Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 n.6 Pretoria Jun. 2020
http://dx.doi.org/10.7196/SAMJ.2020.v110i6.14357
RESEARCH
A retrospective cohort study comparing pregnancy outcomes and neonatal characteristics between HIV-infected and HIV-non-infected mothers
G GoldmanI; S BudhramII, III
IMB ChB; Discipline of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIMB BCh, MMed (O&G), FCOG (SA), FCMFM (SA), MPhil (Maternal Fetal Medicine); Discipline of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIIMB BCh, MMed (O&G), FCOG (SA), FCMFM (SA), MPhil (Maternal Fetal Medicine); Department of Maternal and Fetal Medicine, Inkosi Albert Luthuli Hospital, Durban, South Africa
ABSTRACT
BACKGROUND. South Africa (SA) has a high disease burden of HIV/AIDS. Previously, studies have shown that HIV-infected women have adverse pregnancy outcomes.
OBJECTIVES. To determine the effect of HIV infection on neonatal birthweight, head circumference, birth length and duration of pregnancy.
METHODS. This was a retrospective study, and data were obtained from the maternity records of women who delivered at Stanger Hospital, SA, from August to December 2016. Pregnancies were dated using an early ultrasound scan. Women with comorbidities that are known to affect birth anthropometry were excluded, as well as all self-reported smokers. Women were divided into HIV-infected and HIV-non-infected groups and compared.
RESULTS. Among the 392 women included in the cohort, 171 (43.6%) were HIV-infected and 221 (56.4%) were non-infected. All HIV-infected women were receiving antiretroviral therapy. There was no significant difference in neonatal birthweight, head circumference, birth length or duration of pregnancy between the groups.
CONCLUSIONS. HIV infection that has been treated does not appear to be an independent risk factor for fetal growth restriction or preterm delivery in an SA population.
The World Health Organization (WHO) estimated that in 2015, 7.1 million people in South Africa (SA) were HIV-infected; of those, 4 million were women >15 years of age.[1] HIV infection has previously been shown to increase the risk of preterm labour and small-for-gestational-age neonates,[2-4] but HIV infection per se has not been shown to directly affect fetal growth.
Investigating fetal growth potential has become significantly more important in detecting aberrant fetal growth. Preventing adverse outcomes is directly dependent on knowing and understanding the normal trajectory of fetal growth. As a developing country, normograms for fetal growth are not available and SA therefore relies on growth curves derived from developed countries, which is not ideal for our purpose. Intrauterine growth restriction is associated with a higher rate of stillbirths,[5,6] and detection of such fetuses remains a top priority in improving maternal and fetal care.
Fetal growth is complex and multifactorial; it is influenced by many known factors, e.g. under- or over-nutrition, chronic inflammation, hypertensive disorders and lifestyle.[7,8] Fetal growth, as assessed by Baschat et al.[9]in 2003, was found to be impaired in HIV-infected women compared with the general population reference curve. Women infected with HIV have underlying chronic inflammation, even with the use of antiretroviral (ARV) therapy.[10] Chronic inflammation during placentation is hypothesised to be one of the reasons for poor trophoblastic invasion, leading to poor placental perfusion and growth restriction. Conditions known to cause this pathological state, such as systemic lupus erythematosus, pre-eclampsia and diabetes mellitus, have been associated with intrauterine growth restriction.[7,11] It is therefore important do determine the effect, if any, of HIV infection on offspring birth arthropometry and on the duration of pregnancy before attempting to describe normal fetal growth in an SA population.
Methods
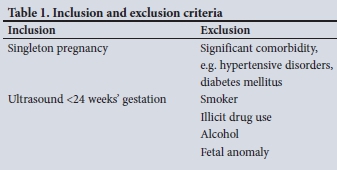
A retrospective cohort study was conducted in KwaDukuza, SA. KwaDukuza forms part of the iLembe health district and has a population of ~630 000, of whom ~53% are women. The sample population was selected from Stanger Hospital, as it is the sole provider of secondary and tertiary obstetric services for all of iLembe district and has efficient ultrasound services for routine early dating of pregnancies. Of the women who delivered at Stanger Hospital, 42% were estimated to be HIV-infected - thus providing a suitable sample of both groups. An independent two-tailed t-test was performed and assumed the following: effect size 0.3, a=0.05, power 0.80, to determine the sample size of 352. Maternity patient records for August - December 2016 were retrieved. Consecutive records of all women with an ultrasound examination at <24 weeks of gestation and of those who met the inclusion/exclusion criteria (Table 1) were selected for the cohort.
HIV infection was diagnosed on a positive rapid third-generation and confirmatory fourth-generation HIV enzyme-linked immunosorbent assay (ELISA) test. Data collected were analysed using Excel 2016 (Microsoft, USA) and SPSS version 22.0 (IBM Corp., USA) to gene-rate descriptive and inferential statistics. The statistically significant difference among groups was determined by the x2 test; Fisher's exact test and univariate analysis were fitted to assess association of the variables, i.e. pregnancy outcome with the pregnancy condition (HIV-infected and HIV-non-infected). The level of significance was set at p<0.05.
Ethical approval
Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (ref. no. BE375/17) before commencement of the study.
Results
The study included 392 women. All HIV-infected women received ARVs - 166 (97%) were on a fixed-dose combination and 5 (3%) on second-line treatment or an unknown combination of ARVs. Ninety-three (54.4%) were virally supressed (<50 copies/mL), 18 (10.5%) had a HIV viral load >1 000 copies/mL and in 60 (35%) the HIV viral load was unknown. In 169 the mean CD4+ count was 459 cells/μL. There was a significant difference in age, parity and gravidity (p<0.01) between the two groups (Table 2). There was no difference in maternal height (p=0.66) or weight (p=0.45) and therefore not in body mass index (BMI) (Table 2).
Both groups had a mean gestational age of 38 weeks at delivery and no significant difference in duration of pregnancy (p=0.26) (Table 3). There was no difference in birthweight between male and female offspring (p=0.87) (Table 4). When comparing birth anthropometry adjusted for parity, there was no significant difference in neonatal birthweight (p=0.31), head circumference (p=0.16) or birth length (p=0.09).
Discussion
In our cohort of 392 women, 171 (43.6%) were HIV-infected. This finding is in keeping with local census data, displaying a high prevalence of HIV in this population.[12] Parity and gravidity increased with increasing age. The HIV-non-infected group included younger and less parous women. The finding of younger women having a lower prevalence of HIV infection could possibly be the result of nationwide initiatives that targeted prevention of HIV infection in the youth. Alternatively, with increasing age, there could be a natural increase in sexual encounters and therefore more exposure, leading to the acquisition of HIV infection. Further investigation is therefore required to draw definitive conclusions.
Previous studies involving birth outcomes have shown a difference in birthweight between HIV-infected and HIV-non-infected women, with the former being more likely to have small-for-gestational-age (SGA) babies. However, these studies were either performed in the era before ARV treatment or tended to be skewed owing to the increased incidence of preterm labour reported in HIV-infected women.[2,3,13,14] In a study conducted in a high-income country,[2] pregnancy outcomes were compared between HIV-infected women (on ARVs) and HIV-non-infected women. It was reported that spontaneous preterm birth occurred more frequently among HIV-infected women and that they had a higher risk for delivery of an SGA baby. [2] It was further suggested that more intensive maternal and fetal surveillance is required in HIV-infected pregnant women. Of note, 25% and 7% of their HIV-infected cohort were smokers and cocaine users, respectively.[2] These are independent factors known to adversely affect pregnancy outcome and represent important confounders. Our study excluded smokers and illicit drug users and showed that, with no difference in gestational age at birth, there was no difference in birth anthropometry (Table 3). These findings are consistent with those of a recent study conducted in India,[15] a low- and middle-income country, as SA.
In the current era of widescale utilisation of ARV therapy, future prospective studies comparing the effects of untreated HIV infection would be unethical, and we can therefore assume that, based on this study, treated HIV infection does not affect fetal growth or pregnancy duration. HIV-infected pregnant women on ARVs might not require increased surveillance of their pregnancies indicated solely on their HIV status, which has important implications in resource-constrained settings. Future research into fetal growth should not discriminate against women based on their HIV status, and women can be reassured that their HIV infection, if treated, will not affect the growth of their fetus or put them at increased risk of a preterm delivery.
There was no significant difference in birthweight between male and female offspring (Table 4). This finding is most likely due to sample size, as much larger studies have demonstrated a difference in birthweight between genders.[16] Local research would need to be conducted to demonstrate a relationship.
Study strengths and limitations
The strengths of this study included a large sample size and strict exclusion criteria. The study was limited by not including sociodemographic data and by compliance and duration of ARV treatment not being assessed.
Conclusions
Women who were HIV-infected and receiving treatment did not appear to have aberrant fetal growth, as evidenced by no significant difference in birth arthropometry compared with their HIV-non-infected counterparts. In the current era of widescale ARV treatment, there appears to be no difference in fetal growth between HIV-infected and HIV-non-infected women or a difference in the duration of pregnany between these groups. In future studies of fetal growth, women who are HIV-infected should not be excluded or adjusted for in determining outcomes.
Declaration. The research for this study was done in partial fulfilment of the requirements for GG's MMed (O&G) degree at the University of KwaZulu-Natal.
Acknowledgements. We acknowledge Dr C Tiloke for assistance with the manuscript preparation.
Author contributions. GG and SB conceptualised and designed the study, and were responsible for data collection, analysis, interpretation, as well as preparation of the manuscript. Both authors read and approved the final manuscript.
Funding. None.
Conflicts of interest. None.
References
1. South Africa UNAIDS. 2017. http://www.unaids.org/en/regionscountries/countries/southafrica (accessed 26 February 2017). [ Links ]
2. Haeri S, Shauer M, Dale M, et al. Obstetric and newborn infant outcomes in human immunodeficiency virus-infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol 2009;201(3):e1-e5. https://doi.org/10.1016/j.ajog.2009.06.017 [ Links ]
3. Malaba T, Phillips T, le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 2017;46(5):1678-1689. https://doi.org/10.1093/ije/dyx136 [ Links ]
4. Xiao P, Zhou Y, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: A meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015;15(1). https://doi.org/10.1186/s12884-015-0684-z [ Links ]
5. Facchinetti F, Alberico S, Benedetto C, et al. A multicenter, case-control study on risk factors for antepartum stillbirth. J Matern Fetal Neonatal Med 2010;24(3):407-410. https://doi.org/10.3109/14767058.2010.496880 [ Links ]
6. Madhi SA, Briner C, Maswime S, et al. Causes of stillbirths among women from South Africa: A prospective, observational study. Lancet Glob Health 2019;7(4):e503-e512. https://doi.org/10.1016/s2214-109x(18)30541-2 [ Links ]
7. Allen VM, Joseph K, Murphy KE, Magee LA, Ohlsson A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: A population based study. BMC Pregnancy Childbirth 2004;4(1):17. https://doi.org/10.1186/1471-2393-4-17 [ Links ]
8. Yan J. Maternal pre-pregnancy BMI, gestational weight gain, and infant birth weight: A within-family analysis in the United States. Econ Hum Biol 2015;18:1-12. https://doi.org/10.1016/j.ehb.2015.03.002 [ Links ]
9. Baschat A, Bukowski R, Kush M, Kriebs J, Harman C. Fetal growth potential in HIV-positive patients. Am J Obstet Gynecol 2003;189(6):S217. https://doi.org/10.1016/j.ajog.2003.10.582 [ Links ]
10. Deeks S, Tracy R, Douek D. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013;39(4):633-645. https://doi.org/10.1016/j.immuni.2013.10.001 [ Links ]
11. Yasmeen S, Wilkins EE, Field NT, Sheikh RA, Gilbert WM. Pregnancy outcomes in women with systemic lupus erythematosus. J Matern Fetal Med 2001;10(2):91-96. https://doi.org/10.1080/jmf.10.2.91.96 [ Links ]
12. National Department of Health. The National HIV and Syphilis Prevalence Survey 2007. Pretoria: NDoH, 2008. [ Links ]
13. Shivamurthy G, Pukale R, Mankhani R. A prospective study of obstetric and new born outcome in a cohort of HIV affected pregnant women. J Evol Med Dent Sci 2015;4(4):514-524. https://doi.org/10.14260/jemds/2015/77 [ Links ]
14. Ellis J, Williams H, Graves W, Lindsay MK. Human immunodeficiency virus infection is a risk factor for adverse perinatal outcome. Am J Obstet Gynecol 2002;186(5):903-906. https://doi.org/10.1067/mob.2002.123407 [ Links ]
15. Patil S, Bhosale R, Sambarey P, et al. Impact of maternal human immunodeficiency virus infection on pregnancy and birth outcomes in Pune, India. AIDS Care 2011;23(12):1562-1569. https://doi.org/10.1080/09540121.2011.579948 [ Links ]
16. Roland MC, Friis CM, Lorentzen B, Bollerslev J, Haugen G, Henriksen T. Gender differences in fetal growth and fetal-placental ratio in preeclamptic and normal pregnancies. Pregnancy Hypertens 2013;3(2):95. https://doi.org/10.1016/j.preghy.2013.04.101 [ Links ]
 Correspondence:
Correspondence:
G Goldman
ggold03@gmail.com
Accepted 31 October 2019














