Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 no.11 Pretoria Nov. 2019
http://dx.doi.org/10.7196/samj.2019.v109i11.14242
IN PRACTICE
CLINICAL ALERT
Intrathecal tranexamic acid during spinal anaesthesia for caesarean delivery: A lethal drug error
D G BishopI; A C LundgrenII; N F MoranIII; I PopovIV; J MoodleyV
IMB ChB, DA, FCA, PhD; Department of Anaesthesia, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IIMB, ChB, DA, FFA, PhD, MSc Med (Health Law and Bioethics); Steve Biko Centre for Bioethics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIBM BCh, MA, FCOG; KwaZulu-Natal Department of Health; and Department of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IVMD, FCOG (SA); Department of Gynaecology and Obstetrics, Port Shepstone Regional Hospital, KwaZulu-Natal, South Africa
VMB ChB, FRCOG, FCOG, MD; Women's Health and HIV Research Group, Department of Obstetrics and Gynaecology, School of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
The National Committee on Confidential Enquiries into Maternal Deaths recently received notification of a death in South Africa caused by inadvertent intrathecal administration of tranexamic acid (TXA). TXA is increasingly used during caesarean delivery following updated recommendations from the World Health Organization in 2017. However, its greater availability has led to an international rise in drug errors during obstetric spinal anaesthesia. This case highlights a growing clinical risk, of which all operating theatre staff should be aware. Review of existing operating theatre drug handling practices is required in order to decrease this risk. Recommendations are made that aim to minimise drug errors associated with the use of this potentially life-saving intervention.
The National Committee on Confidential Enquiries into Maternal Deaths recently received notification of a death in South Africa (SA) caused by inadvertent intrathecal administration of tranexamic acid (TXA). This case highlights a growing clinical risk, of which all operating theatre staff should be aware. Review of existing operating theatre drug handling practices is required in order to minimise this risk.
TXA is included in the World Health Organization (WHO) essential medicines list (EML)[1] as well as the SA National Department of Health EML.[2] It is a synthetic lysine analogue that acts to reduce fibrinolysis through competitive inhibition of plasminogen binding sites. TXA is increasingly being used in the perioperative setting as a result of recently updated WHO guidelines recommending early use of intravenous TXA during caesarean delivery (CD) when excessive bleeding occurs.[3] The key messages from this guideline are summarised in Table 1. This change in practice is largely due to the results of the WOMAN trial, a landmark multicentre study including 20 000 patients that showed reduced maternal mortality due to bleeding with the early administration of TXA in the setting of postpartum haemorrhage.[4] The WOMAN trial showed that if TXA was given intravenously within 3 hours of bleeding following normal vaginal delivery or CD, maternal mortality was reduced by 31%, although the absolute reduction was small (1.7 - 1.2%, risk ratio 0.69, 95% confidence interval 0.52 - 0.91; p=0.008). These benefits were most pronounced in low- and middle-income settings such as SA.[4] The WHO states that 'regardless of the level of health system resources, TXA should be recognized as a lifesaving intervention and be made readily available for the management of postpartum haemorrhage in settings where emergency obstetric care is provided'.[3]
SA TXA recommendations
It has been suggested that the high number of deaths due to obstetric haemorrhage (OH) at or after CD in SA is a national emergency,[5] and despite a recent downward trend, OH remains the third most common cause of maternal mortality at ~17%.[6] Accordingly, the nationally endorsed training programme for obstetric emergencies (Essential Steps in the Management of Obstetric Emergencies:
ESMOE)[7] has been revised to recommend early intravenous administration of 1 g TXA for bleeding during or after CD. Excessive bleeding is now defined as >500 mL in the suction bottle, or a decrease in blood pressure accompanied by a rise in heart rate associated with bleeding, as detected by the anaesthetist. This is earlier than the traditional description of at least 1 000 mL blood loss during CD, and TXA is therefore being used with greater frequency. While there may be a role for the administration of TXA before CD,[8,9] there is not yet enough evidence from high-quality research to recommend such prophylaxis at a national level.[10] In particular, there is no evidence that prophylactic TXA before CD reduces maternal death.
With increased availability and use of TXA during and immediately following CD, the risk of drug error increases. Our case of maternal death was assessed by independent experts to be due to intrathecal TXA, and occurred in the context of a disturbing international trend. A recent review in Anaesthesia[11] highlighted 21 such cases between 1988 and 2018, 10 of which were fatal. Twenty were due to 'ampoule error'. An accompanying editorial[12] entitled 'Spinal tranexamic acid - a new killer in town' highlighted the dramatic increase in the number of cases since 2009. Seven cases involved CD, 6 of which resulted in death: it appears that mortality is higher following CD than following other surgery. The authors mention anecdotal reports of further cases that have not been formally reported, making the true incidence hard to estimate. Clinicians are understandably reluctant to submit case reports relating to serious medical error. Additionally, cases such as ours that come to light through a confidential enquiry process cannot be published in detail owing to requirements to maintain anonymity. The incidence is therefore probably far higher than currently reported.
Consequences of intrathecal TXA administration
Intrathecal TXA is a potent neurotoxin and neurological sequelae dominate the clinical presentation, usually with refractory seizures. Massive sympathetic stimulation frequently occurs, often leading to lethal cardiac arrhythmias such as ventricular fibrillation. Treatment is mainly supportive and should occur in an intensive care setting, including antiepileptics such as diazepam, thiopentone and magnesium sulphate[13] and appropriate antiarrhythmic medication. Early cerebrospinal fluid (CSF) lavage is also recommended, following success in the management of similar cases.[11,14,15] CSF lavage consists of removing 10 mL of CSF and replacing this with 10 mL of saline, repeated up to four times.[14,15] The increased mortality rate in the obstetric population following intrathecal TXA is possibly due to decreased CSF volume in pregnancy, leading to increased drug concentrations.[11]
Given the consequences of inadvertent intrathecal TXA administration, is the increased risk justifiable? TXA has become an integral part of the management of OH: the WOMAN trial[4] suggested that a maternal life could be saved with every 267 usages following OH. The potential 'number needed to harm' is difficult to estimate: Palanisamy and Kinsella[12] estimate the risk due to drug error to be <1 in 10 000 spinal anaesthetics, although this is necessarily based on a large degree of conjecture. In Africa, the incidence of severe bleeding during or after CD is almost 6%, while 70% of all complications and 25% of all deaths are secondary to bleeding complications.[16] With a lower recommended threshold for the use of TXA, it is likely that the drug will be given in >6% of cases. The benefits clearly outweigh the risks: the focus therefore needs to be on minimising or eliminating drug error.
Minimising the risk of intrathecal drug error
The incidence of perioperative drug errors ranges from one in 133 anaesthetics in retrospective studies[17] to one in two operations in prospective studies (one in 20 drug administrations).[18] Obstetric neuraxial drug administration errors in particular may result in devastating consequences.[19] There is a lack of randomised controlled trials that examine specific techniques and their ability to reduce drug error; recommendations are therefore based on expert opinion and best available evidence.[20,21]
All health facilities should ensure that they have clearly written policies that minimise medication errors, and then audit and appraise errors that do occur.[22] This approach should nurture a culture of drug safety, including multidisciplinary involvement, ongoing education and specific evidence-based interventions.[22] However, despite vociferous calls for changes in practice, merely exhorting doctors to be more careful is often inadequate.[23] Ideally, system changes should make it impossible for error to occur. A similar problem has been encountered with epidural anaesthesia, where the use of Luer universal connectors has allowed for cross-connectivity, resulting in drug errors. This problem is easily preventable with the use of non-Luer connectors, although uptake has been slow.[23] Non-Luer connectors will not prevent a single-shot spinal anaesthesia drug error, however, as occurs with TXA.
The risk of accidental use of the wrong drug increases when ampoules look similar, or are physically available in close proximity. [24] There are now a large number of generic versions of TXA, and changes in supplier and the appearance of ampoules are increasingly common. Human error is to some degree unavoidable, and rather than attempting to eliminate all mistakes, strategies should aim to reduce predictable errors. Solutions that minimise the possibility of human error should be given highest priority.[24] Technology-assisted drug identification, using barcode readers, is one such intervention, although it is unlikely to be immediately available in SA facilities. Pre-filled syringes may be another, although this may be problematic for manufacturers, as each drug must be tested for stability in a pre-filled syringe. Other solutions include the careful reading and labelling of syringes, and a second person or device checking the drug.[19] More costly methods, such as commercially prepared spinal anaesthesia trays including bupivacaine, are unlikely to represent solutions for low- and middle-income countries such as SA. Most importantly, the physical location of TXA must minimise the potential for drug error. There are numerous drugs in theatre that should never be injected intrathecally; we need to ensure that TXA is one of these. Avoiding a drug substitution error mandates meticulous attention to drug checking systems, and above all ensuring that TXA is not kept on or near the spinal anaesthesia trolley. Consideration should be given to storing TXA out of theatre, provided that the drug will be available immediately when requested.
We have made recommendations in Table 2 summarising key interventions aimed at reducing drug error from the relevant literature.[12,19-22,25]
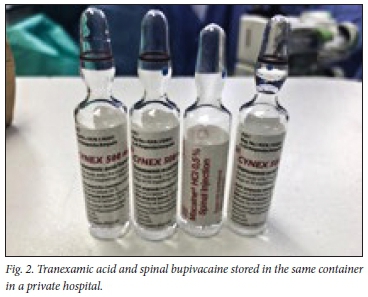
Importantly, this clinical alert applies equally to both the private and public sectors in SA, where different versions and appearances of the drug ampoules are available. Fig. 1 illustrates the current appearances of TXA and bupivacaine in the state sector in KwaZulu-Natal Province. Fig. 2 illustrates the TXA used by one of the private hospitals in KwaZulu-Natal. This image was taken after discovering these ampoules in the same container, illustrating the potential for drug error.

Conclusions
The indications for TXA during and after CD continue to expand. The increased use and availability of the drug have led to a concerning increase in inadvertent intrathecal administration worldwide - an error that always results in harm. We need to urgently raise awareness of this potentially lethal mistake and take steps to ensure that we have no further such cases in SA. The first step is to store TXA in a separate location from spinal bupivacaine, and ensure that the drug is never present on the spinal anaesthesia trolley.
Declaration. None.
Acknowledgements. The authors thank the National Committee on Confidential Enquiries into Maternal Deaths for permission to divulge information on this case.
Author contributions. The first draft of the manuscript was written by DGB. All authors participated in critical review of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. World Health Organization. WHO Model List of Essential Medicines. 2017. https://www.who.int/medicines/publications/essentialmedicines/en/ (accessed 23 June 2019). [ Links ]
2. Republic of South Africa. Essential Drugs Programme. Standard Treatment Guidelines and Essential Medicines List: Hospital Level (Adults). 4th ed Pretoria: National Department of Health, 2015. http://www.health.gov.za/index.php/standard-treatment-guidelines-and-essential-medicines-list/category/286-hospital-level-adults?download=2409:hospital-level-adult-2015-v5-0 (accessed 23 June 2019). [ Links ]
3. World Health Organization. WHO Recommendation on Tranexamic Acid for the Treatment of Postpartum Haemorrhage. Geneva: WHO, 2017. https://www.who.int/reproductivehealth/publications/tranexamic-acid-pph-treatment/en/ (accessed 23 June 2019). [ Links ]
4. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389(10084):2105-2116. https://doi.org/10.1016/S0140-6736(17)30638-4 [ Links ]
5. Fawcus S, Pattinson RC, Moodley J, et al Maternal deaths from bleeding associated with caesarean delivery: A national emergency. S Afr Med J 2016;106(5):53-57. https://doi.org/10.7196/SAMJ.2016.v106i5.10821 [ Links ]
6. National Committee on Confidential Enquiries into Maternal Deaths. Saving Mothers 2014 - 2016: Seventh Triennial Report on Confidential Enquiries into Maternal Deaths in South Africa: Executive Summary. Pretoria: National Department of Health, 2018. http://www.health.gov.za/index.php/hiv-aids-tb-and-maternal-and-child-health/category/160-child-and-school-health?download=2644:saving-mothers-2014 (accessed 23 June 2019). [ Links ]
7. Moran NF, Naidoo M, Moodley J. Reducing maternal mortality on a countrywide scale: The role of emergency obstetric training. Best Pract Res Clin Obstet Gynaecol 2015;29(8):1102-1118. https://doi.org/10.1016/j.bpobgyn.2015.08.002 [ Links ]
8. Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2015, Issue 6. Art. No.: CD007872. https://doi.org/10.1002/14651858.CD007872.pub3 [ Links ]
9. Franchini M, Mengoli C, Cruciani M, et al Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: An updated systematic review and meta-analysis. Blood Transfus 2018;16:329-337. https://doi.org/10.2450/2018.0026-18 [ Links ]
10. Ker K, Shakur H, Roberts I. Does tranexamic acid prevent postpartum haemorrhage? A systematic review of randomised controlled trials. BJOG 2016;123(11):1745-1752. https://doi.org/10.1111/1471-0528.14267 [ Links ]
11. Patel S, Robertson B, McConachie I. Catastrophic drug errors involving tranexamic acid administered during spinal anaesthesia. Anaesthesia 2019;74(7):904-914. https://doi.org/10.1111/anae.14662 [ Links ]
12. Palanisamy A, Kinsella SM. Spinal tranexamic acid - a new killer in town. Anaesthesia 2019;74(7):831-833. https://doi.org/10.1111/anae.14632 [ Links ]
13. Hatch DM, Atito-Narh E, Herschmiller EJ, Olufolabi AJ, Owen MD. Refractory status epilepticus after inadvertent intrathecal injection of tranexamic acid treated by magnesium sulfate. Int J Obstet Anesth 2016;26:71-75. https://doi.org/10.1016/j.ijoa.2015.11.006 [ Links ]
14. Tsui BC, Malherbe S, Koller J, Aronyk K. Reversal of an unintentional spinal anesthetic by cerebrospinal lavage. Anesth Analg 2004;98(2):434-436. https://doi.org/10.1213/01.ane.0000095152.81728.dc [ Links ]
15. Ting HY, Tsui BC. Reversal of high spinal anesthesia with cerebrospinal lavage after inadvertent intrathecal injection of local anesthetic in an obstetric patient. Can J Anaesth 2014;61(11):1004-1007. https://doi.org/10.1007/s12630-014-0219-5 [ Links ]
16. Bishop D, Dyer RA, Maswime S, et al. Maternal and neonatal outcomes after caesarean delivery in the African Surgical Outcomes Study: A 7-day prospective observational cohort study. Lancet Glob Health 2019;7(4):e513-e522. https://doi.org/10.1016/S2214-109X(19)30036-1 [ Links ]
17. Webster CS, Merry AF, Larsson L, McGrath KA, Weller J. The frequency and nature of drug administration error during anaesthesia. Anaesth Intensive Care 2001;29(5):494-500. https://doi.org/10.1177/0310057X0102900508 [ Links ]
18. Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology 2016;124(1):25-34. https://doi.org/10.1097/ALN.0000000000000904 [ Links ]
19. Patel S, Loveridge R. Obstetric neuraxial drug administration errors: A quantitative and qualitative analytical review. Anesth Analg 2015;121(6):1570-1577. https://doi.org/10.1213/ANE.0000000000000938 [ Links ]
20. Wahr JA, Abernathy JH 3rd, Lazarra EH, et al Medication safety in the operating room: Literature and expert-based recommendations. Br J Anaesth 2017;118(1):32-43. https://doi.org/10.1093/bja/aew379 [ Links ]
21. Jensen LS, Merry AF, Webster CS, Weller J, Larsson L. Evidence-based strategies for preventing drug administration errors during anaesthesia. Anaesthesia 2004;59(5):493-504. https://doi.org/10.1111/j.1365-2044.2004.03670.x [ Links ]
22. Risk Management Analysis Committee of the French Society for Anesthesia and Critical Care, French Society for Clinical Pharmacy. Preventing medication errors in anesthesia and critical care (abbreviated version). Anaesth Crit Care Pain Med 2017;36(4):253-258. https://doi.org/10.1016/j.accpm.2017.04.002 [ Links ]
23. Birnbach DJ, Brull SJ, Prielipp RC. J'accuse! Failure to prevent epidural and spinal catheter misconnections. A A Case Rep 2016;6(5):107-110. https://doi.org/10.1213/XAA.0000000000000260 [ Links ]
24. Litman RS. How to prevent medication errors in the operating room? Take away the human factor. Br J Anaesth 2018;120(3):438-440. https://doi.org/10.1016/j.bja.2018.01.005 [ Links ]
25. Marshall SD, Chrimes N. Medication handling: Towards a practical, human-centred approach. Anaesthesia 2019;74(3):280-284. https://doi.org/10.1111/anae.14482 [ Links ]
 Correspondence:
Correspondence:
D G Bishop
bishop@ukzn.ac.za
Accepted 23 July 2019














