Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 n.11 Pretoria Nov. 2019
http://dx.doi.org/10.7196/samj.2019.v109i11.14365
CME
Venous thromboembolism in pregnancy
P F Wessels
MB ChB, MMed (Haem), Cert Clin Haematology (SA); Ampath Laboratories; and Department of Medical Oncology, Faculty of Health Sciences, University of Pretoria, South Africa
ABSTRACT
Pregnancy-related venous thromboembolic events are important preventable causes of morbidity and mortality in South Africa. All pregnant patients should be evaluated for thrombotic risk at different stages of their pregnancy and appropriate preventive steps taken. Maternal and fetal wellbeing must be kept in mind, as well as physiological changes leading to altered drug pharmacokinetics. Managing the patient with thrombotic risk in pregnancy, diagnosing venous thromboembolism (VTE) during pregnancy and treatment of venous thromboembolic events should be managed by a team. Excellent recent reviews on this subject are available, including risk factor stratification in anticoagulant therapy; managing the patient at time of labour; diagnosing VTE; and managing neuraxial anaesthesia in the pregnant patient on anticoagulant therapy.
The risk of venous thromboembolism (VTE) increases 5 - 10-fold during pregnancy, and 15 - 35-fold postpartum compared with that in non-pregnant women of the same age.[1] High-quality data specific to the pregnant patient are lacking, as pregnancy is an exclusion criterion in most randomised clinical VTE trials. Most guidelines are therefore based on expert opinion and observational studies. Pregnancy also deals with maternal and fetal wellbeing. Despite these issues, several excellent publications have appeared in the past year. The aim of this review is not to replace any of these papers, but rather summarise some of the points made. The interested reader is advised to read some (if not all) of these recommendations, which include those on thromboprophylaxis in obstetrics and gynaecology; management of thromboembolism in pregnancy; and evidence-based anticoagulation in pregnancy (see references 12, 13 and 14).
Why are patients prone to VTE during pregnancy?
Various physiological changes[2] that prevent haemorrhage during birth and in the weeks after pregnancy lead to a natural hyper-coagulable state in the pregnant patient (Table 1). Pregnant women also experience venous stasis (the enlarging uterus compresses the inferior vena cava and pelvic veins). Another VTE risk factor (in the well-known Virchow's triad) is also present, with endothelial changes described in pregnancy[4] (including endothelial trauma during delivery).
How many patients develop VTE in pregnancy?
Depending on the population studied, VTE complicates 0.5 - 2.2 per 1 000 deliveries.[1] Pregnancy-related VTE is one of the 10 most important potentially preventable causes of maternal death in South Africa (SA).[5] In the UK, VTE is the leading direct cause of maternal death (12.9% of patients between 2013 and 2015) and the fifth most common cause of death overall in pregnancy[6] Approximately 80% of venous thrombotic events are deep-vein thrombosis (DVT) and 20% pulmonary embolism.[7] The risk of VTE increases from the time of conception, with the highest risk at the time of delivery and immediately thereafter, especially in the first 3-6 postpartum weeks, with approximately half of VTE events occurring during that period. Arterial thrombotic events also increase during pregnancy, but are 4 times less common than VTE.[8]
How do we identify pregnant patients at risk of VTE?
All patients should be risk stratified for VTE early in pregnancy if admitted to hospital or confined to bedrest during pregnancy, as well as in the postpartum period. Various risk factors have been classified as high, intermediate or low.[9] Complex scoring systems[9,10] have been developed, but no randomised clinical trials have been performed to validate these. Patients at high risk of VTE in pregnancy should be managed by a team, including a clinical haematologist. The risk of bleeding should always be evaluated prior to prescribing thromboprophylaxis.
VTE events are classified as unprovoked events (-50%), indicating that there is no known clinical risk factor (e.g. trauma, surgery, active cancer, immobility, pregnancy or hormonal usage). Provoked events, however, may be due to a transient factor or persistent risk factor Risk of recurrence is highest with provoked persistent risk factors, very high with unprovoked risk factors, and lower with transient risk factors.[11] In pregnancy, this means that a previous oestrogen-related VTE event (persistent provoked risk) and previous unprovoked VTE event have a very high risk of recurrence during pregnancy
Evaluation of risk factors at time of first consultation or antepartum
The patient can be assessed according to risk factors mentioned in Royal College of Obstetricians and Gynaecologists (RCOG)[9] and/ or South African Society of Thrombosis and Haemostasis (SASTH)[12] articles (Table 2).
Three important questions must be answered (Fig. 1):

Has the patient previously experienced VTE?
If confirmative, at least 6 weeks' postpartum VTE prophylaxis should be given. If the event was oestrogen related, unprovoked or more than one event occurred, antepartum and postpartum prophylaxis are required.[9] Another approach to the recurrent risk in cases of a previous VTE, is that all pregnant patients with a previous VTE event should receive antepartum and postpartum prophylaxis, unless the single previous VTE event was due to a major risk factor.
A familial or acquired clotting tendency (thrombophilia) represents a consistent relative increased risk for a first VTE. There is much controversy regarding managing patients with known thrombophilia and whether they should undergo thrombophilia testing. The second question is therefore:
Does the patient have a family history (first degree) of thrombosis or known thrombophilia? Which specific thrombophilia is present (including acquired antiphospholipid syndrome)?
One approach has been to grade the different types of thrombophilia as severe, intermediate or minor types, and to look at events in other family members with the same thrombophilic abnormality. Table 3[13,14] summarises the recommendations by various authors. It is imperative that the total patient 'package must be evaluated, e.g. even though carriers of the factor V Leiden mutation may only need careful observation antepartum, the patient may have additional risk factors, such as a high body mass index (BMI), thus also putting them into a higher risk grade and need for antepartum prophylaxis.
The question arises whether patients should be tested for thrombophilia before pregnancy if there is a family history of VTE. A positive family history of VTE is a poor predictor of the presence of thrombophilia.[15] In any patient with a first-degree relative with VTE, whether a thrombophilic tendency has been proven or not, the VTE risk doubles, and in cases where the VTE event occurred in a family member <50 years of age, the risk rises up to 3-fold.[15] The RCOG guideline[9] therefore suggests testing the patient with a first-degree relative who had an unprovoked or oestrogen-related VTE before the age of 50 years. Patients may choose to undergo thrombophilia screening or specific testing for a thrombophilic abnormality that has been diagnosed in their family. Pretest counselling is necessary, because of the implications for the patient and her family. The RCOG guideline![9] suggests testing for antiphospholipid syndrome if an unprovoked VTE has occurred in a pregnant patient in the past (this may influence management of anticoagulation).
Various other riskfactors (personal risks (e.g. BMI, smoking), pregnancy-related risks (e.g. caesarean section) and medical comorbidities (e.g. autoimmune disorder)) are known to increase the risk of developing VTE, and thus the third question to ask the patient is the following:
Are there additional risk factors present?
The magnitude of this last group depends on the number of risk factors present, as well as the nature of the specific risk factors. Assigning weight to factors has been attempted, but not validated. It is also unclear whether risk factors have an additional or multiplicative role.[13] The 2018 American Society of Hematology (ASH) guideline[13] suggests considering (without previous VTE or thrombophilia) prophylaxis - only if more than one additional risk factor is present. The RCOG Green-top guideline[9] and the SASTH guideline[12] suggest considering thromboprophylaxis in intermediate risk factors (hospital admission, non-obstetric surgery during pregnancy, medical comorbidities and ovarian hyperstimulation syndrome (first-trimester-only prophylaxis)) and when multiple (>4) minor risk factors (age >35 years, BMI >30 kg/m2, parity >3, smoker (at least 10 cigarettes per day), gross varicose veins, current systemic infection or perioperative infection, immobility, e.g. paraplegia, long-distance travel (>8 hours), strict bedrest >7 days, pre-eclampsia with intrauterine growth restriction, multiple pregnancy, in vitro fertilisation and dehydration/hyperemesis) are present.
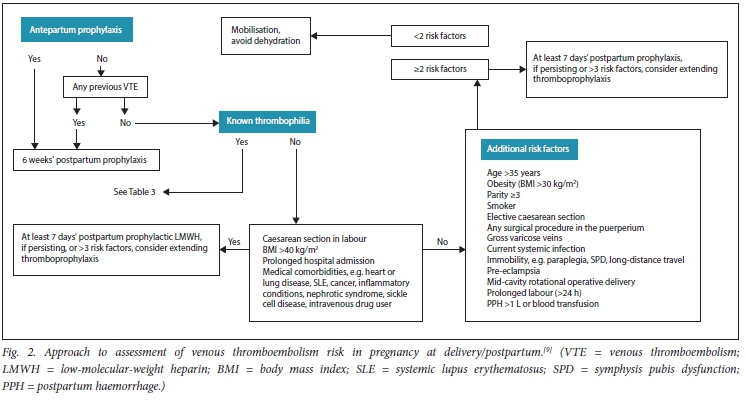
Evaluation of risk factors at the time of delivery/ postpartum
There are three groups of risk factors that should be considered at this time of pregnancy (Table 4 and Fig. 2).
Which anticoagulant drugs are safe in pregnancy and during breastfeeding?[14]
Drugs that are safe during pregnancy and breastfeeding are listed in Table 5.
How are patients on anticoagulation managed if they are contemplating pregnancy?
When contemplating pregnancy, preconception counselling regarding risks for mother and baby is essential. The International Society on Thrombosis and Haemostasis (ISTH) specifically recommends that women of childbearing age be counselled regarding risk of novel oral anticoagulants (NOACs) (dabigatran, rivaroxaban, apixaban) in pregnancy owing to possible teratogenic effects.[16] The suggested management of a patient on anticoagulants contemplating pregnancy is shown in Table 6.[14]
Practical aspects of low-molecular-weight heparin prophylaxis in pregnancy
Which patients should not receive low-molecular-weight heparin as the drug of choice?
Unfractionated heparin (UFH) is indicated in patients with significant renal dysfunction and may be used if a shorter period of anticoagulation/urgent reversal is contemplated (peripartum). Fondaparinux (or danaparoid, which is currently not available South Africa (SA)) is the drug of choice in patients with heparin-induced thrombocytopenia (HIT) or a history of HIT
What dosage of low-molecular-weight heparin should patients receive for VTE prophylaxis? Should one measure effect?
Physiological changes (increased volume of distribution and renal clearance) during pregnancy alter the pharmacokinetics of drugs,[16] but until the study[17] evaluating required dosages for VTE prophylaxis in pregnancy is completed, the 2018 ASH guidelines suggest against the use of intermediate dosages in antepartum prophylaxis compared with standard dosages, and for the use of standard- or intermediate-dose low-molecular-weight heparin (LMWH) for postpartum VTE prophylaxis.[13] The SASTH guideline[12] also suggests standard prophylactic dosages, but in view of the physiological changes in pregnancy, recommends that regular anti-factor X activity (FXa) measurement be done, especially in women at extremes of weight, with renal disease and severe pre-eclampsia. The RCOG guideline[9] suggests that routine monitoring of the platelet count not be done in pregnant patients, unless UFH is used. Both the SASTH guideline[12] and the Society of Obstetricians and Gynaecologists of Canada[18] suggest that baseline platelet count be done, followed a week later with screening for HIT.
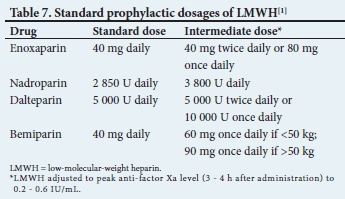
Standard dosages of LMWH are given in Table 7.
How long should VTE prophylaxis be continued?
Patients with a high VTE risk (such as those needing antepartum prophylaxis, previous VTE event, multiple risk factors) should continue with prophylaxis for 6 weeks postpartum, and at least for 10 days if at intermediate risk, with low-risk patients receiving thromboprophylaxis until discharged from hospital.[12]
Which patients should receive thromboprophylaxis after caesarean delivery?
There is no clear consensus regarding which patients have a low enough risk not to receive thromboprophylaxis after elective caesarean delivery (CD). Emergency CD alone puts the patient in the intermediate-risk group of the RCOG[9] (with thromboprophylaxis indicated). This intermediate-risk group also includes patients with elective CD and an additional risk factor. Another guidance paper[1] suggests VTE prophylaxis if one of the following is present: prior VTE, antepartum immobilisation (strict bedrest >1 week), significant postpartum infection, postpartum haemorrhage (PPH) of at least 1 000 mL that requires reoperation, pre-eclampsia with growth restriction, significant medical comorbidities (systemic lupus erythematosus (SLE), heart disease, sickle cell disease) or known thrombophilia. Thromboprophylaxis is also indicated if >2 of the following are present (in emergency CD >1): PPH >1 000 mL without
rupture) or any vaginal bleeding. Switching from LMWH to UFH closer to delivery has been suggested in the very-high-risk patient to reduce the 'time without anticoagulation period', but this showed increased bleeding risk.[26] (UFH can be discontinued 4-6 hours before delivery[27]) Attention should be paid to the mechanism of clotting (formation of thrombin), as well as to the other haemostatic factors (known as the 4 Ts).[3] The ESC guideline suggests active management of the third stage of labour with modified doses of oxytocin.[28] Wound drains (abdominal and rectus sheath) should be considered with CD and skin closure should be with interrupted sutures or staple clips.[9] Reintroduction of treatment should be started 4-6 hours after vaginal delivery or 6 -12 hours after CD (American College of Obstetricians and Gynecologists)[1] in the absence of postpartum haemorrhage and regional anaesthesia[9] The Royal College
of Obstetricians and Gynaecologists[9] guideline suggests avoiding the use of warfarin in the first 5 days after delivery, and for a longer period in the patient at risk of postpartum haemorrhage. Mechanical prophylactic measures should be left in situ until the patient is ambulatory and on anticoagulation again.
Managing neuraxial anaesthesia
With CD, the risk for adverse intraoperative and postoperative events is lower with neuraxial anaesthesia than with general anaesthesia. It is also often the choice for anaesthesia during labour, because it contributes to minimising the urge to bear down before complete cervical dilation and decreases circulating catecholamines.[29] If the patient is on anticoagulation, it is crucial to evaluate the specific drug, dosage and time since administration before neuraxial anaesthesia.
Knowledge of the patient's platelet count and possible drugs that interfere with platelet function is necessary before administering neuraxial anaesthesia. Extreme caution is needed if any other drugs influencing coagulation are prescribed,[12] and concomitant antiplatelet therapy (aspirin/non-steroidal anti-inflammatory drugs (NSAIDs)) should not be administered with heparin if a neuraxial catheter is left in situ[30] Aspirin should not be given for 5-7 days prior to neuraxial anaesthesia if the patient is receiving anticoagulant therapy. Women who have received neuraxial anaesthesia should reoperation necessary, BMI >30 kg/m2, fetal growth restriction, pre-eclampsia, multiple pregnancies and tobacco use during pregnancy (>10 cigarettes per day).
A practical approach is recommended by both SASTH[12] and the European Society of Anaesthesiology (ESA) VTE guideline task force:[19] all patients should receive thromboprophylaxis in hospital (ESA -except low-risk patients with elective CD), with the duration of thromboprophylaxis at least 6 weeks for high-risk patients and at least 7 days for low-risk patients. Mechanical prophylaxis after surgery and before starting LMWH should be left in place until the patient is mobilised and the LMWH effect is present, Managing neuraxial blockade is discussed below.
What should be done if patients on low-molecular-weight heparin develop side-effects?
• Even though the risk of HIT in pregnancy is low with the use of LMWH (estimated at <0.1%),[21] if the diagnosis of HIT is considered (using the 4Ts score: tone of the uterus, trauma to the birth canal, tissue (retained placenta or abnormal placentation and thrombin)), the patient should be switched to a non-heparin anticoagulant (fondaparinux), and the appropriate testing done to confirm the diagnosis. If ruled out, the patient may continue receiving LMWH.
• Heparin-induced skin reaction is often seen (1.8 - 42.0% of patients)[14] and switching to another LMWH often resolves the issue.
• Bleeding risk depends on the dosage of the anticoagulant and patient factors. Prior to prescribing thromboprophylaxis,[22] the patient's bleeding history should always be evaluated. Risk of bleeding is highest at the time of delivery and options to reduce this risk are discussed below under management of labour.
• Osteoporosis risk is estimated to be low with prophylactic dosages of LMWH, but data are needed on therapeutic LMWH use in pregnancy. This risk is much higher with the use of UFH. Routine screening for osteoporosis in the pregnant patient is not mandatory.[14]
Specific hypercoagulable difficult situations
• Antithrombin (AT) deficiency. Heparin (especially UFH) acts as an anticoagulant via AT, and VTE despite adequate LMWH prophylaxis has been described.[23] Another problem is that some of the anti-FXa laboratory methods add AT during testing; the laboratory value would then be unreliable in AT deficiency. These patients may need AT supplementation (not available in SA), and testing the effect of LMWH needs to be done with a method that does not add external AT in the laboratory.
• Antiphospholipid syndrome (API.S).[14] This acquired thrombophilia has a high risk of pregnancy complications. Patients with APLS (even without a previous history of VTE and not on long-term prophylactic anticoagulation before pregnancy) should receive prophylactic LMWH antepartum and up to 6 weeks postpartum (there is an ongoing study to find the optimal dose).[241 Patients receiving therapeutic anticoagulation before pregnancy should continue with therapeutic LMWH antepartum, postpartum and during pregnancy. Additional aspirin (<100 mg daily) should also be given. Catastrophic APLS, although rare, seems to be triggered in pregnancy, and a specialist team should manage these patients.
Diagnosis of VTE in pregnancy
A pregnant patient with signs or symptoms suggestive of DVT should be evaluated promptly and LMWH should be administered until the diagnosis has been excluded, unless there are contraindications to LMWH (15 - 24% of patients with DVT develop pulmonary embolism (PE), which is tatal in almost 15% of pregnant patients).[22] Until recently, pretest probability scoring systems (such as the modified Wells scoring system and the YEARS criteria, which include DVT present, haemoptysis and PE the most likely diagnosis) have not been used in the diagnosis of VTE during pregnancy. D-dimer testing has limited use to exclude VTE in pregnancy, as D-dimer values increase in pregnancy. Furthermore, the use of D-dimer testing in pregnancy remains controversial, as the RCOG guideline advises against such testing in this setting (a negative D-dimer is inadequate proof to exclude PE in pregnancy).[22]Fig. 3 shows the RCOG approach: if a DVT is clinically suspected, compression duplex ultrasonography should be undertaken. If this is negative, but there is a high level of suspicion, a repeat ultrasound examination should be done after 3 and 7 days.[22] If iliac vein thrombosis is suspected magnetic resonance imaging (MRI) or a venogram may be done if ultrasonography is negative.

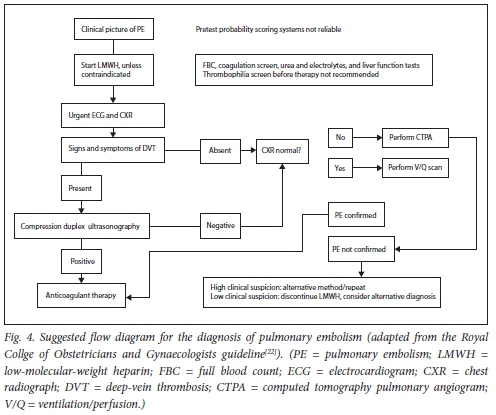
For the work-up of the pregnant woman presenting with a possible PE, the RCOG Green-top guideline[22] suggests the following (Fig. 4): Unless contraindicated, consider giving LMWH during work-up. The patient should have a chest radiograph and electrocardiogram (ECG) done urgently. If signs and symptoms of DVT are present, an ultrasound examination should be done, and if positive, no further testing is required (but the patient must be assessed for severity of the PE). If the ultrasound findings are negative, confirmation of the PE is either with a ventilation/perfusion (V/Q) scan or computed tomography pulmonary angiogram (CTPA). Radiation risk to the mother is the highest with CTPA, and radiation risk to the fetus is highest with a V/Q scan (radiation risk below the threshold for fetal teratogenicity, death or growth restriction, but a very small increased risk of childhood cancer development). Informed consent must be obtained from the mother before performing any of these tests. If the chest radiograph is abnormal, a CTPA is suggested rather than a V/Q (more specific for other pathological conditions). If the clinical suspicion is still high after the CTPA or V/Q test, alternative testing or repeat testing may be done, but anticoagulation must be continued until the diagnosis is definitely excluded. The ASH guideline[13] suggests a V/Q scan rather than CTPA for exclusion of PE. The European Society of Cardiology (ESC) 2019 acute pulmonary embolism management guideline,[25] however, suggests using the assessment of clinical probability, D-dimer measurement, compression ultrasound examination, and CTPA to possibly safely exclude PE in pregnancy. With new CTPA techniques, radiation to the breasts of the mother is much less (such as reducing kilovoltage, reducing the anatomical coverage of the scan and reducing the contrast-monitoring component of the CTPA).[25] If a PE is diagnosed, its severity and the risk of early death should be assessed as soon as possible, and the patient referred to a multidisciplinary team with experience in the management of PE.
Treatment of VTE in pregnancy
All guidelines state that treatment of VTE in pregnancy should be with LMWH as the preferred drug.[13] UFH may be used in massive PE (cardiovascular collapse), or when VTE occurs at term.[3, 22] Acute superficial vein thrombosis should also be treated with LMWH.[13] The dosage of LMWH needs to be therapeutic, and although traditionally given as a twice-daily dose, the ASH guideline[13] (low certainty) recommends that either once-daily or twice-daily LMWH may be administered. Due to the half-life of LMWH, a twice-daily dosage is a more rational choice pharmacokineti-cally.[3] Doses are titrated against the patient's booking or early pregnancy weight. Routine anti-FXa measurement is not indicated (ASH guideline),[13] but regular anti-FXa measurement is suggested in the SASTH guideline[12] (for prophylaxis and therefore also for treatment). The RCOG Green-top guideline[22] suggests anti-FXa measurement in cases of extremes of body weight (<50 kg or >90 kg), and in the presence of complicating factors (e.g. renal dysfunction). Recommended baseline blood measurements are full blood count, coagulation screening, urea and electrolytes and liver function tests, but thrombophilia screening should not be done at the time of VTE.[22] Most patients with VTE need to be admitted to hospital. Treatment should be continued with therapeutic doses during the remainder of the pregnancy and at least for 6 weeks postpartum. If the patient has a high risk of osteoporosis, bleeding or an isolated distal DVT, 1 month of full-dose therapy decreased to 75% of the therapeutic dose may be considered.[1] If the VTE event occurred during the last trimester, at least 3 months of therapy should be given. With VTE at term, consideration should be given to UFH (much easier to use therapeutically).[22] Thrombolysis should only be considered in life-threatening VTE (massive PE, limb-threatening DVT), and must be managed by a team of experienced clinicians.[22] The 2018 ASH guideline[13] states that pregnant patients with acute PE and right ventricular dysfunction in the absence of haemodynamic instability should not receive thrombolysis (systemic thrombolytic therapy only in acute PE and life-threatening haemodynamic instability). Graduated elastic compression stockings to reduce oedema are suggested.[22] The only indication for the insertion of an inferior vena cava filter would be an acute VTE and if anticoagulation is contraindicated.[1]
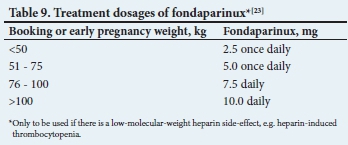
Suggested dosages of LMWH and fonda-parinux according to weight are described in Tables 8 and 9.
Managing labour in patients on anticoagulation
Anticoagulation at the time of labour poses a risk of bleeding, and all pregnant patients receiving antepartum thromboprophylaxis need a delivery plan.[12] This should be discussed early (-30 weeks' gestation)[14] and includes mode of delivery, e.g. CD, induction of labour (scheduled delivery) or spontaneous labour and delivery. The decision should be based on obstetric indication,[12] but mostly depends on patient and physician preference. The ASH guideline[13] suggests scheduled delivery with prior discontinuation of anticoagulation in patients receiving therapeutic dosages of LMWH (24 hours before delivery). In patients receiving prophylactic dosages of LMWH, the ASH guideline suggests against scheduled delivery (conditional recommendation, with very little evidence), but LMWH should be discontinued 12 hours prior to delivery. If spontaneous labour occurs, the patient must be advised to discontinue LMWH at the first sign of labour (contraction, membrane be monitored closely for the development of spinal haematoma (mandatory for a minimum of 12 hours; ideally for 72 hours).[12] Table 10 shows some of the suggested guidelines that have been published in the past year, but the SOAP guideline states clearly that it 'is not intended to set out a legal standard of care and does not replace medical care or the judgment of the responsible medical professional considering all the circumstances presented by an individual patienf.[30] It is also applicable to the patient with normal renal function in the context of pregnancy and body weight >40 kg, with no other known contraindications to neuraxial anaesthesia.[30] The ASRA guideline[28] is also incorporated in Table 10. It is important that each unit has an easily accessible protocol for managing these patients. If spontaneous labour occurs while the patient is on anticoagulation, neuraxial anaesthesia should not be administered if labour is within the contraindicated timeframe.
Summary
Managing VTE in the pregnant patient is complex, and although guidelines have been discussed in this article, a team approach, taking all factors in the individual patient into consideration, is essential. It is impossible to discuss all the permutations of thrombotic and bleeding risk factors in every patient. The reader is advised to read the excellent recent reviews, including the first statement by SOAP regarding anaesthetic management in pregnant patients receiving thromboprophylaxis.[30]
Declaration. None.
Acknowledgements. None.
Author contributions. Sole author.
Funding. None.
Conflicts of interest. I have been on advisory boards and received honoraria from Sanofi and Acino pharmaceutical companies.
References
1. Bates SM, MiddeJdorp S, Rodger M, James AH, Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 2016,41(1).92-128. https://doi.org/10.1007/sll239-015-1309-0 [ Links ]
2. Thornton P, Douglas J. Coagulation in pregnancy. Best Pract Res Clin Obstet Gynaecol 2010,24(3). 339-352. https://doi.org/10.1016/j.bpobgyn.2009.11.010 [ Links ]
3. Fogerty AE. Challenges of anticoagulation therapy in pregnancy. Curr Treat Options Cardiovasc Med 2017,19(10).76. https://doi.org/10.1007/sll93 [ Links ]
4. Bravo MC, Bernstein I, McBride C, et al. Hemostatic activation and glycocalyx shedding during pregnancy. Blood 2018,132.3794. https://doi.org/10.1182/blood-2018-99-119304 [ Links ]
5. Moodley J, Fawcus S, Pattinson R. Improvements in maternal mortality in South Africa. S Afr Med J 2018-,108(3a):s4-s8. https://doi.org/10.7196/SAMJ.2018.vl08i3.12770 [ Links ]
6. Speed V, Roberts LN, Patel JP, Arya R. Venous th rombo em bolism and women's health. Br J Haematol 2018-,183(3).346-363. https://doi.org/10.1111/bjh.l5608 [ Links ]
7. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period. Incidence, risk factors, and mortality Am J Obstet Gynecol 2006,194(5).1311-1315. https://doi.org/10.1016/j.ajog.2005.11.008 [ Links ]
8. McLean K, Cushman M. Venous th rombo em holism and stroke in pregnancy. Hematology 2016,2016(1).243-250. https://doi.org/10.1182/asheducation-2016.1.243 [ Links ]
9. Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-Top Guideline No. 37a, 2015. https://www.rcog.org.uk/globalassets/documents/guidelin es/gtg-37a.pdf (accessed 30 September 2019). [ Links ]
10. Dargaud Y, Rugeri L, Fleury C, et aL Personalized thromboprophylaxis using a risk score for the management of pregnancies with high risk of thrombosis. A prospective clinical study. J Thromb Haemost2017-,15(5)*97-906. https://doi.org/10.1111/jth.13660 [ Links ]
11. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing G-J, Kyrle PA, for the Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease. Categorization of patients as having provoked or unprovoked venous thromboembolism. Guidance from the SSC of ISTH. J Thromb Haemost 2016,14(7).1480-1483. https://doi.org/10.llll/jth.1333e [ Links ]
12. Schapkaitz E, de Jong PR, Jacobson BF, Bülier HR. Recommendations for thromboprophylaxis in obstetrics and gynaecology. S Afr J Obstet Gynaecol 2018,24(1).27-31. https://doi.org/10.7196/sajog.1312 [ Links ]
13. Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism. Venous thromboembolism in the context of pregnancy. Blood Advances 2018,2(22).3317-3359. https://doi.org/10.1182/bloodadvances.2018024802 [ Links ]
14. Scheres LJJ, Bistervels IM, Middeldorp S. Everything the clinician needs to know about evidence-based anticoagulation in pregnancy. Blood Rev 2019-,33.82-97. https://doi.org/10.1016/j.blre.2018.08.001 [ Links ]
15. Bleker SM, Coppens M, Middeldorp S. Sex, thrombosis and inherited thrombophilia. Blood Rev 2014,28.123-133. https://doi.org/10.1016/j.blre.2014.03.005 [ Links ]
16. Anderson GD. Pregnancy-induced changes in pharmacokinetics. Clin Pharmacokinet 2005*14(10). 989-1008. https://doi.org/10.2165/00003088-200544100-00001 [ Links ]
17. Bleker SM, Buchmüller A, Chauleur C, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy. Rationale and design of the Highlow study, a randomised trial of two doses. Thromb Res 2016,144:62-68. https://doi.org/10.1016/j.thromres.2016.06.001 [ Links ]
18. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can 2014,36(6).527-553. https://doi.org/10.1016/S1701-2163(15)30569-7 [ Links ]
19. Ducloy-Bouthors A-S, Baldini A, Abdul-Kadir R, Nizard J, for the ESA VTE Guidelines Task Force. European guidelines on perioperative venous thromboembolism prophylaxis. Surgery during pregnancy and the immediate postpartum period. Eur J Anaesthesiol 2018,35(2).130-133. https://doi.org/10.1097/eja.0000000000000704 [ Links ]
20. James A, Committee on Practice Bulletins. Obstetrics Practice Bulletin No. 123·. Thromboembolism in pregnancy. Obstet Gynecol 2011,118(3).718-729. [ Links ]
21. Linkins L-A, Dans AL, Mores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia. Antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012,141(2 Suppl).e495S-e530S. https://doi.org/10.1378/chest.l1-2303 [ Links ]
22. Royal College of Obstetricians and Gynaecologists. Thrombosis and embolism during pregnancy and the puerperium. Acute management (Green-top Guideline No. 37b). 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37b.pdf (accessed 30 September 2019). [ Links ]
23. Fogerty AE. Management of venous thromboembolism in pregnancy. Curr Treat Options Cardiovasc Med 2018-,20(8).69. https://doi.org/10.1007/sll936-018-0658-3 [ Links ]
24. Bleker SM, Buchmuller A, Chauleur C, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy. Rationale and design of the highlow study, a randomised trial of two doses. Thromb Res 2016,144:62-68. https://doi.org/10.1016/j.thromres.2016.06.001 [ Links ]
25. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines tor the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2019.1-61. https://doi.org/10.1093/eurhearti/ehz405 [ Links ]
26. Wang EHZ, Marnoch CA, Khurana R, Sia W, Yuksel N. Haemorrhagic complications of peripartum anticoagulation. A retrospective chart review. Obstet Med 2014,7(2).77-83. https://doi.org/10.1177%2F1753495X14520849 [ Links ]
27. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy. Antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012,141(2 Suppl).e691S-e736S. https://doi.org/10.1378/chest.ll-2300 [ Links ]
28. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018,39(34).3165-3241. https://doi.org/10.1093/eurhearti/ehy340 [ Links ]
29. Horlocker TT, Vandermeuelen E, Kopp S, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anesth Pain Med 2018,43(3).263-309. https://doi.org/10.1097/AAR0000000000000763 [ Links ]
30. Leffert L, Butwick A, Carvalho Β, et al. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg 2018,12(3).928-944. https://doi.org/10.1213/ane.0000000000002530 [ Links ]
 Correspondence:
Correspondence:
Ρ F Wessels
wesselspf@mweb.co.za
Accepted 28 August 2019














