Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 n.7 Pretoria Jul. 2019
http://dx.doi.org/10.7196/samj.2019.v109i7.13705
RESEARCH
doi:10.7196/samj.2019.v109i7.13705
Characterisation of protease resistance mutations in a South African paediatric cohort with virological failure, 2011 - 2017
Z MakatiniI; S MdaII; O TowobolaIII; H MthiyaneIV; P MilesV; J T BlackardVI
IMSc, MB ChB, DTM&H, Dip HIV Man, FCPath (SA) Viro; Department of Virology, Faculty of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa; Department of Virology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB, PhD; Department of Virology, Faculty of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IIIPhD; Department of Virology, Faculty of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
IVMSc; Department of Virology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VMSc; Waltham Forest Clinical Commissioning Group, Leytonstone, London, UK
VIPhD; Department of Internal Medicine, College of Medicine, University of Cincinnati, Ohio, USA
ABSTRACT
BACKGROUND: Advances in HIV management have improved treatment outcomes in the HIV-infected population. However, these advances have not been without multifaceted challenges. In sub-Saharan Africa, their impact is reflected in the increased emergence of HIV drug resistance mutations. With the rise in exposure of children to protease inhibitors (PIs), the possibility of increasing PI resistance remains a concern
OBJECTIVES: To describe a group of antiretroviral-experienced children with PI drug resistance mutations after failure on first- or second-line regimens in a public sector setting in South Africa
METHODS: This was a retrospective cohort study of 22 children perinatally infected with HIV who had HIV genotyping conducted between January 2011 and December 2017
RESULTS: Of the 236 children who had HIV genotyping conducted, 22 (9.3%) had evidence of HIV PI resistance mutations. Twenty-one of the 22 children (95.5%) had major mutations in the protease region of the HIV genome. Of these children, 66.7% (14/21) had loss of response to both boosted lopinavir and atazanavir, with boosted darunavir remaining susceptible in only 12 (57.1%). The most frequent major PI mutations were V82A (76.2%), M46I/M46L (76.2%), I54V (62.0%) and L76V(33.3%
CONCLUSIONS: We observed a high rate of PI resistance mutations, with a resulting loss of PIs that could be used in construction of third-line regimens. To build on improvements from the introduction of antiretroviral therapy, increased efforts are needed by both health professionals and caregivers to improve adherence measures in children perinatally infected with HIV
The introduction and scale-up of combination antiretroviral therapy (cART) has resulted in a significant decrease in morbidity and mortality rates in children perinatally infected with HIV.[1,2] These children are now surviving into adolescence and adulthood with lifelong chronic HIV infection.[3] Challenges persist, however, in part owing to the limited availability of antiretroviral treatment options for paediatric patients.[4] Further, inequalities persist in the South African (SA) public sector healthcare system, and limited clinical HIV expertise remains a key concern.[5] In poorly resourced public sector settings in SA, with higher rates of virological failure[6-8] and longer duration of a failing regimen,[4,9,10] the emergence of drug resistance mutations poses a threat to future antiretroviral options.
SA national antiretroviral guidelines for the treatment of HIV-infected children have evolved from cART initiation with stavudine (d4T) as part of the nucleoside reverse transcriptase inhibitor (NRTI) backbone to abacavir (ABC).[11] Single dose-ritonavir (RTV) was routinely prescribed to children aged <3 years for the first 3 years of the antiretroviral roll-out programme in SA.[12] Prior exposure to RTV-based regimens has been reported to be associated with suboptimal treatment responses, thus increasing the risk of acquisition of protease inhibitor (PI) resistance mutations.[13] Boosted lopinavir (LPV) for children aged <3 years and efavirenz (EFV) for children aged >3 years has remained unchanged. A number of strategies, including lamivudine (3TC) monotherapy, have been widely employed to manage the complex adherence challenges in children failing first- or second-line therapy in the public sector setting. As a result, a large proportion of children are exposed to a wide array of antiretroviral agents with a potential of emergence of HIV drug resistance, resulting in limited options for the future. Access to salvage therapy in the SA public sector was implemented in 2015 by the National Department of Health and allows for construction of an appropriate regimen determined by an HIV genotype test.[11]
PIs are an important foundation for the management of HIV-infected children in SA. The type of PI exposure and whether unboosted PIs are used influence HIV resistance PI mutations and patterns that emerge.[14] Despite the high genetic barrier of PIs, major PI resistance mutations have been reported in several SA studies (involving 89, 75 and 50 children), with frequencies of 6.3%, 10.7% and 14%, respectively[14-16]
Objectives
The current study focuses on a group of heavily experienced children managed in the public sector with resource constraints, with virological failure of either first-line or second-line regimens, longer duration of a failing regimen, exposure to various treatment strategies to mitigate virological failure, and the risk of increasing acquisition of PI resistance.
Methods
Study population
This was a retrospective cohort study of children perinatally infected with HIV attending Dr George Mukhari Academic Hospital (DGMAH), a public sector hospital located outside Pretoria, SA.
Approximately 2 200 children are actively managed in the DGMAH HIV paediatric clinic. Children are transferred to the adult HIV clinic on reaching the age of 16 years. The HIV package of care includes routine monthly clinic visits, follow-up CD4+ T-cell counts and HIV viral load (VL) testing, as well as safety bloods as defined by the SA National Antiretroviral Treatment Guidelines.[12] Ongoing academic research activities provided access to HIV genotyping for individuals with a VL >1 000 copies/mL. The majority of the paediatric attendees were initiated on an ABC with 3TC NRTI backbone with either EFV or boosted LPV depending on age. However, a number of children were initiated on treatment prior to 2010 and remained on d4T with 3TC as part of the NRTI backbone. Enrolment of the cohort was through convenience sampling of children aged <16 years who had been on cART for ≥1 year, had developed virological failure, and had HIV genotyping conducted from January 2011 to December 2017. Children with missing clinical files or HIV genotype reports conducted prior to 2011 were excluded from the cohort.
Ethical considerations
The Sefako Makgatho Health Sciences University Research and Ethics Committee approved collection of clinical and genotype data (ref. no. MREC/P/83/2009). Written informed consent was obtained from each child's caregiver.
HIV genotyping
HIV genotyping was performed using an in-house drug resistance assay. Briefly, a 1.7 kb amplicon was generated by reverse transcriptase (RT)-initiated polymerase chain reaction of the entire protease (PR) and partial RT-coding regions. The amplicon was sequenced using five primers from codons 1 - 99 of PR and codons 1 - 230 of RT.
Sequencing was performed with either an ABI PRISM 3730 or an ABI PRISM 3100-Avant Genetic Analyzer (Applied Biosystems, USA). To identify drug resistance mutations and predict drug susceptibility, the Stanford HIV Drug Resistance Database version 8.3 (Stanford University, USA) was used. Susceptibility to boosted LPV, atazanavir (ATV) and darunavir (DRV) was determined using the penalty score where, from the total score for each of the three PIs, five levels of inferred drug resistance were reported: susceptible, potential lowlevel resistance, low-level resistance, intermediate resistance and high-level resistance.
Statistical analysis
Clinical baseline and demographic variables were compared using medians with interquartile ranges (IQRs) for numerical data or proportions for categorical data, with genotype data presented as proportions. A two-sided Fisher's exact test was used to compare the association of categorical variables, and p-values <0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, USA).
Results
Patient characteristics
Demographic and baseline clinical characteristics are presented in Tables 1 and 2. Of 236 children who had HIV genotyping conducted over the 6-year study period, 22 (9.3%) had evidence of PI HIV resistance mutations and were included in the study; 54.5% of all children were female. World Health Organization (WHO) clinical stage at cART initiation was III or IV in 91.0% of the children. WHO stage III was the most common stage at 77.2%, representing most of the children noted to have been placed on concurrent TB treatment at cART initiation. The median age at initiation was 3 years (IQR 1.25 - 8.6), with most of the children (45.5%) initiating cART between the ages of 0 and 3 years and ~14% at an advanced age of 10 - 16 years.
All children were documented to have been infected perinatally. Prior to treatment initiation, 40.9% of children had laboratory evidence of severe immunosuppression, with CD4+ T-cell percentages between 0% and 10%. Review of CD4+ T-cell absolute counts revealed that 27.3% had CD4+ T-cell counts <100 cells/μL. Overall, the median duration of a failing regimen was 22 months (IQR 6 - 66), and the median VL at genotype was 4.5 log10 copies/mL.
Virological failure
The median age of all 22 children at genotyping was 8 years (IQR 4.35 - 11.7), with a duration of 22 months on a failing regimen.
The median HIV VL at failure was 4.52 log10 (IQR 3.38 - 4.8). All 22 children with PI resistance mutations had been on a boosted LPV regimen at the time of genotyping. The predominant NRTI backbone in use in this cohort was zidovudine (AZT) with 3TC (n=7, 31.8%) and ABC with 3TC (n=7, 31.8%). The remaining children were on AZT with ABC (n=5, 22.7%) and d4T with 3TC (n=3, 13.6%). In addition, 6 of the children (22.3%) had prior exposure to single-dose RTV for a median duration of 3 months as well as 3TC monotherapy as a holding regimen.
Drug resistance mutations
All genotyped samples belonged to HIV subtype C. Twenty-one of the 22 children (95.5%) had major mutations in the PR region.
The distribution of all PI mutations is reflected in Figs 1 and 2.
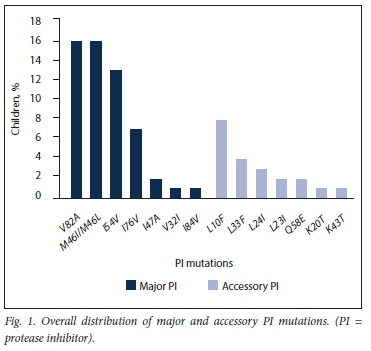

The most frequently occurring major PI mutations were V82A (76.2%), I54V (62.0%), M46I (52.4%), L76V (33.3%) and M46L (23.8%). The remaining major PI mutations I47A, I84V and V32I were detected at frequencies of 9.5%, 4.7% and 4.7%, respectively.
The median number of major PI mutations was 3 (IQR 2 - 4), with the predominance of 3 mutations seen in 6 children. The major LPV resistance mutations detected were M46I, L76V and V82A, occurring alone or in combinations of up to 5 mutations in 7 children. Fourteen of the 21 children with major mutations (66.7%) experienced loss of activity to both boosted LPV and ATV. However, boosted DRV remained susceptible in 12 (57.1%).
Mutations classified as 'accessory' PI resistance mutations by the IAS (International Antiviral Society)-USA were detected in 10/22 children (45.5%), with only one (4.5%) having a Q58E non-polymorphic accessory mutation exclusively.
'Other' PI mutations A71V and L10V were observed with a frequency of 2 each. Further analysis of children with prior exposure to RTV revealed no association between duration of single-dose RTV and emergence of PI mutations (p=0.275). There was no association between duration of a failing regimen (<2 years v. >2 years on failing regimen) and the emergence of additional PI mutations (p=0.6032).
The most common NRTI mutation was the M184V mutation, detected at a frequency of 17 (77.3%). L74V and L74I - both with compensatory effects on viral fitness - were detected in children on ABC, with a frequency of 2 (9.1%) and 1 (4.5%), respectively. The combination of L74V plus M184V occurred in 3 children (13.6%) in our cohort. Viruses carrying the K65R resistance mutation were observed in only 3 children (13.6%). Of interest is that none of these children were documented to have been on tenofovir disoproxil fumarate (TDF), but all had exposure to ABC at the time of HIV genotyping. Thymidine analogue mutations (TAMs) were observed in 63.6% of the children, with 4 (18.2%), 3 (13.6%) and 6 (27.3%) harbouring 1, 2 and >3 TAMs, respectively. A distinct TAMs 1 pathway pattern was detected in 3 patients, with the rest displaying overlapping TAMs 1 and 2 pathways. Non-nucleoside reverse transcriptase inhibitor (NNRTI) mutations were detected in 14 children (63.6%), at the following frequencies: K103N n=5, 22.7%; V106M n=3, 13.6%; G190A n=5, 22.7%; Y188C/L n=5, 22.7%; and E138A/Q n=2, 9.1%. Cross-level resistance to first-generation NNRTIs was evident in 12 of these children (85.7%), with only 7 of them remaining fully susceptible to etravirine, a second-generation NNRTI often used for construction of salvage therapy.
Discussion
With high levels of virological failure in our cohort, convenience sampling of 236 children for HIV genotyping was conducted over a 6-year period. We focused on the 9.3% (22/236) who displayed PI resistance mutations and were on PIs at the time of HIV genotyping. Our findings of increasing PI resistance mutations are consistent with other SA studies of similar size conducted between 2000 and 2012, in which rates of PI resistance mutations were 6.3%,[15] 10.7%[14] and 14%.[16] However, another study reported a high prevalence of major PI mutations (49%)[17] in a public sector setting in SA.
Baseline clinical information on the study participants reflects a pattern consistent with poor outcomes.[18] While the overall median age of initiation of cART was 3 years, 13.6% of the children were initiated at ages between 10 and 16 years and 91.0% were clinically staged as WHO III or IV. In addition, 40.9% of the children had a nadir CD4+ T-cell percentage between 0% and 10%. It has been suggested and shown that a low CD4+ T-cell count may be associated with an increased probability of virological failure on PI-based regimens.[19-21]
A factor contributing to the emergence of major PI resistance mutation is prior exposure to an unboosted PI during ongoing viraemia.[17,22,23] With the SA national antiretroviral treatment guidelines in the past (2004 - 2007) having advocated full-dose RTV in children aged <6 months and those on concomitant rifampicin-based antituberculosis therapy,[12] 6 (27.3%) of the children in our cohort with major PI mutations had been on full-dose RTV. Similar findings from a number of small studies of children on full-dose RTV support the development of major PI resistance mutations, with reported rates of 37.1%,[24] 50%[18] and 72%.[14] However, the high proportion of children with major PI-associated resistance mutations in our cohort (95.5%) cannot be explained by prior use of RTV alone. Three of the 21 children with major PI mutations (14.3%) also had natural polymorphisms that on their own would not be expected to affect the efficacy of boosted PIs.[25-27]
Knowledge of the effect of various HIV resistance mutations on antiretroviral susceptibility can facilitate selection of the most active second- or third-line regimen, especially if options involving other classes of drugs have been exhausted. In our cohort, major PI mutations were detected at a high rate of 95.5%, resulting in 66.7% of children losing activity of both boosted LPV and ATV, with 12 (57.1%) remaining fully susceptible to second-generation DRV. V82A - a non-polymorphic mutation selected primarily by boosted LPV - was one of the most frequently occurring PI mutations in this cohort, detected in 76.2% of the children with major PI mutations. Emergence of V82A resulted in loss of activity of boosted LPV with cross-level resistance to boosted ATV.[28-30] However, L76V, a PI mutation selected for by LPV and DRV,[31,32] was observed in 7 (33.3%) of these children, resulting in loss of activity of both boosted LPV and DRV. The presence of the major PI mutations with resulting loss to both LPV and ATV, as well as DRV, accessible only through the SA National Department of Health third-line committee as part of salvage therapy, remains a serious concern. The fact that none of the children in our cohort were presented to the third-line committee highlights the need for increased awareness of availability of access to this committee.
Study limitations
A limitation of this study is the fact that it was conducted at a single site, an academic hospital, so the findings may not be generalisable to other settings. The small cohort size also means that caution should be exercised when interpreting these findings, and a study with a much larger cohort may be needed to corroborate the findings.
Conclusions
Despite gains made in HIV management, HIV resistance continues to be a significant challenge in HIV-infected children, limiting current and future options. Of concern is the loss of antiretroviral agents typically used to construct a regimen for salvage therapy and the fact that none of the children in the study had accessed third-line committee-recommended therapy.
Declaration. The publication of this article was a requirement for ZM's PhD degree.
Acknowledgements. We acknowledge the children and staff at DGMAH.
Author contributions. ZM contributed to the study design, analysis and writing of the manuscript. JTB, SM and OT contributed to the study design, analysis and manuscript review. PM and HM largely contributed to the manuscript review.
Funding. We thank the Discovery Foundation Fellowship Award for financial contribution towards this study.
Conflicts of interest. None.
References
1. Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 2008;8(8):477-489. https://doi.org/10.1016/S1473-3099(08)70180-4 [ Links ]
2. World Health Organization, United Nations Children's Fund, Joint United Nations Programme on HIV and AIDS. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. HIV Treatment. Geneva: WHO, 2013. https://www.who.int/hiv/pub/progressreports/update2013/en/(accessed 25 August 2018). [ Links ]
3. Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: Keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc 2013;16:18555. https://doi.org/10.7488/IAS.16.1.18555 [ Links ]
4. Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, Sherman G. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis 2007;19ó(Suppl 3):S474-S481. https://doi.org/10.1086/521116 [ Links ]
5. Borghi J, Ataguba J, Mtei G, et al. Methodological challenges in evaluating health care financing equity in data-poor contexts: Lessons from Ghana, South Africa and Tanzania. In: Chernichovsky D, Hanson K, eds. Innovations in Health System Finance in Developing and Transitional Economies (Advances in Health Economics and Health Services Research, vol. 21). Bingley, UK: Emerald Group Publishing, 2009:133-156. [ Links ]
6. Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J 2011;30(11):974-979. https://doi.org/10.1097/INF.0b013e31822539f6 [ Links ]
7. Sigaloff KC, Calis JC, Geelen SP, van Vugt M, de Wit TF. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: A systematic review. Lancet Infect Dis 2011;11(10):769-779. https://doi.org/10.1016/S1473-3099(11)70141-4 [ Links ]
8. Gupta RK, Gibb DM, Pillay D. Management of paediatric HIV-1 resistance. Curr Opin Infect Dis 2009;22(3):256-263. https://doi.org/10.1097/QCO.0b013e3283298f1f [ Links ]
9. Janssen N, Ndirangu J, Newell ML, Bland RM. Successful paediatric HIV treatment in rural primary care in Africa. Arch Dis Child 2010;95(6):414-421. https://doi.org/10.1136/adc.2009.169367 [ Links ]
10. Green TN, Archary M, Gordon ML, et al. Drug resistance and coreceptor usage in HIV type 1 subtype C-infected children initiating or failing highly active antiretroviral therapy in South Africa. AIDS Res Hum Retroviruses 2012;28(4):324-332. https://doi.org/10.1089/aid.2011.0106 [ Links ]
11. National Department of Health, South Africa. National Consolidated Guidelines for Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of Children, Adolescents and Adults. April 2015. https://aidsfree.usaid.gov/sites/default/files/tx_south_africa_pmtct_2015 (accessed 7 June 2017). [ Links ]
12. National Department of Health, South Africa. National Antiretroviral Treatment Guidelines. 2004. http://apps.who.int/medicinedocs/documents/s17758en/s17758en.pdf (accessed 26 May 2019). [ Links ]
13. Fatti G, Bock P, Grimwood A, Eley B. Increased vulnerability of rural children on antiretroviral therapy attending public health facilities in South Africa: A retrospective cohort study. J Int AIDS Soc 2010;13:46. https://doi.org/10.1186/1758Pe-2652-13-46 [ Links ]
14. Orrell C, Levison J, Ciaranello A, et al. Resistance in pediatric patients experiencing virologic failure with first-line and second-line antiretroviral therapy. diatr Infect Dis J 2013;32(6):644-647. https://doi.org/10.1097/INF.0b013e3182829092 [ Links ]
15. Pillay S, Bland RM, Lessells RJ, Manasa J, de Oliveira T, Danaviah S. Drug resistance in children at virological failure in a rural KwaZulu-Natal, South Africa, cohort. AIDS Res Ther 2014;11(1):3. https://doi.org/10.1186/1742-6405-11-3 [ Links ]
16. Rossouw TM, Feucht UD, Melikian G, et al. Factors associated with the development of drug resistance mutations in HIV-1 infected children failing protease inhibitor-based antiretroviral therapy in South Africa. PLoS One 2015;10(7):e0133452. http://doi.org/10.1371/journal.pone.0133452 [ Links ]
17. Le Moing V, Chene G, Carrieri MP, et al. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. AIDS 2002;16(1):21-29. https://doi.org/10.1097/00002030-200201040-00004 [ Links ]
18. Nuttall J, Pillay V. Characteristics and early outcomes of children and adolescents treated with darunavir/ritonavir-, raltegravir- or etravirine-containing antiretroviral therapy in the Western Cape Province of South Africa. S Afr Med J 2018;108(2):105-110. https://doi.org/10.7196/SAMJ.2018.v108i2.12573 [ Links ]
19. Ananworanich J, Kosalaraksa P, Siangphoe U, et al. A feasibility study of immediate versus deferred antiretroviral therapy in children with HIV infection. AIDS Res Ther 2008;5:24. https://doi.org/10.1186/1742-6405-5-24 [ Links ]
20. Paredes R, Mocroft A, Kirk O, et al. Predictors of virological success and ensuing failure in HIVpositive patients starting highly active antiretroviral therapy in Europe: Results from the EuroSIDA study. Arch Intern Med 2000;160(8):1123-1132. https://doi.org/10.1001/archinte.160.8.1123 [ Links ]
21. Grabar S, Pradier C, le Corfec E, et al. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS 2000;14(2):141-149. https://doi.org/10.1097/00002030-200001280-00009 [ Links ]
22. Van Zyl GU, van der Merwe L, Claassen M, et al. Protease inhibitor resistance in South African children with virologic failure. Pediatr Infect Dis J 2009;28(12):1125-1127. https://doi.org/10.1097/INF.0b013e3181af829d [ Links ]
23. Van Zyl GU, van Mens TE, McIlleron H, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011;56(4):333-339. https://doi.org/10.1097/QAI.0b013e31820dc0cc [ Links ]
24. Taylor BS, Hunt G, Abrams EJ, et al. Rapid development of antiretroviral drug resistance mutations in HIV-infected children less than two years of age initiating protease inhibitor-based therapy in South Africa. AIDS Res Hum Retroviruses 2011;27(9):945-956. https://doi.org/10.1089/aid.2010.0205 [ Links ]
25. Brumme CJ, Harrigan PR. No inherent association between minor mutations in HIV protease at baseline and selection of the L90M mutation at the time of the first virological failure. J Infect Dis 2005;191(10):1778-1779; author reply 9-80. https://doi.org/10.1086/429672 [ Links ]
26. Champenois K, Deuffic-Burban S, Cotte L, et al. Natural polymorphisms in HIV-1 protease: Impact on effectiveness of a first-line lopinavir-containing antiretroviral therapy regimen. J Med Virol 2008;80(11):1871-1879. https://doi.org/10.1002/jmv.21315 [ Links ]
27. Frater AJ, Beardall A, Ariyoshi K, et al. Impact of baseline polymorphisms in RT and protease on outcome of highly active antiretroviral therapy in HIV-1-infected African patients. AIDS 2001;15(12):1493-1502. https://doi.org/10.1097/00002030-200108170-00006 [ Links ]
28. Rhee SY, Liu T, Ravela J, Gonzales MJ, Shafer RW. Distribution of human immunodeficiency virus type 1 protease and reverse transcriptase mutation patterns in 4,183 persons undergoing genotypic resistance testing. Antimicrob Agents Chemother 2004;48(8):3122-3126. https://doi.org/10.1128/AAC.48.8.3122-3126 [ Links ]
29. Vermeiren H, van Craenenbroeck E, Alen P, et al. Prediction of HIV-1 drug susceptibility phenotype from the viral genotype using linear regression modeling. J Virol Methods 2007;145(1):47-55. https://doi.org/10.1016/j.jviromet.2007.05.009 [ Links ]
30. Kempf DJ, Isaacson JD, King MS, et al. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J Virol 2001;75(16):7462-7469. https://doi.org/10.1128/JVI.75.16.7462-7469.2001 [ Links ]
31. Barber TJ, Harrison L, Asboe D, et al. Frequency and patterns of protease gene resistance mutations in HIV-infected patients treated with lopinavir/ritonavir as their first protease inhibitor. J Antimicrob Chemother 2012;67(4):995-1000. https://doi.org/10.1093/jac/dkr569 [ Links ]
32. Nijhuis M, Wensing AM, Bierman WF, et al. Failure of treatment with first-line lopinavir boosted with ritonavir can be explained by novel resistance pathways with protease mutation 76V. J Infect Dis 2009;200(5):698-709. https://doi.org/10.1086/605329 [ Links ]
 Correspondence:
Correspondence:
Z Makatini
zinhle@wol.co.za
Accepted 7 January 2019














