Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 n.12 Pretoria Dec. 2019
http://dx.doi.org/10.7196/samj.2019.v109i12.13893
RESEARCH
Prevalence of low serum testosterone levels among men with type 2 diabetes mellitus attending two outpatient diabetes clinics in KwaZulu-Natal Province, South Africa
I M ParukI; F J PirieII;N M NkwanyanaIII;A A MotalaII
IMB BCh, FCP (SA), Cert Endocrinol Metab (SA), MMedSci; Department of Diabetes and Endocrinology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIMB BCh, FCP (SA), PhD: Department of Diabetes and Endocrinology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIIBSc, BSc Hons, MSc, PhD; A A; Department of Public Health Medicine, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. The reported prevalence of low testosterone among men with type 2 diabetes mellitus (T2DM) is high. However, there is a dearth of information on the prevalence of androgen deficiency symptoms and low serum testosterone levels in men with T2DM from sub-Saharan Africa. Scanty data are available from Nigeria, Ghana and South Africa (SA).
OBJECTIVES. To determine the prevalence of low serum testosterone and associated risk factors and the prevalence of androgen deficiency symptoms in men with T2DM.
METHODS. In a cross-sectional observational study, androgen deficiency symptoms in men with T2DM attending two outpatient diabetes clinics in Durban, KwaZulu-Natal Province, SA, were assessed using the Ageing Males' Symptoms Scale (AMS) questionnaire and direct enquiry. Serum total testosterone (TT), sex hormone-binding globulin (SHBG), luteinising hormone (LH), fructosamine, serum lipids and glycated haemoglobin (HbAlc) were measured and free testosterone (FT) was calculated. TT, SHBG and FT levels were measured in control subjects with no history of diabetes.
RESULTS. There were 148 men with T2DM in the study group and 50 control subjects in the control group. In the study group, the majority were black Africans (58.8%); Indians (39.2%) and whites (2.0%) constituted the remainder. The mean (standard deviation (SD)) age was 57.5 (11.2) years, the mean duration of diabetes 11.4 (8.9) years and the mean HbAlc 8.6% (1.9%). Of the study group, 85.8% had metabolic syndrome. Mean TT, SHBG and FT and median LH (interquartile range) in the study group were within normal ranges. However, mean (SD) serum TT and FT were lower in the study group than in the control subjects (14.5 (5.8) v. 18.8 (7.2) nmol/L;p<0.001 and 265.9 (90.4) v. 351.7 (127.3) pmol/L; p<0.001, respectively). The prevalence of low serum total testosterone (LSTT) and low serum free testosterone (LSFT) in the study group was 35.8% and 16.2%, respectively. The prevalence of androgen deficiency symptoms using the AMS questionnaire was 74.5% and correlated poorly with LSTT or LSFT. In multivariate analysis, LSFT was significantly associated with age (odds ratio (OR) 1.05, 95% confidence interval (CI) 1.02 - 1.218; p=0.043) and waist circumference (WC) (OR 1.033, 95% CI 0.999 - 1.068; p=0.059). LSTT was associated with body mass index (BMI) only (OR 1.138, 95% CI 1.063 - 1.218; p<0.0001). TT correlated inversely with BMI, WC and the number of metabolic syndrome criteria. FT correlated inversely with BMI, WC and WHR.
CONCLUSIONS. There was a high prevalence of LSTT, LSFT and androgen deficiency symptoms in this study. Serum TT and FT were lower in men with T2DM than in control subjects. Risk factors associated with LSFT or LSTT included higher BMI and WC and older age. The AMS score was a poor predictor of low testosterone. More research is required locally before any screening policy can be recommended.
Male androgen deficiency is an international health-related issue that has received increased attention over the past two decades, with considerable epidemiological evidence demonstrating a decline in testosterone levels with ageing.[1] The prevalence of androgen deficiency in middle-aged and older men was found to be between 6% and 12% in the Massachusetts Male Aging Study[2] Testosterone deficiency has been associated with a plethora of disorders including type 2 diabetes mellitus (T2DM), obesity, cardiovascular disease and the metabolic syndrome. The reported prevalence of low testosterone levels among men with T2DM is high and ranges between 30% and 50%.[3,4] The reason for the association between T2DM and low testosterone remains uncertain, but it has been postulated that a hypothalamic or pituitary cause is likely, given the predominant finding of normal or low luteinising hormone (LH) levels with low testosterone levels.[4] Evidence from meta-analyses has shown that T2DM is associated with lower total testosterone (TT) levels after adjusting for age and body mass index (BMI)[5] Despite the available evidence, current international diabetes guidelines do not advocate routine screening for testosterone levels in male patients with diabetes.
When assessing testosterone status, international guidelines advocate measuring TT, but recommend calculating free testosterone (FT) levels in patients with TT levels near the lower limit of the normal range or those in whom alterations of sex hormone-binding globulin (SHBG) are suspected.[3] A diagnosis of hypogonadism requires an unequivocally low testosterone level in conjunction with typical signs or symptoms of hypogonadism. A number of screening tools in the form of self-administered questionnaires have been developed, including the Ageing Males' Symptom Scale (AMS) questionnaire, Massachusetts Male Aging Study questionnaire and Androgen Deficiency in the Ageing Male (ADAM) questionnaire.[6] However, none of these questionnaires have been validated in black African men.
There is a dearth of information on the prevalence of androgen deficiency symptoms and low serum testosterone levels in men with T2DM from sub-Saharan Africa (SSA). To date published data are available only from three countries, Nigeria, Ghana and South Africa (SA) (Table 1).[7-13] These studies report a high prevalence of low serum testosterone, from 29.5% to 50%. However, only a few studies have investigated the risk factors associated with low testosterone.[7,810,11]
Objectives
To determine the prevalence of and risk factors associated with low serum testosterone levels in men with T2DM attending two outpatient diabetes clinics in KwaZulu-Natal Province, SA.
Methods
This was a cross-sectional study undertaken at two hospitals in Durban. All males aged >18 years with a diagnosis of T2DM according to the World Health Organization definition[14] who were attending the adult diabetes clinic at Inkosi Albert Luthuli Central Hospital (IALCH) or the medical outpatient diabetes clinic at Prince Mshiyeni Memorial Hospital (PMMH) were invited to participate. Patients with diabetes classified as other than type 2 and those with disorders of sexual development were excluded. Healthy adult men employed at IALCH and PMMH or relatives of patients attending the diabetes clinic were recruited as control subjects. Control subjects were excluded if they had a history of glucose intolerance, low testosterone or disorder of sexual development. Informed consent was obtained from all subjects.
At study entry, a detailed history was obtained from all study subjects by questionnaire and patient folder review. Information collected included demographic details, symptoms of androgen deficiency, smoking history, current drug therapy, past medical history, and microvascular or macrovascular diabetes complications. Symptoms of androgen deficiency were assessed via direct patient enquiry (change in growth of facial hair/beard growth; loss of libido: erectile dysfunction) and with the AMS questionnaire.[6] The AMS questionnaire includes three subcategories consisting of a defined set of items, and each question is rated on a scale of 1 - 5 points. Patients with an AMS score of <27 were classified as having few or no symptoms. A score of 27 - 36 was graded as mild symptoms, 37 - 49 as moderate and >50 as severe. Anthropometric measurements (weight height, and waist and hip circumference) and blood pressure were recorded on the day of serum testosterone sample collection.
Laboratory tests performed on all study subjects included serum TT, SHBG, LH, serum lipids (Advia 1800 Clinical Chemistry System; Siemens, Germany), glycated haemoglobin (HbAlc) (G8 HPLC Analyser; Tosoh Bioscience, USA) and serum fructo-samine (Dimension EXL 200 Integrated Chemistry System: Siemens, Germany). FT was calculated using the Vermeulen equation. Venous blood samples were collected between 08h00 and lOhOO from a forearm vein. Specimens for LH, SHBG and TT were centrifuged and the plasma was stored at -70°C until analysed as a single batch.
Low serum total testosterone (LSTT) was defined as a measured serum TT <12.0 nmol/L.[15] Low serum free testosterone (LSFT) was defined as a calculated FT <180 pmol/L (reference range 180 -739 pmol/L). Using FT as the criterion, hypogonadism was defined as LSFT < 180 pmol/L and an AMS score >27. Using TT as the criterion, hypogonadism was defined as LSTT <12 nmol/L[15] and an AMS score >27. Overt hypogonadism was defined as TT <8 nmol/L and an AMS score >27. LH levels between 3.8 mlU/L and 7.3 mlU/L were classified as normal, levels <3.8 mlU/L as low and levels >7.3 mlU/L as high. Metabolic syndrome was defined by the criteria of the Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention.[16]
The sample size was calculated using an open-source calculator (OpenEpi, version 3; Open Source Epidemiologic Statistics for Public Health, www.OpenEpi.com). The study aimed to recruit an equal number of cases and controls and with power set at 80%, a hypothetical proportion of controls with exposure at 10% and a hypothetical proportion of cases with exposure at 33% (using data from the literature), the required number of cases was 49 and that of control subjects 49. The study was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (ref. no. BF 225/13) and the KwaZulu-Natal Department of Health (ref. no. HRKM 322/13).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 23 (IBM, USA) was used for data analysis. Descriptive analysis of the data was performed (means, standard deviations (SDs), ranges, frequencies and percentages). Independent f-tests were used to compare continuous normal variables between binary groups. Pearsons χ2 tests were used to compare categorical variables between groups. Missing continuous data were replaced by the series mean. Univariate logistic regression analysis was used to screen all risk factors at the 0.1 level of significance for inclusion into a multiple logistic regression model. Once predictors were chosen, they were entered into a backwards stepwise model with entry and exit probabilities set at 0.05 and 0.1, respectively.
Results
The total study population for analysis comprised 148 male subjects with T2DM (study group) and 50 control subjects (control group), recruited between January 2014 and March 2015. Of 157 patients who consented to participate in the study, 97 were recruited at IALCH and 60 at PMMH; 9 were excluded because of known hypothalamic or pituitary disease. Table 2 shows the characteristics of the study group. The mean (SD) age was 57.5 (11.2) years. The majority were black African (58.8%, n=87), followed by Indian (39.2%, n=58); white patients were in the minority, constituting only 2.0% (n=3) of the study population. The mean (SD) duration of diabetes was 11.4 (8.9) years with 46.3% of the group having had T2DM for >10 years. The majority (81.8%) of the patients were overweight or obese, and 85.8% fulfilled the criteria for metabolic syndrome. The microvascular complications of retinopathy microalbuminuria and proteinuria were noted in 23.6%, 22.9% and 18.2% of patients, respectively. The prevalence of known ischaemic heart disease (IHD), peripheral vascular disease (PVD) and cerebrovascular disease (CVD) was 17.6%, 7.4% and 4.1%, respectively.
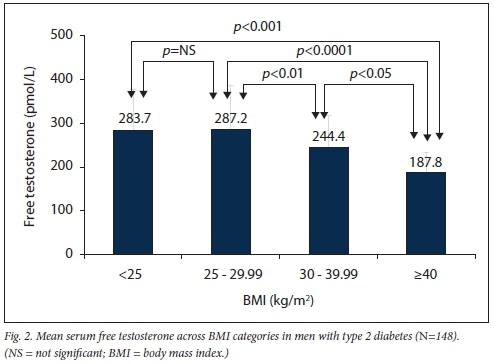
The mean (SD) HbAlc in the study group was 8.6% (1.9%), mean serum total cholesterol 4.2 (1.3) mmol/L and mean serum low-density cholesterol 2.5 (1.41) mmol/L (Table 2). Mean serum FT levels were significantly lower with increasing age and higher BMI (Figs 1 and 2). Mean FT was lowest in the >40 kg/m2 category and significantly lower when compared with all other BMI categories (v. <25 kg/m2p<0.001: v. 25 - 29.9 kg/m2p<0.0001; v. 30 - 39.9 kg/ m2p<0.05).
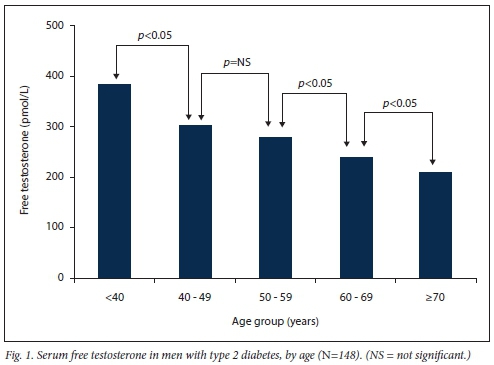
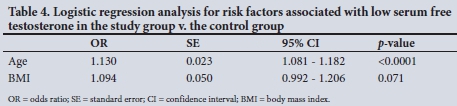
When compared with the control group, the study group (T2DM) was significantly older (mean (SD) 57.7 (11.1) v. 43.9 (10.7) years; p<0.001) and had a higher BMI (29.8 (6) v. 27.1 (4.2) kg/m2; p=0.01). Mean (SD) serum TT (14.5 (5.8) v. 18.8 (7.2) nmol/L; p<0.001) and FT (265.9 (90.4) v. 351.7 (127.3) pmol/L; p<0.001) were significantly lower in the study group, while the prevalence of LSTT and LSFT was significantly higher in the study group (p=0.0087 and p=0.0088, respectively) (Table 3). In logistic regression analysis, FT remained significantly lower in the study group v. the control subjects (p=0.001) after adjusting for other variables such as age and BMI (Table 4).
In the study group, the prevalence of androgen deficiency symptoms was higher using the AMS questionnaire (74.5%) (mild 36.2%, moderate 26.9%, severe 11.4%) compared with direct patient enquiry (68.9%), although this difference was not statistically significant. The most common symptom on direct enquiry was erectile dysfunction (58.8%); loss of libido, decrease in beard growth and infertility were reported in 56.1%, 2.7% and 2.0%, respectively (Table 5, Fig. 3).
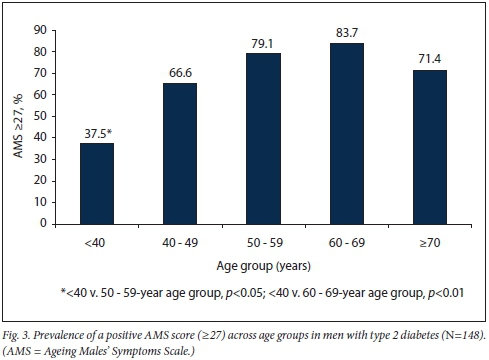
The prevalence of LSFT was 16.2%, and it rose significantly with increasing age and BMI. The prevalence of LSTT was 35.8%; however, with a more stringent cut-point of <8 nmol/L, the prevalence was 10.1%. Hypogonadism was present in 11.5% using FT and in 26.3% using TT <12 nmol/L.
Using TT <8 nmol/L and an AMS score >27, overt hypogonadism was observed in 7.4% (Table 5).
In univariate analysis, LSFT was associated with age, a decrease in beard growth, BMI, waist circumference (WC), hip circumference and albumin level. In multivariate analysis, the significant independent risk factors associated with LSFT were age (odds ratio (OR) 1.05, 95% confidence interval (CI) 1.02 - 1.218; p=0.043) and WC (OR 1.033, 95% CI 0.999 - 1.068; p=0.059). LSTT was associated with microalbuminuria, proteinuria, weight, BMI, WC, hip circumference and metabolic syndrome on univariate analysis. In multivariate analysis, the only significant independent risk factor associated with LSTT was BMI (OR 1.138, 95% CI 1.063 - 1.218; p<0.0001).
There was no significant difference in the total or individual domain AMS questionnaire scores between patients with LSFT and those with normal FT. Receiver operating characteristic curve analysis showed that the AMS score was not a good predictor of LSFT or LSTT (area under the curve 0.5 (95% CI 0.37 - 0.64) and 0.49 (95% CI 0.39 - 0.59), respectively). There was a poor correlation between TT or FT and the AMS scores (r=0.1, p=0.3; r= -0.003, p=0.9, respectively).
A moderate inverse correlation was found between TT and BMI as well as WC (r=-0.34, p<0.001; »•=-0.35, p<0.001, respectively).
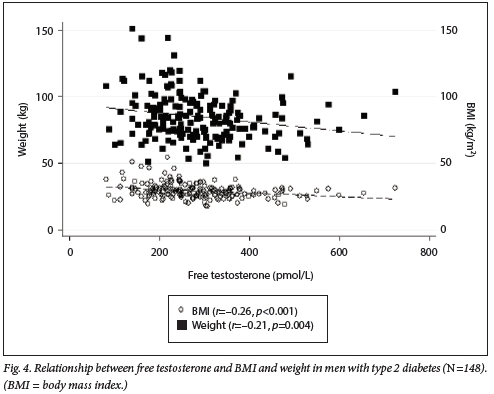
Regarding FT, a moderate inverse correlation with age and WC (r=-0.45, p<0.001; r=-0.33, p<0.001) was noted. The scatter plot in Fig. 4 shows the negative correlation of FT with BMI and weight (r=-0.27, p<0.001; r=-0.21, p<0.01, respectively). For both FT and TT, no significant correlation was observed with HbAlc.
Mean (SD) serum LH levels were significantly lower in the control group than in the study group (4.5 (2.3) IU/L v. 5.8 (3.1) IU/L; p=0.006), but levels were within the normal range for both groups. The majority of patients with LSTT or LSFT had low or normal LH levels (79.2% and 81.1%, respectively), compatible with secondary hypogonadism; the remainder (20.8% and 18.9%, respectively) had a high LH level compatible with primary hypogonadism (Table 3).
Discussion
This study showed a high prevalence of LSFT and LSTT in men with T2DM. The prevalence of androgen deficiency symptoms was high using either the AMS questionnaire or direct enquiry. The AMS questionnaire was found to be a poor predictor of low serum testosterone, and there was a weak correlation between AMS scores and serum testosterone. LH levels were either normal or low in the majority of patients with LSFT or LSTT, compatible with hypogonadotropic hypogonadism. In multivariate analysis, there was an independent negative association with age and WC in diabetic men with LSFT.
The high prevalence of low serum testosterone observed in this study is in accordance with the reported prevalence (17 - 33%) from cross-sectional studies in Western countries such as the USA.[4,17] Similarly high prevalences have also been reported from India (26.3%), Jordan (36.5%) and Poland (46%).[18-20] Furthermore, the observation of significantly lower TT and FT in the diabetic men compared with the control subjects is compatible with the findings of a large meta-analysis.[5]
There are limited data on the prevalence of low serum testosterone in men with T2DM from SSA, with only a few studies from Nigeria and a single study each from Ghana and SA.[7-13] Such studies report the prevalence of LSTT as 29.5 - 55%.[7-10] Compared with the present study, Kemp and Rheeder[7] reported a higher prevalence of LSTT (50%) in 150 men attending a tertiary diabetes clinic in Pretoria, the majority of whom (91%) had T2DM. This finding could be related to the characteristics of the study population, who were older than our subjects (62 v. 57.5 years) and had a longer duration of diabetes (15 v. 11.4 years), a higher mean WC (112 v. 103 cm) and a higher burden of CVD (41% v. 18%). A finding common to the Pretoria study and the present study was the strong association between WC and LSTT.
Screening for symptoms of androgen deficiency has been recommended by some organisations to justify measurement of serum testosterone.[21] The few available studies in diabetic men have shown that the sensitivity of using questionnaires is high, but that they lack specificity; also, they correlate poorly with serum testosterone, similar to the findings of the present study[19,22,23] The Pretoria study[7] found that the ADAM questionnaire had a high sensitivity (95%) and low specificity (5%), similar to the findings of a cross-sectional survey among 200 Nigerian men with T2DM.[7,24] The value of the AMS and ADAM questionnaires in SSA populations therefore remains unresolved, and they require further validation before they can be recommended in routine practice. Direct patient enquiry for symptoms such as loss of libido and erectile dysfunction should be considered.
From the available literature, the prevalence of hypogonadism among men with T2DM in Western countries ranges from 20% to 42%.[17,25] The prevalence of 11.5% in the present study (defined as LSFT with a positive AMS score) is lower than that reported in three studies from Nigeria (29.5 -65.3%), and is probably related to differences in the definition of hypogonadism used in these studies.[9-11] It is well recognised that the majority of diabetic men with low serum testosterone have normal or low levels of LH (67 - 83%), and the findings of the present study are compatible with these reports. [4,17,19,26,27] These findings suggest that the majority of men with low testosterone have features compatible with hypogonadotropic hypogonadism as the underlying defect.
The significant risk factors associated with LSFT and LSTT in the present study were WC and BMI, similar to reports from other studies.[28,29] Although lower SHBG concentrations related to higher obesity levels in diabetics may partly explain the lower TT, they do not account for the lower FT levels. Other factors such as elevated circulating adipokines or higher oestradiol levels may explain the effect in men with diabetes owing to the association of this disease with obesity[30,31] T2DM in itself is an inflammatory state that may suppress gonadotropin-releasing hormone (GnRH). However, the hypogonadal-obesity-adipokine hypothesis implicates the proinflammatory adipokines elaborated by adipose tissue depots as the inciting agents that suppress GnRH secretion, thus lowering testosterone synthesis.[32] Of particular concern for SSA are rising obesity rates, especially in SA, which has the highest prevalence of obesity, because many more men will be at risk of developing low testosterone and the consequences thereof.[33]
The effect of ageing on lowering testosterone levels among men in the general population has clearly been shown in landmark studies.[1,2] In an SA cohort of the Transition and Health during Urbanisation of South Africans (THUSA) study that investigated 364 male subjects between the ages of 20 and 82 years, testosterone levels also declined with age.[34] This finding was supported by the present study, but of note was the absence of LSFT among all patients aged <40 years. If screening for androgen deficiency is being considered in this population, routinely screening patients aged <40 years may not be cost-effective.
No association between serum testosterone and HbAlc was noted in our cohort, similar to findings in other studies.[4,7,8,10,29] Although we observed no association between serum lipids and serum testosterone, there was a weak inverse correlation between serum TT and serum total triglycerides, similar to two other studies from SSA.[8,11]
Current international guidelines recommend screening men with T2DM for low testosterone.[3] The SA guideline for the management of T2DM published by the Society for Endocrinology Metabolism and Diabetes of South Africa (SEMDSA) in 2017 states that screening for low serum testosterone levels in men with T2DM and symptoms of hypogonadism is mandatory[21] The available evidence suggests that screening may be justified in this high-risk population and that men most likely to benefit from screening would be older and overweight with predominant central adiposity. Furthermore, appropriate treatment in the form of testosterone replacement is available. More local data are required, including cost-benefit analysis, before recommendations can be made regarding a screening policy for SA men with T2DM. The poor performance of the ADAM and AMS questionnaires highlights the need for further studies to validate their use. Alternatively, direct enquiry into symptoms such as erectile dysfunction and loss of libido can be considered.
Study limitations and strengths
The major limitations of this study were its cross-sectional design and the small sample of white men. Although IHD, PVD and CVD may be associated with hypogonadism, statistical analysis to control for these variables was not done. Study strengths include the measurement of both FT and TT and inclusion of men from district-level and tertiary-level clinics.
Conclusions
There was a high prevalence of low serum TT and FT in this study. Serum testosterone was lower in men with T2DM than in control subjects. Risk factors associated with low serum testosterone included older age and higher BMI and WC. The prevalence of androgen deficiency symptoms was high, but the AMS score was a poor predictor of low testosterone. Screening may be justifiable in this population, but further research is required locally to conduct a cost-benefit analysis before any policy can be advocated.
Declaration. This study was submitted as a thesis for IMP's MMedSci degree.
Acknowledgements. Support was received from Lancet Laboratories for laboratory analysis. Ms Nonhlanhla Nombula assisted with data collection. Dr Β Janowski and Dr Y Shode assisted with co-ordination of research at PMMH.
Author contributions. IMP: study concept and design, acquisition of data, study supervision, analysis and interpretation of data, drafting of the manuscript; FJP: acquisition of data, critical revision of the manuscript for important intellectual content; NMN: statistical design; AAM: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content.
Funding. The study was supported by a grant that was made available via SEMDSA through sponsorship from Bayer Southern Africa.
Conflicts of interest. None.
References
1. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackmail MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001,86(2).724-731. https://doi.org/10.1210/jcem.86.2.7219 [ Links ]
2. Araújo AB, O'Donnell AB, Brambilla DJ, et al Prevalence and incidence of androgen deficiency in middle-aged and older men. Estimates from the Massachusetts Male Aging Study J Clin Endocrinol Metab 2004;9(12)5920-5926. https://doi.org/10.1210/jc.2003-031719 [ Links ]
3. Bhasin S, Cunningham GR, Hayes FJ, et al Testosterone therapy in men with androgen deficiency syndromes. An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010,95(6).25362559. https://doi.org/10.1210/jc.2009-2354 [ Links ]
4. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004,89(11).5462-5468. https://doi.org/10.1210/jc.2004-0804 [ Links ]
5. Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone. A meta-analysis study Int J Androl 2011;34(6 pt 1).528-540. https://doi.org/10.1111/j.1365-2605.2010.01117.x [ Links ]
6. Heinemann LAJ, Zimmermann Τ, Vermeulen Α, Thiel C, Hummel W. A new aging males1 symptoms' rating scale. Aging Male 1999;2(2).105-114. https://doi.org/10.3109/13685539909003173 [ Links ]
7. Kemp T, Rheeder P. The prevalence and associations of erectile dysfunction in a South African male diabetic urban population. J Endocrinol Metab Diabetes S Afr 2015;20(3).134-139. https://doi.org/10.1080/16089677.2015.1090185 [ Links ]
8. Asare-anane H, Ofori E, AgyemangY, etal. Obesity and testosterone levels in Ghanaian men with type 2 diabetes. Clin Diabetes 2014;32(2).61-65. https://doi.org/10.2337/diadin.32.2.61 [ Links ]
9. Ogbera AO. Relationship between serum testosterone levels and features of the metabolic syndrome defining criteria in patients with type 2 diabetes mellitus. West Afr J Med 2011,30(4).277-281. [ Links ]
10. Ugwu T, Ikem R Kolawole B, Ezeani I. Clinicopathologic assessment of hypogonadism in men with type 2 diabetes mellitus. Indian J Endocrinol Metab 2016,20(5).667. https://doi.org/10.4103/2230-8210.190554 [ Links ]
11. Akinloye O, Popoola BB, Ajadi MB, Uchechukwu JG, Oparinde DR Hypogonadism and metabolic syndrome in Nigerian male patients with both type 2 diabetes and hypertension. Int J Endocrinol Metab 2014;12(1)Tel0749. https://doi.org/10.5812/ijem.l0749 [ Links ]
12. Ubajaka CF, Meludu SC, Dioka CE, et al. Evaluation of male sex hormones and trace elements in male type 2 diabetic patients attending Nnamdi Azikiwe university teaching hospital diabetic clinics. Nigel I Med 2015,24(2).162-168. [ Links ]
13. Fabian UA, Chailes-Davies MA, Fasanmade AA, et al. Male sexual dysfunction, leptin, pituitaiy and gonadal hoimones in Nigeiian males with metabolic syndiome and type 2 diabetes mellitus. J Repiod Infertil 2016;17(l)17-25. [ Links ]
14. World Health Organization and International Diabetes Fedeiation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Report of a WHO/IDF Consultation. Geneva. WHO, 2006. https://www.who.int/diabetes/publications/Definition_and_diagnosis_of_diabetes_new.pdf (accessed 14 October 2019). [ Links ]
15. Nieschlag Ε, Swerdloff R, Behre HM, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males. ISA, ISSAM, and EAU recommendations. J Androl 2006,27(2).135-137. https://doi.org/10.2164/jandrol.05047 [ Links ]
16. Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity. Circulation 2009,120(16).1640-1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644 [ Links ]
17. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes. Correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007,30(4).911-917. https://doi.org/10.2337/dc06-1426 [ Links ]
18. Agarwal P, Singh P, Chowdhury S, et al. A study to evaluate the prevalence of hypogonadism in Indian males with type-2 diabetes mellitus. Indian J Endocrinol Metab 2017,21(1).64-70. http://doi.org/10.4103/2230-8210.196008 [ Links ]
19. Al Hayek A, Ajlouni Κ, Khader Y, Jafai S, Khawaja N, Robert A. Prevalence of low testosterone levels in men with type 2 diabetes mellitus. A cross-sectional study. J Fam Community Med 2013,20(3).179-186. https://doi.org/10.4103/2230-8229.122006 [ Links ]
20. Rabijewski M, Papierska L, Zgliczynski W, Pidtkiewicz P. The incidence of hyp ο gonadotropic hypogonadism in type 2 diabetic men in Polish population. Biomed Res Int 2013, article ID 767496. https://doi.org/10.1155/2013/767496 [ Links ]
21. SEMDSA Type 2 Diabetes Guidelines Expert Committee. SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus. J Endocrinol Metab Diabetes S Afr 2017,22(1, Suppl 1).S1-S196. https://doi.org/10.1080/16089677.2015.1056468 [ Links ]
22. Biswas M, Hampton D, Newcombe RG, Rees DA. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-relate d quality of life. Clin Endocrinol (Oxf) 2012,76(5)-665-673. https://doi.org/10.1111/j.1365-2265.2011.04196.x [ Links ]
23. El Saghier EOA, Shebl SE, Fawzy OA, Eltayeb lhab M, Bekhet LMA, Gharib A. Androgen deficiency and erectile dysfunction in patients with type 2 diabetes. Clin Med Insights Endocrinol Diabetes 2015;8:CMED.S27700. https://doi.org/10.4137/CMed.s27700 [ Links ]
24. Ugwu ET, Ikem RT. Androgen deficiency in aging male questionnaire for the clinical detection of testosterone deficiency in a population of black sub-Saharan African men with type 2 diabetes mellitus. Is it a reliable tool? Curr Diabetes Rev 2016;14(3).280-285 https://doi.org/10.2174/1573399812666161228152036 [ Links ]
25. Corrales JJ, Burgo RM, Garca-Berrocal B, et al. Partial androgen deficiency in aging type 2 diabeticmen and its relationship to glycemic control. Metabolism 2004,53(5).666-672. https://doi.org/10.1016/j.metabol.2003.12.016 [ Links ]
26. Hamilton EJ, Davis WA, Makepeace A, et aL Prevalence and prognosis of a low serum testosterone in men with type 2 diabetes, the Fremantle Diabetes Study Phase II. Clin Endocrinol (Oxf) 2016;85(3):444-452. https://doi.org/10.1111/cen.l3087 [ Links ]
27. Dandona P, Dhindsa S, Chandel A, Chaudhuri A. Hyp o gonadotropic hypogonadism in men with type 2 diabetes. Postgrad Med 2009;121(3).45-51. https://doi.org/10.3810/pgm.2009.05.2001 [ Links ]
28. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med 2013;10(6).1612-1627. https://doi.org/10.1111/jsm.l2146 [ Links ]
29. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154(6).899-906. https://doi.org/10.1530/eje.l.02166 [ Links ]
30. Grossmann Μ, GianattiEJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17(3).247-256. https://doi.org/10.1097/MED.0b013e32833919cf [ Links ]
31. Cohen PG. The hypogonadai-obesity cycle. Role of aromatase in modulating the testosterone-estradiol shunt - a major factor in the genesis of morbid obesity. Med Hypotheses 1999;52(1).49-51. https://doi.org/10.1054/mehy.l997.0624 [ Links ]
32. Jones TH. Testosterone associations with erectile dysfunction, diabetes, and the metabolic syndrome. Eur Urol Suppl 2007;6(16):847-857. https://doi.org/10.1016/j.eursup.2007.07.002 [ Links ]
33. Mbanya JC, Assah FK, Saji J, Atanga EN. Obesity and type 2 diabetes in sub-Sahara Africa. Curr Diab Rep 2014,14(7).501. https://doi.org/10.1007/sll892-014-0501-5 [ Links ]
34. Gray PB, Kruger A, Huisman HW, Wissing MP, Vorster HH. Predictors of South African male testosterone levels-. The THUSA study. Am J Hum Biol 2006-,18(1).123-132. https://doi.org/10.1002/ajhb.20471 [ Links ]
 Correspondence:
Correspondence:
IΜ Paruk
paruki@ukzn.ac.za
Accepted 9 May 2019














