Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 n.7 Pretoria Jul. 2019
http://dx.doi.org/10.7196/samj.2019.v109i7.13997
IN PRACTICE
CASE REPORT
doi:10.7196/samj.2019.v109i7.13997
Recurrent idiopathic spontaneous coronary artery dissection
P MkokoI; S PandieI; M NtsekheII
IMB ChB, FCP (SA), MMed (Internal Medicine); Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
IIMD, PhD; Division of Cardiology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
Spontaneous coronary artery dissection (SCAD) is a relatively rare cause of acute coronary syndrome and sudden cardiac death, which frequently affect young women in the absence of established cardiovascular risk factors. Advancements in cardiovascular imaging account for the increasing recognition of this diagnosis and associated diagnoses, although classic diagnostic modalities such as electrocardiography remain of paramount importance. We present a young woman with recurrent SCAD and briefly discuss her management and its outcome.
Case report
A 38-year-old single mother of 2 (a current smoker) presented to our unit in February 2015 with a history of acute severe central chest pressure that woke her during her sleep. It lasted for 1 hour and was associated with diaphoresis and nausea. She did not present to a hospital, hoping that her chest pain would diminish with the use of analgesia. However, intolerable dyspnoea forced her to present to our unit 3 days after the onset of symptoms. Her clinical examination did not reveal any signs of heart disease. Her laboratory results showed normal renal function and a highly sensitive troponin T of 1 677 ng/L (normal <14 ng/L). An ECG done at presentation showed sinus rhythm, ST elevation in the anterior-lateral leads (V2 - V6, aVL and lead I) and inferior leads (II, III and aVF). A coronary angiogram and a left ventriculogram were done to exclude acute coronary syndrome (ACS). These procedures revealed a distal spontaneous coronary artery dissection of the left anterior descending (LAD) branch of the left coronary artery, a non-dilated left ventricle with apical akinesia and an apical filling defect suggestive of a left ventricular apical thrombus.
Investigations for fibromuscular dysplasia, thyrotoxicosis, HIV and pregnancy were all negative. We treated the patient conservatively with aspirin, atenolol, enalapril, simvastatin and warfarin. Her follow-up visits in the cardiac clinic were unremarkable - there was no angina or dyspnoea and subsequent cardiac echocardiography suggested resolution of the apical thrombus. Anticoagulation with warfarin was discontinued 3 months after the index presentation.
Twenty-four months later she presented to our emergency department with an abrupt onset of central chest pressure radiating to the neck while walking. On clinical examination, her blood pressure was 122/58 mmHg, pulse 53/min, there were no signs suggestive of heart failure and no audible bruits suggestive of fibromuscular dysplasia. The highly sensitive troponin T was significantly raised (456 ng/L), and an ECG performed in the emergency department revealed a regular sinus rhythm of 78/min, QRS of 70 ms, with axis at +120°, and deep T-wave inversion from V2 to V6, including leads I, II and aVL. In light of her high-risk presentation, she underwent a coronary angiogram, which revealed a long segment of spontaneous coronary artery dissection extending from the mid- to distal LAD artery, a severe lesion compared with the distal LAD dissection she suffered 24 months earlier.
As with the first presentation, a decision was taken to manage the patient conservatively, with dual antiplatelet therapy (aspirin and clopidogrel) for 6 months, as well as atenolol, enalapril and simvastatin. At follow-up visits, she has been asymptomatic with no signs of heart failure.
Discussion
First presented as a case report >80 years ago,[1] spontaneous coronary artery dissection (SCAD) is a relatively rare cause of ACS. SCAD has an overwhelming preponderance in middle-aged women, with some studies suggesting a prevalence of 22 - 43% of ACS in women <50 years of age. SCAD may account for up to 3% of non-atherosclerotic coronary artery disease responsible for sudden cardiac death.[2-4]
Two pathogenic mechanisms of SCAD have been described: (i) the intimal tear hypothesis, whereby an interruption in the intimal-luminal interface forms an entry point for intramural haematoma accumulation inside the false lumen, leading to separation of the arterial wall; and (ii) the medial haemorrhage hypothesis that postulates that bleeding into the arterial wall due to spontaneous rupture of the vasovasorum is the primary pathogenic mechanism. However, bleeding within the false lumen compresses the true lumen and impairs myocardial perfusion.[5-7] This occurs in the absence of fibroatheromatous plaque rupture characteristic of type 1 ACS.[8]
Although SCAD is idiopathic in the majority of patients, an association has been described with fibromuscular dysplasia (FMD);[9] there are also case reports of SCAD in patients with HIV.[10] In a cohort (N=50) from Vancouver General Hospital, Canada, 86% of patients with SCAD had evidence of FMD in at least 1 non-coronary artery territory and 46% in >2 non-coronary territories.[9] Although the study was limited by small patient numbers and potential referral bias, it highlights the importance of FMD as a risk factor for SCAD.
During pregnancy and shortly after delivery women are vulnerable to develop SCAD. In up to 17% of patients with SCAD in the recently published Mayo clinic registry data, the condition was pregnancy related.[11] In this registry, 7.4% of patients were pregnant at the time of SCAD and in 92.6% SCAD occurred from delivery to 12 weeks postpartum. However, pregnancy-related SCAD can occur as late as 24 months postpartum.[12] Patients suffering from SCAD during pregnancy are more likely to be older at the time of first childbirth and are more frequently multiparous.[11] Furthermore, up to 11.1% of patients with pregnancy-related SCAD have history of pre-eclampsia. Advanced maternal age, multiparity and pre-eclampsia are well-established risk factors for peripartum cardiomyopathy.[13] Therefore, a pathophysiological relationship between SCAD, pre-eclampsia and peripartum cardiomyopathy is plausible and remains to be elucidated. Pregnancy-related SCAD is often marked by severe presentation characterised by ST elevation myocardial infarction (STEMI), lower left ventricular ejection fraction and left mainstem involvement or multiple coronary artery territories when compared with non-pregnancy-related SCAD.[11] Other notable risk factors for SCAD are multiple pregnancies (n>4), use of hormone-replacement therapies, infertility therapies, connective tissue disease and emotional and physical stress.[5,11,12,14]
Most patients with SCAD present with ACS. Some SCAD patients (24 - 87%) present with STEMI.[5,9,11,15,16] Non-STEMI (NSTEMI) is the presenting ACS in 18 - 70% of SCAD patients.[5,9,11,15,16] A small proportion of SCAD patients experience ventricular arrhythmias, cardiogenic shock and sudden cardiac death.[5]
Coronary angiography is readily and relatively widely available and is therefore the first modality used to diagnose SCAD. Three angiographic findings of SCAD have been well characterised (Fig. 1):
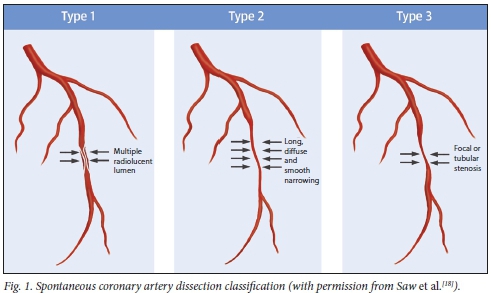
• Type 1 describes contrast dye staining of the arterial wall with multiple radiolucent lumens - the pathognomonic angiographic appearance of SCAD. However, it accounts for only 29 - 48% of SCAD cases on coronary angiography.[5,6,17]
• Type 2 lesions are characterised by diffuse smooth and long stenoses of varying severity. These lesions commonly involve the mid- and distal coronary segments of the coronary arteries. In type 2a, the segment of stenosis is followed distally by a normal-calibre vessel. In type 2b, the stenosis extends distally to affect the entire length of the coronary artery. Type 2 accounts for up to 67% of SCAD presentations.[5,6,14,17]
• Type 3 is not distinguishable from coronary atherosclerosis. However, the absence of atherosclerotic changes in other coronary vessels, presence of a long lesion (>11 mm), hazy lesions and linear lesions are suggestive of the presence of SCAD.[5,17] Type 3 lesions are the least common angio-graphic manifestations of SCAD (up to 4%).[6]
Coronary angiography offers a 2-dimensional view of the coronary arteries and does not show an image of the arterial walls. Consequently, coronary angiography will readily diagnose type 1 SCAD and potentially miss the other variants. Optical coherence topography (OCT) and intravascular ultrasound (IVUS) offer better imaging of the arterial wall (Table 1). OCT readily visualises intimal tears, intramural thrombi, false lumen and intramural haematomas.[15] Furthermore, OCT assists in adequate and superior stent implantation in SCAD management.[15] IVUS provides deeper-vessel visualisation and a better appreciation of the intramural haema-toma.[5] At the time of our patient's presentation, our unit did not have access to IVUS or OCT.
The management of SCAD is mostly based on expert opinion. In the absence of mainstem dissection or ongoing ischaemic symptoms, most patients are managed conservatively. However, revascularisation with either percutaneous coronary intervention or coronary artery bypass grafting is advocated for patients with mainstem dissection, ongoing ischaemic symptoms and haemodynamic instability.[5,6] SCAD patients generally have a favourable outcome, with in-hospital mortality rates as low as 5%. Recurrence of myocardial infarction in conservatively treated patients occurs in 5 -10%.[5] Recurrence of SCAD has been reported with increasing frequency when patients are followed up for a longer period. Recurrence is -15% after 2 years and up to 27% after 5 years.[5] In a prospective follow-up study of 327 patients with SCAD, Saw et al.[11]reported recurrence in 10.4% of patients in a median follow-up of 3.1 (interquartile range 1.49 - 5.49) years. Most importantly, these authors reported that the use of beta-blockers is protective against SCAD recurrence and that the presence of systemic hypertension is detrimental.
Conclusion
SCAD is an important cause of ACS in young women, especially those using oral contraceptive or hormone replacement therapy or women in the peripartum period.
Clinicians must maintain a high index of suspicion when a young woman with no atherosclerotic risk factors presents with ACS.
Declaration. None.
Acknowledgements. None.
Author contributions. PM wrote the manuscript, with MN and SP as supervisors.
Funding. None.
Conflicts of interest. None.
References
1. Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42: Rupture. Br Med J 1931;1:667. https://doi.org/10.n36/bmj.L3667.667 [ Links ]
2. Rashid HNZ, Wong DTL, Wijesekera H, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome: A single-centre Australian experience. Int J Cardiol 2016;202:336-338. https://doi.org/10.1016/j.ijcard.2015.09.072 [ Links ]
3. Elkayam U, Jalnapurkar S, Barakkat MN, et al. Pregnancy-associated acute myocardial infarction. A review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014;129:1695-1702. https://doi.org/10.1161/CIRCULATIONAHA.113.002054 [ Links ]
4. Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden cardiac death. Heart 2010;96(14):1119-1125. https://doi.org/10.1136/hrt.2009.185157 [ Links ]
5. Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol 2016;68(3):297-312. https://doi.org/10.1016/j.jacc.2016.05.034 [ Links ]
6. Al-Hussaini A, Adlam D. Spontaneous coronary artery dissection. Heart 2017;103(13):1043-1051. https://doi.org/10.1136/heartjnl-2016-310320 [ Links ]
7. Alfonso F, Bastante T, Rivero F, et al. Spontaneous coronary artery dissection. Circulation 2014;78:2099-2110. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001984 [ Links ]
8. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60(16):1581-1598. https://doi.org/10.1161/CIR.0b013e31826e1058 [ Links ]
9. Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: Prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013;6(1):44-52. https://doi.org/10.1016/j.jcin.2012.08.017 [ Links ]
10. Tariq U. Spontaneous coronary artery dissection (SCAD) in an HIV positive young female. Chest 2015;148(1):70A. https://doi.org/10.1378/chest.2277541 [ Links ]
11. Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol 2017;70(4):426-435. https://doi.org/10.1016/j.jacc2017.05.055 [ Links ]
12. Vijayaraghavan R, Verma S, Gupta N, Saw J. Pregnancy-related spontaneous coronary artery dissection. Circulation 2014;130:1915-1920. https://doi.org/10.1161/CIRCULATIONAHA.114.011422 [ Links ]
13. Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J Am Coll Cardiol 2013;62(18):1715-1723. https://doi.org/10.1016/j.jacc.2013.08.717 [ Links ]
14. Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29(9):1027-1033. https://doi.org/10.1016/j.cjca.2012.12.018 [ Links ]
15. Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 2012;59(12):1073-1079. https://doi.org/10.1016/j.jacc.2011.08.082 [ Links ]
16. Alfonso F, Paulo M, Lennie V, et al. Spontaneous coronary artery dissection. JACC Cardiovasc Interv 2012;5(10):1062-1070. https://doi.org/10.1016/j.jcin.2012.06.014 [ Links ]
17. Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84(7):1115-1122. https://doi.org/10.1002/ccd.25293 [ Links ]
18. Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection: Clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70(9):1148-1158. https://doi.org/10.1016/j.jacc.2017.06.053 [ Links ]
 Correspondence:
Correspondence:
P Mkoko
mkoko25@me.com
Accepted 27 March 2019














