Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 n.8 Pretoria Aug. 2020
http://dx.doi.org/10.7196/SAMJ.2020.v110i8.14898
CORRESPONDENCE
Fine-needle aspiration cytology of head and neck masses: Is ultrasound guidance routinely warranted?
To the Editor: Fine-needle aspiration cytology (FNAC) is a simple, cheap and quick diagnostic procedure to differentiate infective from benign from malignant head and neck masses, and to direct management.[1] It also reduces surgical interventions and surgery-related morbidity and anxiety.[2]
Palpation-guided FNAC has a high sensitivity, specificity and accuracy in excess of 90%, and non-diagnostic rates are <20%.[3] However, routine use of ultrasound guidance has been advocated in FNAC of palpable masses, especially with palpable thyroid nodules.[4]
Oncology and otolaryngology trainees at some South African (SA) training hospitals no longer perform freehand, palpation-guided FNAC of masses in the head and neck, but refer patients for ultrasound-guided FNAC (USGFNAC). One of us (JJF) has also noted this to be the case in the private sector. USGFNAC adds expense, time and hospital visits for patients and hospitals and places an additional burden on radiology and pathology resources.
We question whether freehand, palpation-guided FNAC of neck masses should be advocated, especially in resource-constrained settings such as SA, and whether it should be included as an entrustable professional activity (EPA) in workplace-based assessments of trainee surgeons and oncologists.
A retrospective observational study was done to determine the non-diagnostic rate of freehand, palpation-guided FNAC with palpable head and neck masses in an SA setting. All patients who underwent FNAC for palpable head and neck masses in JJF's surgical practice from 1 January 2014 to 31 December 2018 were included. A non-aspiration technique with a 23-gauge needle was used; only when there was a negative yield of material was an aspiration technique employed. Local anaesthesia was not used. The aspirate was ejected onto a glass microscope slide, a second microscope slide was used to smear the cellular material into a monolayer of cells, and a spray fixative was applied.
Of 281 aspirates, most were sampled from cervical lymph nodes (36%) and other cervical masses (27%) (Table 1). Thirty-seven aspirates were non-diagnostic (13%). One hundred and forty-two aspirates (51%) were benign and 102 (36%) were malignant. Pleomorphic adenoma or reactive nodes were the most common benign diagnoses, and primary or metastatic squamous cell carcinoma was the most common malignant diagnosis.
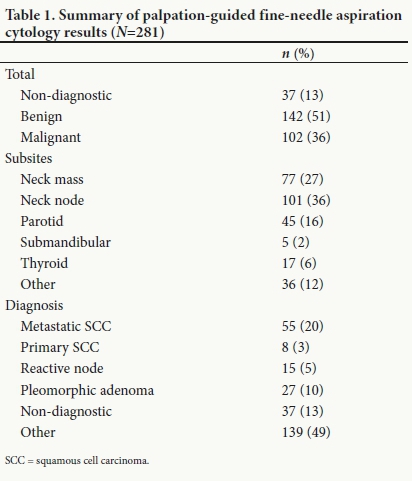
The 87% diagnostic yield of freehand palpation-guided FNAC achieved in this study is similar to that reported in a large meta-analysis by Gharib and Goellner[5] (83%). Choong et al.[3]found no statistically significant difference in non-diagnostic rates between palpation and USGFNAC when done for thyroid nodules (5.0% v. 4.5%; p=0.53).
While USGFNAC adds value for non-palpable masses, cystic masses with a small solid component and non-diagnostic palpation-guided FNAC, we believe that it should not be routinely used (unless available in the doctor's surgery) considering the high yield of freehand, palpation-guided FNAC, as well as the expense, which in the USA is four times that of palpation-guided FNAC,[3] and the training and equipment required, including maintenance and cost of the machines. [6] Added to this is the need to prioritise use of available ultrasound machines and expertise for obstetrics and trauma in developing countries.[7] USGFNAC also adds expense, time and hospital visits for both patients and hospitals, places an additional burden on radiology and pathology services, and causes diagnostic delays.
Factors other than ultrasound guidance may affect the non-diagnostic rate, such as the experience of the doctor performing the FNAC and the experience of the cytopathologist interpreting the biopsy.[8] The rate of non-diagnostic FNAC is also influenced by the number of needle passes, with 10 - 15 deemed adequate.[9] Learning and practising correct palpation-guided FNAC techniques should therefore be integral to training of oncologists and surgeons, and should form part of workplace-based assessment.
The results of this study support the use of freehand, palpation-guided FNAC of neck masses, especially in resource-constrained settings such as SA. It should be included as an EPA in workplace-based assessments of trainee surgeons and oncologists.
Gerrit Viljoen
Registrar, Division of Otorhinolaryngology, Faculty of Health Sciences, University of Cape Town, South Africa dr.gerrit.viljoen@gmail.com
Nandi Viljoen
Registrar, Division of Anatomical Pathology, Faculty of Health Sciences, University of Cape Town, South Africa
Ellen Bolding
Consultant, Division of Anatomical Pathology, PathCare, Cape Town, South Africa
Johannes J Fagan
Professor and Chair, Division of Otorhinolaryngology, Faculty of Health Sciences, University of Cape Town, South Africa
References
1. Iacob A, Zazgyva A, Ormenisan A, Mezei T, Sin A, Tilinca M. Effectiveness of fine-needle aspiration cytology in the diagnosis of lateral cervical nonthyroid tumors. Medicine (Baltimore) 2016;95(31):e4448. https://doi.org/10.1097/MD.0000000000004448 [ Links ]
2. Tandon S, Shahab R, Benton JI, Ghosh SK, Sheard J, Jones TM. Fine-needle aspiration cytology in a regional head and neck cancer center: Comparison with a systematic review and meta-analysis. Head Neck 2008;30(9):1246-1252. https://doi.org/10.1002/hed.20849 [ Links ]
3. Choong KC, Khiyami A, Tamarkin SW, McHenry CR. Fine-needle aspiration biopsy of thyroid nodules: Is routine ultrasound-guidance necessary? Surgery 2018;164(4):789-794. https://doi.org/10.1016/j.surg.2018.04.047 [ Links ]
4. Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 1998;8(1):15-21. https://doi.org/10.1089/thy.1998.8.15 [ Links ]
5. Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: An appraisal. Ann Intern Med 1993;118(4):282-289. https://doi.org/10.7326/0003-4819-118-4-199302150-00007 [ Links ]
6. Shah S, Bellows BA, Adedipe AA, Totten JE, Backlund BH, Sajed D. Perceived barriers in the use of ultrasound in developing countries. Crit Ultrasound J 2015;7:11. https://doi.org/10.1186/s13089-015-0028-2 [ Links ]
7. Sippel S, Muruganandan K, Levine A, Shah S. Review article: Use of ultrasound in the developing world. Int J Emerg Med 2011;4:72. https://doi.org/10.1186/1865-1380-4-72 [ Links ]
8. Ghofrani M, Beckman D, Rimm DL. The value of onsite adequacy assessment of thyroid fine-needle aspirations is a function of operator experience. Cancer Cytopathol 2006;108(2):110-113. https://doi.org/10.1002/cncr.21715 [ Links ]
9. Rausch P, Nowels K, Jeffrey RB Jr. Ultrasonographically guided thyroid biopsy: A review with emphasis on technique. J Ultrasound Med 2001;20(1):79-85. https://doi.org/10.7863/jum.2001.20.L79 [ Links ]














