 2016;2:15082. https://doi.org/10.1038/nrdp.2015.82 [ Links ]
7. Morrell DS, Pearson JM, Sauser DD. Progressive bone and joint abnormalities of the spine and lower extremities in cerebral palsy. Radiographics 2002;22(2):257-268. https://pubs.rsna.org/doi/10.1148/radiographics.22.2.g02mr19257 [ Links ]
8. Dickson RA. Spinal deformity-basic principles. Curr Orthop 2004;18(6):411-425. https://doi.org/10.1016/j.cuor.2004.12.001 [ Links ]
9. Shakil H, Iqbal ZA, Al-Ghadir AH. Scoliosis: Review of types of curves, etiological theories and conservative treatment. J Back Musculoskelet Rehabil 2014;27(2):111-115. https://doi.org/10.3233/BMR-130438 [ Links ]
10. McCarthy JJ, D'Andrea LP, Betz RR, Clements DH. Scoliosis in the child with cerebral palsy. J Am Acad Orthop Surg 2006;14(6):367-375. https://doi.org/10.5435/00124635-200606000-00006 [ Links ]
11. Kane WJ. Scoliosis prevalence: A call for a statement of terms. Clin Orthop Relat Res 1977;126:43-46. [ Links ]
12. Koop SE. Scoliosis in cerebral palsy. Dev Med Child Neurol 2009;51(4):92-98. http://www.ncbi.nlm.nih.gov/pubmed/19740215 [ Links ]
13. Bertoncelli C, Solla F, Loughenbury PR, Tsirikos AI, Bertoncelli D, Rampal V. Risk factors for developing scoliosis in cerebral palsy: A cross-sectional descriptive study. J Child Neurol 2017;32(7):657-663. https://doi.org/10.1177/0883073817701047 [ Links ]
14. Bonnett C, Brown JC, Grow T. Thoracolumbar scoliosis in cerebral palsy - results of surgical treatment. J Bone Joint Surg Am 1976;58(3):328-336. [ Links ]
15. Majd ME, Muldowny DS, Holt RT. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine (Phila Pa 1976) 1997;22(13):1461-1466. https://doi.org/10.1097/00007632-199707010-00007 [ Links ]
16. Balmer G., MacEwen GD. The incidence and treatment of scoliosis. J Bone Joint Surg Am Br 1970;52(1):134-137. [ Links ]
17. Persson-Bunke M, Hägglund G, Lauge-Pedersen H, Wagner P, Westbom L. Scoliosis in a total population of children with cerebral palsy. Spine (Phila Pa 1976) 2012;37(12):708-713. https://doi.org/10.1097/BRS.0b013e318246a962 [ Links ]
18. Robson P. The prevelance of scoliosis in adolescents and young adults with cerebral palsy. Dev Med Child Neurol 1968;10(4):447-452. https://doi.org/10.1111/j.1469-8749.1968.tb02917.x [ Links ]
19. Madigan RR, Wallace SL. Scoliosis in institutionalized cerebral palsy population. Spine (Phila Pa 1976) 1981;11(6):583-590. https://doi.org/10.1097/00007632-198111000-00009 [ Links ]
20. Collins DK, Ponseti I V. Long-term follow-up of patients with idiopathic scoliosis not treated surgically. J Bone Joint Surg Am 1969;51(3):425-445. [ Links ]
21. Weinstein SL, Zavala DC, Ponseti I V. Idiopathic scoliosis: Long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am 1981;63(5):702-712. [ Links ]
22. Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet 1998;351(9117):1687-1692. https://doi.org/10.1016/S0140-6736(98)01302-6 [ Links ]
23. Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am 1988;70A(9):1290-1296. [ Links ]
24. Gu Y, Shelton JE, Ketchum JM, Cifu DX, Palmer D, Sparkman A, et al. Natural history of scoliosis in nonambulatory spastic tetraplegic cerebral palsy. PMR 2011;3:27-32. https://doi.org/10.1016/j.pmrj.2010.09.015 [ Links ]
25. Spiegel D, Loder RT, Alley K, et al. Spinal deformity following selective dorsal rhizotomy. J Pediatr Orthop 2003;24(1):30-36. https://doi.org/10.1007/s00381-009-0993-5 [ Links ]
26. Johnson MB, Goldstein L, Thomas SS, Piatt J, Aiona M, Sussman M. Spinal deformity after selective dorsal rhizotomy in ambulatory patients with cerebral palsy. J Pediatr Orthop 2004;24(5):529-536. https://doi.org/10.1097/00004694-200409000-00013 [ Links ]
27. Steinbok P, Hicdonmez T, Sawatzky B, Beauchamp R, Wickenheiser D. Spinal deformities after selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg 2005;102(4):363-373. https://doi.org/10.3171/ped.2005.102.4.0363 [ Links ]
28. Golan JD, Hall JA, O'Gorman G, Poulin C, Benaroch TE, Cantin MA, et al. Spinal deformities following selective dorsal rhizotomy. J Neurosurg 2007;106(6):441-449. https://doi.org/10.3171/ped.2007.106.6.441 [ Links ]
29. Langerak NG, Vaughan CL, Hoffman EB, Figaji AA, Fieggen AG, Peter JC. Incidence of spinal abnormalities in patients with spastic diplegia 17 to 26 years after selective dorsal rhizotomy. Child's Nerv Syst 2009;25(12):1593-603. https://doi.org/10.1007/s00381-009-0993-5 [ Links ]
30. Renshaw TS, Green NE, Griffin PP, Root L. Cerebral palsy: Orthopaedic management. Instr Course Lect 1996;45:475-490. [ Links ]
31. Terjesen T, Lange JE, Steen H. Treatment of scoliosis with spinal bracing in quadriplegic cerebral palsy. Dev Med Child Neurol 2000;42:448-454. https://doi.org/10.1017/s0012162200000840 [ Links ]
32. Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet 2004;363:1619-1631. https://doi.org/10.1016/S0140-6736(04)16207-7 [ Links ]
33. Roussouly P, Nnadi C. Sagittal plane deformity: An overview of interpretation and management. Eur Spine J 2010;19(11):1824-1836. https://doi.org/10.1007/s00586-010-1476-9 [ Links ]
34. Vedantam R, Lenke LG, Keeney JA, Bridwell KH. Comparison of standing sagittal spinal alignment in asymptomatic adolescents and adults. Spine (Phila Pa 1976) 1998;23(2):211-215. https://doi.org/10.1097/00007632-200009010-00011 [ Links ]
35. Joseph SA, Moreno AP, Brandoff J, Casden AC, Kuflik P, Neuwirth MG. Sagittal plane deformity in the adult patient. J Am Acad Orthop Surg 2009;17(6):378-388. https://doi.org/10.5435/00124635-200906000-00006 [ Links ]
36. Gelb DE, Lenke LG, Bridwell KH, Blanke K, Mcenery KW. An analysis of sagittal spinal alignment in 100 asymptomatic middle and older aged volunteers. Spine (Phila Pa 1976) 1995;20(12):1351-1358. [ Links ]
37. Boseker EH, Moe JH, Winter RB, Koop SE. Determination of 'normal' thoracic kyphosis: A roentgenographic study of 121 'normal' children. J Pediatr Orthop 2000;20(6):796-798. https://doi.org/10.1097/00004694-200011000-00019 [ Links ]
38. Bernhardt M, Bridwell K. Semental analysis of the sagital plane alignmnet of the normal thoracic and lumbar spines and thoracolumbar junction. Spine (Phila Pa 1976) 1989;14:717-721. https://doiorg/10.1097/00007632-198907000-00012 [ Links ]
39. Voutsinas SA, MacEwen GD. Sagittal Profiles of the Spine. Clin Orthop Relat Res 1986;(210):235-242. [ Links ]
40. Stagnara P, de Mauroy J, Dran J, et al Reciprocal angulation of vertebral bodies in a sagital plane: Approach to reference for the evaluation of kyphosis and lordosis. 1982;17(4):335-342. https://doi.org/10.1097/00007632-198207000-00003 [ Links ]
41. Propst-Proctor S, Bleck EE. Radiographic determination of lordosis and kyphosis in normal and scoliotic children. J Pediatr Orthop 1983;3(3):344-346. [ Links ]
42. Tsirikos AI. Development and treatment of spinal deformity in patients with cerebral palsy. Indian J Orthop 2010;44(2):148-158. https://doi.org/10.4103/0019-5413.62052 [ Links ]
43. Lipton GE, Letenhoff EJ, Darbey KW, McCarthy CH. Correction of sagittal plane spinal deformities with unit rod instrumentation in children with Cerebral palsy. J bone Joint Surg 2003;85(12):2349-2358. https://doi.org/10.2106/00004623-200312000-00012 [ Links ]
44. Health Quality Ontario. Lumbosacral dorsal rhizotomy for spastic cerebral palsy: A health technology assessment. Ont Health Technol Assess Ser 2017;17(10):1-186. [ Links ]
45. Lee SY, Chung CY, Lee KM, Kwon SS, Cho KJ, Park MS. Annual changes in radiographic indices of the spine in cerebral palsy patients. Eur Spine J 2016;25(3):679-686 https://doi.org/10.1007/s00586-014-3746-4 [ Links ]
46. Been E, Kalichman L. Lumbar lordosis. Spine J 2014;14(1):87-97. https://doi.org/10.1016/j.spinee.2013.07.464 [ Links ]
47. Harada T, Ebara S, Anwar MM, et al. The lumbar spine in spastic diplegia. A radiographic study. J Bone Joint Surg Br 1993;75(4):534-537. [ Links ]
48. Vialle R, Khouri N, Glorion C, Lechevallier J, Morin C. Lumbar hyperlordosis of neuromuscular origin: Pathophysiology and surgical strategy for correction. Int Orthop 2007;31(4):513-523. https://doi.org/10.1007/s00264-006-0218-4 [ Links ]
49. Song EW, Lenke LG, Schoenecker PL. Isolated thoracolumbar and lumbar hyperlordosis in a patient with cerebral palsy. J Spinal Disord 2000;13(5):455-460. [ Links ]
50. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sport Med Rep 2007;6(1):62-66. [ Links ]
51. Amato M, Tottty WC, Gilula LA. Spondylolysis of the lumbar spine: Demonstration of defects and laminal fragmentation. Radiology 1984;153(3):627-629. https://doi.org/10.1148/radiology.1533.6494460 [ Links ]
52. Auerbach JD, Ahn J, Zgonis MH, Reddy SC, Ecker ML, Flynn JM. Streamlining the evaluation of low back pain in children. Clin Orthop Relat Res 2008;466(8):1971-1977. https://doi.org/10.1007/s11999-008-0296-2 [ Links ]
53. McTimoney M, Micheli L. Current evaluation and management of spondylolysis and spondylolisthesis. Curr Sports Med Rep 2003;2(1):41-46. [ Links ]
54. Beck NA, Miller R, Baldwin K, Zet al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am 2013;95(10):e65. https://doi.org/10.2106/JBJS.L.00824 [ Links ]
55. Hu S, Tribus C, Diab M, Ghanayem A. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am 2008;90:656-671. [ Links ]
56. Fredrickson B, Baker D, McHolck W, Yuan H, Lubicky J. The natural history of spondylosis and spondylolisthesis. J Bone Joint Surg Am. 1984;66(5):699-707. [ Links ]
57. Beuttler W, Fredrickson B, Murttland A, Sweeney C, Grant W, Baker B. The natural history of spondylolysis and spondylolisthesis. Spine (Phila Pa 1976) 2003;28:1027-1035. https://doi.org/10.1097/01.BRS.0000061992.98108.A0 [ Links ]
58. Hennrikus WL, Rosenthal RK, Kasser JR. Incidence of spondylolisthesis in ambulatory cerebral palsy patients. J Pediatr Othopaedics 1993;13:37-40. https://doi.org/10.1097/01241398-199301000-00008 [ Links ]
59. Rosenberg N, Bargar W, Friedman B. The incidence of spondylosis and spondylolisthesis in nonambulatory patients. Spine (Phila Pa 1976) 1981;6(1):36-38. https://doi.org/10.1097/00007632-198101000-00005 [ Links ]
60. Langerak NG, Britz E, Dix-Peek S, du Toit J, Fieggen AG, Lamberts RP. Incidence of spinal deformities and the relationship with physical status and back pain in ambulant adults with cerebral palsy and spastic diplegia. Eur Spine J 2020;29(6):1416-1423. https://doi.org/10.1007/s00586-019-06235-3 [ Links ]
61. Kim H, Kim HS, Moon ES, Choon-Sik Y, Chung T-S, Song HT, et al. Scoliosis imaging: What radiologists should know. Radiographics 2010;30(7):1823-1842. https://doi.org/10.1148/rg.307105061 [ Links ]
62. Roghani T, Khalkhali Zavieh M, Rahimi A, et al. The reliability of standing sagittal measurements of spinal curvature and range of motion in older women with and without hyperkyphosis using a skin-surface device. J Manipulative Physiol Ther 2017;40(9):685-691. https://doi.org/10.1016/j.jmpt.2017.07.008 [ Links ]
63. Marchetti BV, Candotti CT, Raupp EG, Oliveira EBC, Furlanetto TS, Loss JF. Accuracy of a radiological evaluation method for thoracic and lumbar spinal curvatures using spinous processes. J Manipulative Physiol Ther 2017;40(9):700-707. https://doi.org/10.1016/j.jmpt.2017.07.013 [ Links ]
 Correspondence:
Correspondence:
R P Lamberts
rplam@hotmail.com
Accepted 19 February 2020














