Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 no.8 Pretoria Ago. 2019
http://dx.doi.org/10.7196/samj.2019.v109i8.13868
RESEARCH
Hepatitis E in pig-derived food products in Cape Town, South Africa, 2014
S KorsmanI; J BloembergII; M BrombacherII; A GiuricichII; R P Halley-StottIII; M KabaIV
IMB ChB, MMed (Virology), FCPath (SA) Viro; National Health Laboratory Service, Groote Schuur Hospital, Cape Town, South Africa; Division of Medical Virology, Department of Pathology, Faculty of Health Sciences, University of Cape Town, South Africa
IIMB ChB; Division of Medical Virology, Department of Pathology, Faculty of Health Sciences, University of Cape Town, South Africa
IIIPhD, MB ChB; Division of Medical Virology, Department of Pathology, Faculty of Health Sciences, University of Cape Town, South Africa
IVMD, PhD; Division of Medical Microbiology, Department of Pathology, and Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Hepatitis E virus (HEV) genotypes 3 and 4 are zoonoses, with domestic pigs being the most important reservoir. A high anti-HEV IgG seroprevalence of 26 - 28% has been found in humans in Cape Town, South Africa (SA). Studies in industrialised countries have indicated a high prevalence of HEV in pigs and their associated food products
OBJECTIVES: To determine whether HEV could be found in pig-derived food products in Cape Town
METHODS: Pork-containing food products were purchased from supermarkets and butcheries around the Cape Town metropolitan area. HEV detection by polymerase chain reaction (PCR) was performed, and an amplified viral genome fragment was sequenced from positive samples. Phylogenetic analysis was done on the sequenced fragment
RESULTS: HEV was detected by PCR in 2/144 food samples - both were liver spread samples. One genome fragment sequence was obtained, which was closely related to HEV sequences obtained from humans in Cape Town
CONCLUSIONS: HEV can be found in pork-containing meat products available for sale in Cape Town, suggesting that these products could be a potential source of HEV transmission in our geographical area. Meat of pig origin should be thoroughly cooked before being consumed
Hepatitis E virus (HEV) is a small, spherical, non-enveloped RNA virus belonging to the family Hepeviridae. HEV, of which four genotypes cause most human disease, is a common causative agent of acute viral hepatitis throughout the world. Genotypes 1 and 2 are associated with waterborne transmission and cause epidemics. Genotypes 3 and 4 are zoonoses, with domestic pigs being the most important reservoir.[1] Infection is usually subclinical and self-limiting but can cause severe jaundice and acute liver failure, particularly in pregnant women and immunocompromised patients.[1] Studies have found a high prevalence (26 - 28%) of anti-HEV IgG in humans in Western Cape Province, South Africa (SA).[2,3] Risk factors associated with HEV seropositivity include exposure to pig meat.[2,3] In animals, HEV infection has been identified in pigs in the Eastern Cape[4] and Western Cape provinces[5] of SA.
Studies in industrialised countries found a high prevalence of HEV in pigs and their associated food products. For example, HEV RNA was detected in pig-derived products sold in stores in the USA,[6] UK[7] and France.[8] In a mini-outbreak of hepatitis E reported in France,[8] 7/13 individuals who ate figatellu (dried sausage made from raw pig liver) had detectable IgM and/or HEV RNA, as opposed to 0/5 individuals from the same families who did not consume the sausage. In that study, HEV sequences from sausage and infected individuals were genetically similar.[8] The risk of contracting the virus by way of pork products is higher when consuming undercooked or raw pork products.[9] Cooking at 71°C for 20 minutes has been found to fully inactivate the virus.[9]
The objective of the current study was to determine whether HEV could be found in pig-derived food products in Cape Town, SA. We hypothesised that HEV RNA could be found in samples from various pig-derived meats purchased in the Cape Town area. This study points to a plausible infective source to explain the high levels of antibody reactivity against HEV seen in humans in Cape Town.
Methods
Pork products (N=144) were purchased from 59 vendors (45 supermarkets and 14 butcheries) around the Cape Town metropolitan area over 3 days in July 2014. Samples were kept in 'cold boxes' during acquisition and stored at 4°C overnight until processing the next day. Most of the samples collected (n=104) were raw pig products (Table 1). The remaining samples (n=40) were processed or partly processed meats (Table 1).
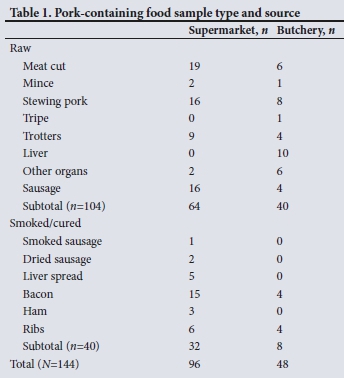
The samples were processed using sterile equipment to prevent cross-contamination. Prior to nucleic acid extraction, ~25 mg of meat from each sample was finely chopped with a scalpel to aid tissue lysis. Nucleic acid was extracted from each sample using the Qiagen QIAamp DNA Mini Kit (Qiagen, Germany) as per the manufacturer's instructions, and eluted in 100 μL. of the buffer provided. Real-time reverse transcription polymerase chain reaction (PCR) was performed on the RotorGene 2000 as described by Garson et al.,[10] using the QuantiFast Pathogen RT-PCR +IC Kit (Qiagen, Germany). Positive samples were amplified as described by Meng et al.,[11] using the SuperScript One-Step RT-PCR System with Platinum Taq (Thermo Fisher Scientific, USA) for the first round of PCR, and SuperTherm Taq DNA Polymerase (Separation Scientific, SA) for the nested reaction, using the following primers:[11] forward outer 3156, reverse outer 3157, forward inner 3158, reverse inner 3159. This region of open reading frame 2 was chosen for analysis based on detection of the four main genotypes, sufficient variability for analysis and ability to compare with other sequences and studies, as this is the most commonly sequenced region. The resulting 348 base-pair product from open reading frame 2 was sent to Inqaba Biotec (SA) for bidirectional sequencing using the nested amplification primers. Chromatograms were edited in FinchTV (Geospiza, USA) and sequences were analysed using MEGA6 (MEGA, USA).[12] The maximum likelihood tree was calculated in MEGA6 using an alignment of 279 nucleotide positions, and bootstrap support was calculated with 1 000 replicates.
Ethical approval
The study was approved by the Human Research Ethics Committee of the University of Cape Town (ref. no. 379/2014).
Results
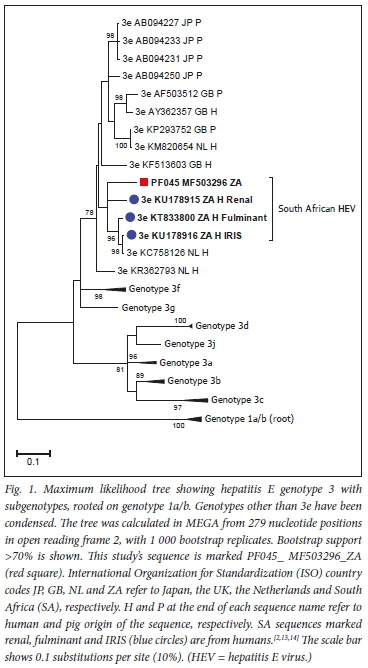
HEV RNA was detected by real-time reverse transcription PCR in two liver spread samples (PF039 and PF045) obtained from different supermarket chains in different suburbs of Cape Town. The cycle threshold values for samples PF039 and PF045 were 37.4 and 34.6, respectively. High-quality HEV sequence (Genbank accession MF503296) was obtained in one of these samples (PF045), and it clustered with other genotype 3e HEV sequences previously reported in humans in Cape Town (Fig. 1). Our HEV sequence shared 87.5 -89.6% similarity with other HEV sequences from Cape Town, and was also closely related (90.4% similarity) to an HEV genotype 3e sequence (Genbank accession KC758126) from the Netherlands (Fig. 1).
Discussion
HEV can be found in pork-containing meat available for sale in Cape Town. This corresponds with what has been reported in industrialised countries.[6-8] The high similarity between our genotype 3 HEV and that of human HEV cases in Cape Town[2,13,14] suggests that pork-containing food products could be a potential source of HEV transmission in our geographical area. Another possible reservoir not yet studied locally could be filter-feeding shellfish, as they feed on sewage entering the ocean; such shellfish are regularly eaten in Cape Town. Although not yet reported in SA, further potential sources of HEV in our study area may include seafood and raw vegetables. HEV has been detected in seafood[15] and vegetables[16] in the UK and Italy, respectively. Finally, blood donor safety needs further consideration.[17]
Conclusions
HEV can be found in pork-containing meat available for sale in Cape Town. Further studies are needed to investigate the foodborne transmission of HEV in SA. Public awareness around pork-containing food safety should be encouraged, especially for high-risk groups,[17] and meat of pig origin should be thoroughly cooked before being consumed.
Declaration. None.
Acknowledgements. None.
Author contributions. MK: study outline, manuscript review; SK: study design, laboratory work, analysis, manuscript writing/review; JB, MB, AG, RPH: contributed equally to sample collection, laboratory work, manuscript writing/review. JB, MB, AG, RPH were undergraduate MB ChB students while the study was being conducted. RPH was in possession of a PhD degree before entering the MB ChB course.
Funding. The project received no specific funding for reagents or laboratory work. MK was a Wellcome Trust (UK) fellow (102429/Z/13/Z) and is currently supported by the Carnegie Corporation of New York (USA) early-career fellowship, the Canadian Institutes for Health Research (CIHR), Canadian HIV Trials Network (CTN) international fellowship, and the US National Institutes of Health (1R01HD093578-01).
Conflicts of interest. None.
References
1. Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med 2012;367(13):1237-1244. https://doi.org/10.1056/NEJMra1204512 [ Links ]
2. Madden RG, Wallace S, Sonderup M, et al. Hepatitis E virus: Western Cape, South Africa. World J Gastroenterol 2016;22(44):9853-9859. https://doi.org/10.3748/wjg.v22.i44.9853 [ Links ]
3. Lopes T, Cable R, Pistorius C, et al. Racial differences in seroprevalence of HAV and HEV in blood donors in the Western Cape, South Africa: A clue to the predominant HEV genotype? Epidemiol Infect 2017;145(9):1910-1912. https://doi.org/10.1017/S0950268817000565 [ Links ]
4. Adelabu OA, Iweriebor BC, Nwodo UU, Obi LC, Okoh AI. Incidence and molecular characterization of hepatitis E virus from swine in Eastern Cape, South Africa. Adv Virol 2017;2017:1073253. https://doi.org/10.1155/2017/1073253 [ Links ]
5. Van Helden L, Korsman S, Grewar J, et al. A One Health approach to investigation of zoonotic hepatitis E virus in Cape Town, South Africa. In: 4th International One Health Congress and 6th Biennial Congress of the International Association for Ecology and Health, 3 - 7 December 2016, Melbourne, Australia. https://link.springer.com/article/10.1007/s10393-016-1191-z (accessed 19 June 2019). [ Links ]
6. Meng XJ. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res 2011;161(1):20-30. https://doi.org/10.1016/j.virusres.2011.01.016 [ Links ]
7. Berto A, Martelli F, Grierson S, Banks M. Hepatitis E virus in pork food chain, United Kingdom, 2009 - 2010. Emerg Infect Dis 2012;18(8):1358-1360. https://doi.org/10.3201/eid1808.111647 [ Links ]
8. Colson P, Borentain P, Queyriaux B, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 2010;202(6):825-834. https://doi.org/10.1086/655898 [ Links ]
9. Barnaud E, Rogée S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol 2012;78(15):5153-5139. https://doi.org/10.1128/AEM.00436-12 [ Links ]
10. Garson JA, Ferns RB, Grant PR, et al. Minor groove binder modification of widely used TaqMan probe for hepatitis E virus reduces risk of false negative real-time PCR results. J Virol Methods 2012;186(1-2):157-160. https://doi.org/10.1016/j.jviromet.2012.07.027 [ Links ]
11. Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA 1997;94(18):9860-9865. https://doi.org/10.1073/pnas.94.18.9860 [ Links ]
12. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013;30(12):2725-2729. https://doi.org/10.1093/molbev/mst197 [ Links ]
13. Andersson MI, Preiser W, Maponga TG, et al. Immune reconstitution hepatitis E: A neglected complication of antiretroviral therapy in Africa? AIDS 2013;27(3):487-489. https://doi.org/10.1097/QAD.0b013e32835b1074 [ Links ]
14. Andersson MI, Stead PA, Maponga T, van der Plas H, Preiser W. Hepatitis E virus infection: An under-diagnosed infection in transplant patients in Southern Africa? J Clin Virol 2015;70:23-25. https://doi.org/10.1016/j.jcv.2015.06.081 [ Links ]
15. Crossan C, Baker PJ, Craft J, Takeuchi Y, Dalton HR, Scobie L. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg Infect Dis 2012;18(12):2085-2087. https://doi.org/10.3201/eid1812.120924 [ Links ]
16. Terio V, Bottaro M, Pavoni E, et al. Occurrence of hepatitis A and E and norovirus GI and GII in ready-to-eat vegetables in Italy. Int J Food Microbiol 2017;249:61-65. https://doi.org/10.1016/j.ijfoodmicro.2017.03.008 [ Links ]
17. Tedder RS, Ijaz S, Kitchen A, et al. Hepatitis E risks: Pigs or blood - that is the question. Transfusion 2017;57(2):267-272. https://doi.org/10.1111/trf.13976 [ Links ]
 Correspondence:
Correspondence:
S Korsman
stephen.korsman@nhls.ac.za
Accepted 4 February 2019.














