Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.110 no.3 Pretoria mar. 2020
http://dx.doi.org/10.7196/samj.2020.v110i3.14279
RESEARCH
Evaluation of the use of low-molecular-weight heparin for venous thromboembolism prophylaxis in medical patients
J A du PlessisI; S A van BlydensteinII; M WongIII
IMB ChB, Dip HIV Man (SA), MMed (Internal Medicine); School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCh, DCH (SA), FCP (SA), MMed (Internal Medicine), Cert Pulm (SA); Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMB BCh, DCH (SA), FCP (SA), FCCP, FRCP (Lond); Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Venous thromboembolism (VTE) complicates a significant proportion of medical admissions. As well as increasing patient morbidity, pulmonary embolism is one of the commonest preventable causes of in-hospital death. An increase in the use of pharmacological preventive measures has been advocated in recent years. South African (SA) and international guidelines have been published in an effort to promote the safe use of VTE prophylaxis.
OBJECTIVES. To describe adherence to both local and international recommendations for VTE prophylaxis in an SA hospital with regard to appropriateness of the decision to prescribe or withhold low-molecular-weight heparin (LMWH), and to observe the practice of dose adjustment in special population groups.
METHODS. This was a prospective, observational study, and data were collected from consenting adults admitted to the medical wards. We assessed the patients' VTE risk, bleeding risk and the presence of contraindications at the time of LMWH prescription as well as the dose prescribed, specifically taking into consideration adjustment for renal dysfunction and obesity.
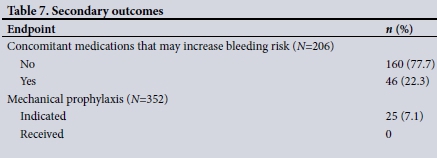
RESULTS. Three hundred and fifty-two patients were enrolled, of whom 51.4% were male and 58.5% received LMWH. Primary outcomes. The appropriate overall decision according to both SA and international guidelines was made in 254 cases (72.2%). The inappropriate decision according to both guidelines was made in 79 cases (22.4%) and the appropriate decision according to one guideline only was made in 18 cases (5.1%), while 1 case (0.3%) was not categorised. Contraindications to VTE prophylaxis were present in 35 patients (9.9%), but 9 of these patients nevertheless received LMWH. An incorrect dose was prescribed in 36 cases (17.5%), the most common reason being an inappropriate reduction in the dose in mild renal dysfunction. Secondary outcomes. Other medications that may have increased bleeding risk were prescribed in 46 patients who received LMWH (22.3%). Mechanical prophylaxis was indicated in 25 (7.1%) of the total sample; however, none received this.
CONCLUSIONS. Overall adherence to published guidelines for VTE prophylaxis has improved compared with other published reviews on the topic, but documentation of patients' VTE risk in files is poor. Overuse in low-risk patients may be an unintended consequence of the widespread advocacy of LMWH use in hospital, highlighting the importance of adequate VTE risk stratification. Incorrect dosing in special population groups is an issue that needs to be addressed, as is non-utilisation of mechanical prophylaxis methods.
Venous thromboembolism (VTE), comprising both deep-vein thrombosis (DVT) andpulmonary embolism (PE), is a complication in up to 6.7% and 4.3% of high-risk medical admissions, respectively[1,2] PE has been described as the most common preventable cause of in-hospital mortality in the USA, killing up to 300 000 patients per annum (1 - 8% of those who develop it).[3-5] Survivors experience significant morbidity in the form of post-phlebitic syndrome (40%) and chronic thromboembolic pulmonary hypertension (4%). Appropriate thromboprophylaxis reduces the risk of VTE occurrence by 45 - 63%, so its importance cannot be overemphasised.[6-8]
Hypercoagulability, venous stasis and vessel wall (endothelial) damage encompass the well-known triad of risk factors for VTE.[9,10] HIV and tuberculosis (TB) are important overlooked risk factors in the South African (SA) setting; however, their impact is not known. For various reasons, low-molecular-weight heparin (LMWH) has largely replaced unfractionated heparin for pharmacological VTE prophylaxis.[11,12] It has proved to be safe[1,13-15] and cost-effective and has been widely advocated[16-20] - a strategy that may also prove to have the unwanted consequence of overuse in low-risk patients.[21,22]
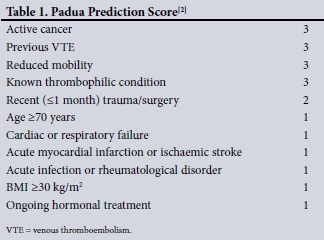
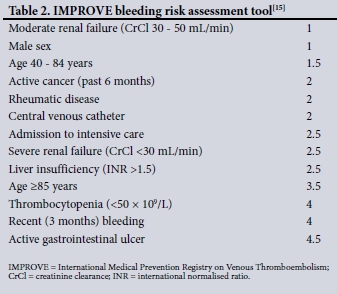
In an effort to promote the safe and appropriate use of anti-coagulants in VTE prophylaxis, both SA and the American College of Chest Physicians (ACCP) have published guidelines aimed at assisting clinicians in decision-making. These guidelines are based on VTE risk stratification and considering the individual bleeding risk. The ACCP published its latest guideline in 2012,[1] promoting the use of the Padua Prediction Score (PPS) that stratifies VTE risk[2] and the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) bleeding risk assessment tool.[15] No risk assessment models (RAMs) are promoted in the 2013 SA published guideline.[23]
Objectives
Some previously published non-SA studies recently showed an overall increase in adherence and improvement in the use of pharmacological prophylaxis for high-risk patients.[21,22,24] Our aim was to describe the use of LMWH at an SA tertiary institution based on adherence to local and ACCP guidelines. To achieve this, the primary study objectives were to evaluate: (i) the patients' VTE risk factors, quantifying them according to the PPS and the local guideline; (ii) bleeding risk according to the IMPROVE bleeding risk tool; (Hi) the presence of contraindications at the time of prescription, i.e. to comment on prescription appropriateness; and (iv) whether correct dosing adjustments were made in special populations, specifically patients with severe renal failure and obesity. Secondary objectives were: (i) to determine whether patients received other drugs at the time of LMWH prescription that could increase bleeding risk; and (ii) to assess whether mechanical preventive measures (including graduated compression stockings (GCS) and intermittent pneumatic compression (IPC) devices) were employed.
Methods
Study design and setting
This was a prospective, observational study at Chris Hani Baragwanath Academic Hospital, Johannesburg, SA, a large academic facility affiliated to the University of the Witwatersrand.
Data collection and sample selection
Data collection took place over July and August 2018 until 352 patients were enrolled. This was done in the adult medical wards by the principal investigator (JAdP). Data were collected from sequential patients in one ward until all the qualifying patients were enrolled before moving on to the next ward, alternating male and female wards. After informed consent had been obtained, all the relevant information needed to complete the RAMs (PPS and IMPROVE) and to meet the other objectives was extracted from patient files and prescription charts. Patients were only seen once at the initial interaction.
Inclusion criteria were all adult patients aged >18 years admitted to the medical wards. Exclusion criteria were patients aged < 18 years, those receiving therapeutic anticoagulation, outlying non-medical patients, and those who did not sign informed consent.
Guidelines, RAMs and special population assessment
The 2012 ACCP guideline[1] and the 2013 SA guideline and recommendations[23] were independently utilised to assess adherence. The ACCP guideline recommends the use of the PPS (Table 1) to quantify VTE risk and the IMPROVE bleeding risk tool (Table 2) to identify patients at high risk of bleeding, while excluding other contraindications. According to the PPS used by the ACCP, a score of >4 denotes a high VTE risk, and if bleeding risk is low (IMPROVE score <7) and no contraindications are present, pharmacological VTE prophylaxis is indicated. If the PPS is >4 but bleeding risk is high (IMPROVE >7) or a contraindication is present, mechanical prophylaxis is indicated until bleeding risk or VTE risk resolves. VTE prophylaxis is not indicated if the PPS is <4, as these patients are at a low risk of VTE.[2,15] The SA guideline recommends that all medically ill, bedridden patients (confined to bed or chair, or only walking to the bathroom and back) with at least one risk factor associated with a high risk of VTE should be given LMWH prophylaxis if no contraindication is present and bleeding risk is low. Alternatively, all medically ill, bedridden patients with other medical conditions, such as acute infections or acute rheumatic disease, with at least one additional associated risk factor, qualify for VTE prophylaxis.[23] The patients were allocated into eight different categories of appropriateness of prescription or omission, based on the guidelines. The SA guideline does not provide an objective way to quantify bleeding risk, so the IMPROVE score was applied for the purpose of this study.
To determine whether appropriate dosage adjustments were correctly made in special populations, patient files were checked for weight and height to determine whether the body mass index (BMI) had been calculated (kg/m2). If had not been calculated, patients were weighed and their height checked to determine the BMI. If the BMI is >35 kg/m2, dosage adjustment to 0.5 mg/kg of enoxaparin daily is recommended, with or without the addition of anti-factor Xa monitoring, rather than the standard 40 mg enoxaparin daily [1,11,23,25,26]However, specific dosing for VTE prophylaxis in obese patients is controversial and no specific dosage is recommended in either the local or the ACCP guideline owing to lack of evidence.[11,25,26] In the present study, if the BMI was >35 kg/m2 the correct dose of enoxaparin was presumed to be 0.5 mg/kg/d, rounded to the nearest commercially available pre-filled syringe formulation.
Renal function was assessed by reviewing the estimated glomerular filtration rate (eGFR) provided by the National Health Laboratory Service (NHLS) laboratory on all creatinine samples submitted to it. The reported eGFR was used instead of creatinine clearance (CrCl) for dose adjustment assessment, even though most of the sourced references refer to CrCl. This is an acceptable approach, supported by some evidence.[27] A dose adjustment to 20 - 30 mg enoxaparin daily is recommended if the CrCl is <30 mL/min according to the latest international recommendations, the South African Medicines Formulary and the local drug package insert.[11,12,14] Adjustment for renal dysfunction, although mentioned, is not specifically addressed in the SA VTE guideline.[23] Forty milligrams of enoxaparin given subcutaneously daily is regarded as the appropriate dose in patients with an eGFR >30 mL/min and a BMI <35 kg/m2.[1,14]
Statistical analysis
Categorical variables including risk factors and dosing were described using proportions, horizontal bar graphs and pie charts. Continuous variables (such as age, weight, height, BMI, PPS) were described using means and standard deviations (SDs). Student's f-test for comparison of means was used to compare gender differences in age, weight, height, BMI and PPS. Pearsons x2test was used to explore associations between categorical variables including SA and international guidelines as well as IMPROVE scores and LMWH prescriptions. Pearsons correlation coefficient was used to explore associations between age and weight. All analysis was done using Excel 2013 (Microsoft, USA) and Stata 15 (StataCorp, USA).
Results
Patient characteristics
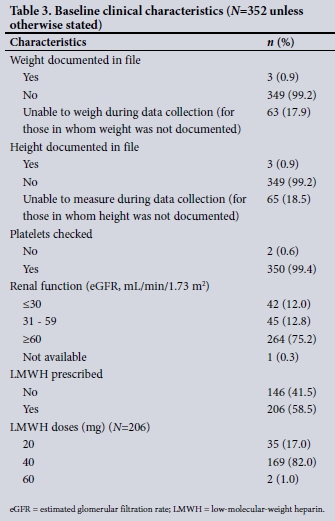
After a total of 596 files were assessed, 352 patients were enrolled (51.4% males). Of the 244 excluded, 8 refused consent. In 132 patients, consent was not obtainable owing to a reduced level of consciousness, confusion or inability to understand the consent process. Fourteen patients were < 18 years of age and 90 patients were receiving therapeutic anticoagulation. No patient withdrew consent after consenting, so all 352 cases were analysed. Table 3 summarises some of the baseline clinical characteristics. The HIV prevalence was 39.2%, and 48 patients (13.6%) were receiving TB treatment.
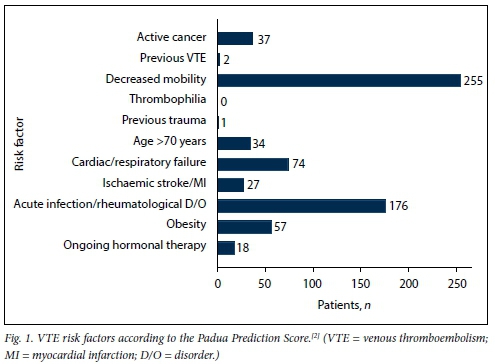
Age was normally distributed, with a mean (SD) of 48.54 (15.35) years for admitted medical patients. There were no significant differences in mean age between males (48.83 (14.38) years) and females (48.22 (16.34) years) (p=0.712). Mean weight was 65.74 (16.54) kg for male patients and 68.36 (20.26) kg for female patients (p=0.230). However, female patients had a significantly higher mean BMI than male patients, 26.34 (7.62) kg/m2 v. 22.13 (5.41) kg/m2, respectively (p<0.001). There was no correlation between age and weight (correlation coefficient R=0.115). Fig. 1 shows the VTE risk factors in the study population according to the PPS, while Figs 2 and 3 summarise the high and other risk factors, respectively, in the study population according to the SA guideline. Fig. 4 indicates the bleeding risk factors according to the IMPROVE score.
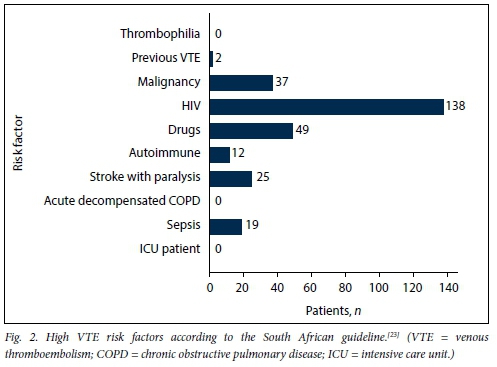
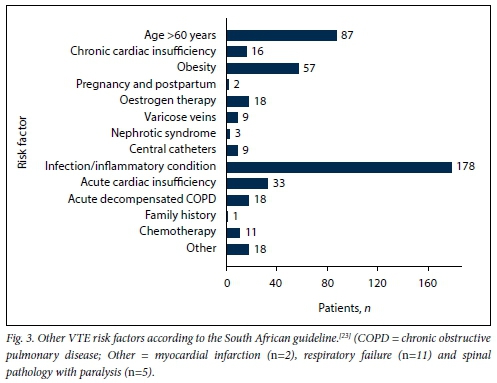
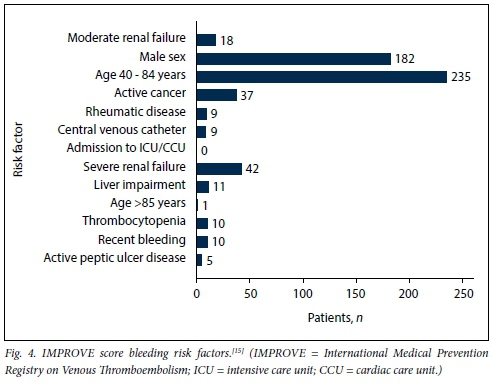
Even though a formal IMPROVE score was not documented in any patient file, clinicians were significantly less likely to prescribe LMWH for patients with a high risk of bleeding (p=0.013). However, 6 (31.6%) of the 19 patients with a high risk of bleeding received LMWH.
Primary and secondary outcomes
Table 4 summarises the primary outcomes, while Tables 5 and 6 describe the eight categories of prescription/omission appropriateness and global clinician decision, respectively. The secondary outcomes are described in Table 7. The mean (SD) PPS was 3.62 (1.90) for males and 3.58 (2.01) for females (p=0.848). There was a strong correlation between the SA and ACCP VTE prophylaxis guidelines, with a 93.1% agreement on LMWH omission and a 94.5% agreement on LMWH prescription (p<0.001). Of the patients, 46 were prescribed medications that potentially increase bleeding risk when given concomitantly with LMWH: these were aspirin (n=38 patients), aspirin and clopidogrel (n=4), warfarin (n=1) and non-steroidal anti-inflammatory drugs (n=3). There was no significant difference in prescription of these medications between patients who were receiving LMWH and those who were not (p=0.066, p=0.621, p=0.585 and p=0.669, respectively, for the abovementioned medications). A total of 52 contraindications to LMWH use were present in 35 patients: IMPROVE score >7 (n=19), international normalised ratio (INR) >1.5 (n=11), thrombocytopenia (n=10), cerebral haemorrhage (n=5), active peptic ulcer disease (n=5), malignant hypertension (n=1) and dissecting aortic aneurysm (n=1). An INR result was not available in 271 (77.0%) of all patients in calculating the IMPROVE score.
A total of 351 of the 352 (99.7%) patients could be allocated to a category, while 1 was excluded because crucial information was not available.
Three patients had a contraindication present at the time of prescription of VTE prophylaxis other than an IMPROVE score of >7: thrombocytopenia (<50 x 10VL) (n=1), coagulopathy with an INR >1.5 without oral anticoagulation (n=1), and hypertensive crisis (n=1).
Discussion
Evaluating the use of anticoagulants and adherence to guidelines are both important to optimise patient care and limit adverse events when VTE prophylaxis is considered. To our knowledge, this is the first SA study to evaluate the use of LMWH for VTE prophylaxis. The study showed 72.4% overall compliance with both a local and an international guideline (prescription and omission of LMWH) and 77.3% compliance with at least one of these guidelines. Dosing was considered separately and was not included in the assessment of prescription appropriateness.
Bateman et al.[28] found that improved adherence to VTE prevention guidelines resulted in an increased rate of VTE risk documentation, rising from 51.5% in 2009 to 71.2% in 2010 (p<0.001), while another before-after observational study, published in 2005 by Scaglione et al.,[24]showed that adherence to VTE prophylaxis guidelines improved prescription in high-risk medical patients from 25% to 41.7%, a significant increase (p=0.0075). In our study, 236 patients were at high risk of VTE according to the ACCP guideline (PPS >4), and 173 (73.3%) of those patients received LMWH. Of the 231 patients who were at high risk according to the SA guideline, 169 (73.2%) received LMWH. The prescription in high-risk VTE patients at this institution is markedly better than the results of Scaglione et al.;[24] however, the National Institute for Health and Clinical Excellence (NICE) guidelines were employed at their institution.[24]
With regard to the overuse of LMWH in low-risk patients, a concern raised in a recent review by Flanders et al.[29]the trend towards improving pharmacological prophylaxis for VTE has also led to an increase in inappropriate prescription in low-risk patients. According to this database review of 44 775 patients, 57.1% of low-risk patients received pharmacological prophylaxis inappropriately[29] This figure was significantly higher than in a smaller cohort study of 80 patients, where 28.8% of patients received LMWH inappropriately based on the same risk predictive score (PPS).[22] Similarly, in our study a total of 37 (30.6%) of the 121 patients at low risk of VTE according to the SA guideline, and a total of 33 (28.5%) of those at low risk according to the PPS, received LMWH. A total of 28/108 (25.9%) who were at low risk of VTE according to both guidelines inappropriately received LMWH.
We also looked at the prescription of LMWH in patients who had a contraindication to administration of the drug. AlHajri and Gebran[22] reported a prevalence of 13.75% in their cohort, but this was proportional to the total of 80 of their patients who received LMWH (both low-and high-risk patients were included). This proportion was similar to those described by Bateman et al.[28]in 2009 and 2010 (14% and 15%, respectively). Flanders et al.[29]reported in their analysis that 12.3% of high VTE risk patients who had a contraindication to VTE prophylaxis nevertheless received it. In our analysis, 24 patients were at high VTE risk (according to both guidelines) and also had a contraindication, and 9 (37.5%) of these patients were prescribed LMWH. Compared with AlHajri and Gebran's[22] and Bateman et al.'s[28] studies, significantly fewer (n=9, 4.4%) of the total number of patients who received LMWH in the present study had a contraindication at the time of prescription.
Underuse of LMWH in high-risk patients has decreased in recent years as a result of widespread advocacy of pharmacological prophylaxis.[30] In the review by Flanders et al.[29] 22% of high-risk patients did not receive LMWH despite no contraindication being present. Similarly, in our study LMWH was inappropriately omitted in 42 (18.8%) of 223 patients who were at high VTE risk according to both guidelines.
An INR was not available in 271 (77.0%) of all patients, and this could have biased the IMPROVE scores. We used multiple imputation to impute missing INR results and calculated a contrived IMPROVE score. There was a 99% agreement on assignment to low bleeding risk, and a 100% agreement on assignment to high bleeding risk. These agreement values confirmed that in our study, although the INR was missing in most cases, the score calculated even without an INR result did not significantly bias our results. As most patients had a low-risk score, the addition of the extra points for a deranged INR did not change the score from <7 to >7. Since an INR is not clinically indicated in most patients, it is unlikely that it would be deranged if the clinician felt it unnecessary to measure the INR. We cannot advocate routine testing for the purposes of calculating an IMPROVE score, particularly in resource-limited settings.
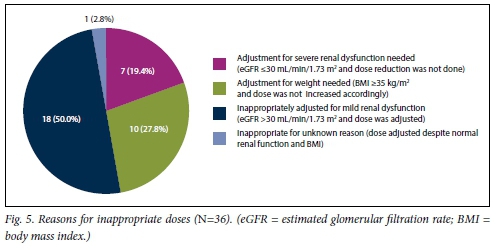
Appropriate dosing is of the utmost importance, and both over- and underdosing is inappropriate, with the concerns being increased risk of bleeding and subtherapeutic anti-factor Xa activity, respectively. In considering dosages, morbidly obese patients and those with severe renal dysfunction have generally been under-represented in clinical trials. [11,12,25,26] The concern in obese patients is that altered pharmacokinetics may lead to reduced heparin activity[11,25,26] LMWH is excreted renally, and pharmacokinetic studies have shown a significantly increased exposure to anti-factor Xa activity with a CrCl <30 mL/min, indicating the need for a dose adjustment.[11,12,14] In the present study, 36 (17.5%) of patients who received LMWH received an incorrect dose. In comparison, in two other trials 28.7% and 40% of patients, respectively, were reported to have received an incorrect dose.[22,31] In 2 cases, no comment was made regarding the appropriateness of the dose of enoxaparin. In both cases, the BMI was >35 kg/m2 (weight 117 kg and 110 kg, respectively), and the GFR was <30 mL/min. One patient received 20 mg enoxaparin and the other 60 mg. We consider that in a setting where numerous reasons for altered pharmacokinetics exist, anti-factor Xa activity should ideally be measured to guide dosing. The reasons for inappropriate dosing are shown in Fig. 5.
Documentation of patients' weight and height was poor, with >99% of patients not having a documented weight or height in the file, in no case with a recorded reason. During our assessment, weight and height was obtainable in >80% of patients, the remainder being too ill to stand upright and unassisted on a scale.
ACCP guidelines make a weak recommendation (grade 2C) for the use of mechanical methods in patients at high risk of VTE and where pharmacological methods are contraindicated or the patient has a significant bleeding risk.[1] According to these criteria, 25 patients in this study qualified for mechanical prophylaxis; however, none received it. The use of mechanical prophylaxis by IPC in a resource-limited setting is inherently challenging. Use of IPC also further limits patient mobilisation, and these devices always need to be in working order. The use of GCS, however, is an alternative that still needs to be explored in our setting.
One of the major drawbacks of the IMPROVE score is that it does not include the use of other medications that may potentially increase bleeding risk.[15] Forty-six of our patients had concomitantly prescribed LMWH and other medications, although we did not assess the effect this had on bleeding events.
Study limitations
The main weakness of this study was that no follow-up was done, so we were not able to assess clinical outcomes. Also, a key population was under-represented: those who were too ill to sign consent. Precise dosing was difficult, especially in obese patients, as pre-filled, fixed-dose syringes were the only formulation available. The duration of adequate prophylaxis was also not assessed in this study. However, the study provides useful data, adds valuable information to the literature topic, and highlights the importance of awareness and adherence to available guidelines.
Conclusions
Various factors account for the inappropriate use of LMWH for VTE prophylaxis. These include lack of adherence to and poor implementation of available guidelines/ recommendations, incorrect dosing in special population groups, and failure to actively exclude contraindications to the prescription of LMWH. At our institution there was 72.4% compliance with both local and ACCP guidelines and 77.3% compliance with at least one of the guidelines, a superior outcome compared with other studies. Various RAMs are available to assist clinicians in decision-making (including the PPS and IMPROVE), but this study has shown that there is no difference between the use of a comprehensive guideline that does not use any RAMs (SA VTE guideline) and a guideline that advocates the PPS (ACCP VTE guideline) in VTE risk stratification. The concern regarding overuse of VTE prophylaxis in low-risk patients was supported by our study, and was a greater problem than LMWH underuse in high-risk patients. Low-risk patients need to be identified more consistently to avoid unnecessary risk and cost.
We recommend that, wherever possible, patients' BMI should be determined on admission to identify those for whom the dose of LMWH requires adjustment. The NHLS also provides a readily available eGFR whenever renal function is assessed by measurement of serum creatinine levels. We recommend that this be used to avoid incorrect dosing in severe renal dysfunction, as well as to ensure correct dosing in patients with mild renal impairment. The use of GCS is an option for mechanical prophylaxis that needs further investigation into its feasibility in a resource-limited environment. Further local studies are needed to assess the impact of implementation of our recommendations as well as the reduction of adverse outcomes due to inappropriate prescription or omission of VTE prophylaxis.
Declaration. This research report was part of the requirements for JAdP's MMed degree at the University of the Witwatersrand.
Acknowledgements. The authors thank the University of the Witwatersrand for conferring this original research project for JAdP's MMed degree, the Department of Internal Medicine at Chris Hani Baragwanath Academic Hospital for allowing and supporting the research project, and the internal examiner as Wits and external examiners appointed by Wits for their suggestions.
Author contributions. JAdP: protocol development, data collection, data processing and article write up. SAvB and MW: acted as first and second supervisors for this dissertation. Both supervisors reviewed and guided this research project from initiation to acceptance for the MMed degree.
Funding. None.
Conflicts of interest. None.
References
1. Kahn SR, Lim W, Dunn AS, et al Prevention of VTE in nonsurgical patients. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl 1):195-226. https://doi.org/10.1378/chest.11-2296 [ Links ]
2. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism. The Padua Prediction Score. J Thromb Haemost 2010;8(11):2450-2457. https://doi.org/10.1111/j.1538-7836.2010.04044.X [ Links ]
3. Anderson FA jr, Zayaruzny M, Heit JA, Fidan D, Cohen AT. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol 2007;82(9):777-782. https://doi.org/10.1002/ajh.20983 [ Links ]
4. Cohen AT, Alikhan R Arcelus JI, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost 2005;94(4):750-759. [ Links ]
5. Prandoni P. Prevention and treatment of venous thromboembolism wih low-molecular-weight heparins. Clinical implications of the recent European guidelines. Thromb J 2008;6:13. https://doi.org/10.1186/1477-9560-6-13 [ Links ]
6. Scarvelis D, Wells PS. Diagnosis and treatment of deep-vein thrombosis. CMAJ 2006;175(9):1087-1092. https://doi.org/10.1503/cmaj.060366 [ Links ]
7. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350(22):2257-2264. https://doi.org/10.1056/NEJMoa032274 [ Links ]
8. Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med 2004; 164(1):17-26. https://doi.org/10.1001/archinte.164.1.17 [ Links ]
9. Anderson AF jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 suppl 1):19-116. https://doi.org/10.1161/01.CIR.0000078469.07362.E6 [ Links ]
10. Anderson FA jr, Wheeler HE. Physician practices in the management of venous thromboembolism. A community-wide survey J Vase Surg 1992;16(5):707-714. https://doi.org/10.1016/0741-5214(92)90225-W [ Links ]
11. Michota F, Merli G. Anticoagulation in special patient populations. Are special dosing considerations required? Cleve Clin J Med 2005;72(1):37-42. https://wwwmdedge.com/ccjm/article/94425/anticoagulation-special-patient-populations-are-special-dosing-considerations (accessed 26 January 2020). [ Links ]
12. Wei MY, Ward SM. The anti-factor Xa range for low molecular weight heparin thromboprophylaxis. Hematol Rep 2015;7(4):5844. https://doi.org/10.4081/hr.2015.5844 [ Links ]
13. Wilbur K, Lynd LD, Sadatsafavi M. Low-molecular-weight heparin versus unfractioned heparin for prophylaxis of venous thromboembolism in medicine patients - a pharmaeconomic analysis. Clin Appl Thromb Hemost 2011;17(5):454-465. https://doi.org/10.1177/1076029610376935 [ Links ]
14. Rossiter D, Barnes K, Decloedt E, et al., eds. South African Medicines Formulary. 10th ed. Cape Town. Health and Medical Publishing Group, 2012. [ Links ]
15. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients. Findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007;132(3):936-945. https://doi.org/10.1378/chest.06-2993 [ Links ]
16. Wadhera R, Piazza G. Cost-effectiveness of LMWH vs. UFH for VTE prophylaxis in critically ill patients. Expert Analysis. American College of Cardiology, 16 December 2014. https://www.acc.org/latest-in-cardiology/articles/2014/12/16/09/55/cost-effectiveness-of-lmwh-vs-ufh-for-vte-prophylaxis-in-critically-ill-patients (accessed 24 January 2020). [ Links ]
17. Gould MK, Dimbitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis-. A cost effectiveness analysis. Ann Intern Med 1999;130(10):789-799. https://doi.org/10.7326/0003-4819-130-10-199905180-00002 [ Links ]
18. Fowler RA, Mittmann N, Geerts W, et al. Cost-effectiveness of dalteparin vs unfractionated heparin for the prevention of venous thromboembolism in critically ill patients. JAMA 2014;312(20):2135-2145. https://doi.org/10.1001/jama.2014.15101 [ Links ]
19. Thirugnanam S, Pinto R, Cook DJ, Geerts WH, Fowler RA. Economic analyses of venous th rombo em bolism prevention strategies in hospitalized patients. A systematic review. Crit Care 2012;16(2):R43. https://doi.org/10.1186/cc11241 [ Links ]
20. Schádlich PK, Kentsch M, Weber M, et aL Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients. Modelling study from the hospital perspective in Germany. Pharmaco economics 2006;24(6):571-591. https://doi.org/10.2165/00019053-200624060-00005 [ Links ]
21. Grant PJ, Conlon A, Chopra V, Flanders SA. Less is more. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022 [ Links ]
22. AlHajri L, Gebran N. The use of low molecular weight heparin for venous thromboembolism prophylaxis in medical patients. How much are we adherent to the guidelines? Open J Int Med 2015;5(4):81-91. https://doi.org/10.4236/ojim.2015.54012 [ Links ]
23. Jacobson BF, LouwS, Bulier H, et aJ. Venous th rombo em bolism. Prophylactic and therapeutic practice guideline. S Afr Med J 2013;103(4):261-267. https://doi.org/10.7196/SAMJ.6706 [ Links ]
24. Scaglione L, Piobbici M, Pagano E, Ballini L, Tamponi G, Ciccone G. Implementing guidelines for venous thromboembolism prophylaxis in a large Italian teaching hospital Lights and shadows. Haematologica 2005;90(5):678-684. http://www.haematologica.org/content/90/5/678.long (accessed 6 February 2020). [ Links ]
25. Freeman AL, Pendleton RC, Rondina MT. Prevention of venous thromboembolism in obesity. Expert Rev Cardiovasc Ther 2010;8(12):1711-1721. https://doi.org/10.1586/erc.10.160 [ Links ]
26. Rondina MT, Wheeler M, Rodgers GM, Draper L, Pendleton RC. Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-ill patients. Thromb Res 2010;125(3):220-223. https://doi.org/10.1016/j.thromres.2009.02.003 [ Links ]
27. Stevens LA, Nolin TD, Richardson MM, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis 2009;54(1):33-42. https://doi.org/10.1053/j.ajkd.2009.03.008 [ Links ]
28. Bateman AG, Sheaff R, Child S, et al. The implementation of NICE guidance on venous thromboembolism risk assessment and prophylaxis. A before-after observational study to assess the impact on patient safety across four hospitals in England. BMC Health Serv Res 2013;13:203. https://doi.org/10.1186/1472-6963-13-203 [ Links ]
29. Flanders SA, Greene M, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism. A cohort study. JAMA Intern Med 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384 [ Links ]
30. Cohen AT, Tapson VF, Bergman JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study). A multinational cross-sectional study. Lancet 2003;371(9610):387-394. https://doi.org/10.1016/S0140-6736(08)60202-0 [ Links ]
31. Pugh MJ, Fincke BG, Bierman AS, et aL Potentially inappropriate prescribing in elderly veterans. Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc 2005;53(8):1282-1289. https://doi.org/10.1111/j.1532-5415.2005.53402.X [ Links ]
 Correspondence:
Correspondence:
J A du Plessis
jadup89@yahoo.com
Accepted 23 August 2019














