Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 n.6 Pretoria Jun. 2019
http://dx.doi.org/10.7196/samj.2019.v109i6.13700
RESEARCH
Audit of diabetic ketoacidosis management at a tertiary hospital in Johannesburg, South Africa
S ThomasI; N A MohamedII, III; S BhanaIV, V
IMB BCh; Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCh, FCP (SA), Cert Endocrinology and Metabolism (SA); Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMB BCh, FCP (SA), Cert Endocrinology and Metabolism (SA); Division of Endocrinology, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IVBSc, MB BCh, MMed (Internal Medicine), FCP (SA), Cert Endocrinology and Metabolism (SA); Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VBSc, MB BCh, MMed (Internal Medicine), FCP (SA), Cert Endocrinology and Metabolism (SA); Division of Endocrinology, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Continuous intravenous infusion (CII) of insulin is the preferred method of treating diabetic ketoacidosis (DKA) worldwide, especially in patients with severe DKA. There is limited evidence evaluating low-dose bolus intravenous (IV) insulin management of DKA out of the intensive care unit (ICU).
OBJECTIVES. To conduct an audit of patients admitted with DKA, who were managed with bolus IV insulin at the medical acute-care unit (MACU), Chris Hani Baragwanath Academic Hospital (CHBAH), Johannesburg, South Africa, over a 4-month period to evaluate whether this is an effective treatment modality, as well as assess patient, disease and management characteristics related to the admissions. Methods. A prospective cross-sectional cohort study was done, interviewing 69 DKA patients from 1 September to 31 December 2017, and collecting relevant biochemical results from their hospital records. The current management protocol at CHBAH was observed, i.e. insulin therapy administered hourly as 10 IU IV insulin. The time to resolution of DKA, complications and deaths were recorded.
RESULTS. Our cohort was predominantly male (60.56%), with an average age of 36 years. All patients were successfully treated with bolus IV insulin, with an average time to resolution of 21 hours. DKA was categorised as mild (19.72%), moderate (50.7%) and severe (29.58). Most patients presented with raised inflammatory markers (64.79%) and some degree of renal impairment (>60%). Complications occurred in 9 patients (12.68%); 7 of these were related to factors precipitating the DKA admission. No deaths occurred. The only factor predicting a longer time to resolution was severity, with an odds ratio of 4.89 (confidence interval 1.04 - 22.84; p=0.044).
CONCLUSIONS. Outcomes are favourable, with bolus IV insulin being used as the treatment modality in patients with mild, moderate and severe DKA at CHBAH. Further studies are needed to corroborate these results in other centres.
Diabetic ketoacidosis (DKA) is a serious manifestation of uncontrolled blood sugar that can occur in patients with type 1 or type 2 diabetes mellitus (DM). There are three cornerstones of therapy: intravenous (IV) fluids, insulin therapy and management of electrolytes. Treatment of DKA has shown many advances over the years. It is now clear that low-dose insulin is as effective as high-dose insulin in treating DKA, with a reduced rate of hypoglycaemia and hypokalaemia.[1] It is also known that an initial IV insulin bolus may not be needed in the management of DKA if insulin therapy is started promptly at a dose of 0.14 IU/kg/h.R[3] There are several ways to administer insulin therapy, which have been evaluated: continuous IV infusion (CII) or bolus IV, subcutaneous (SC) or intramuscular (IM) insulin therapy. It is generally accepted that CII of insulin is the preferred method of treating DKA; this has been incorporated into treatment protocols worldwide.[4] Evidence evaluating different routes of insulin administration is weak or of low quality, and generally comprises consensus opinion.[5] High-quality randomised controlled trials (RCTs) are lacking and needed in this regard.[6] There have been only 5 RCTs evaluating SC bolus management of DKA compared with CII, which have found the latter to be an acceptable alternative in patients with mild to moderate DKA.[7] Specifically, there is a paucity of evidence supporting the bolus IV insulin route.
The rationale behind evaluating the bolus insulin management of DKA stems from an anticipated cost and resource benefit in managing patients out of intensive care units (ICUs). CIIs require flow regulators and constant monitoring of the infusion - both are expensive and require a low nurse-to-patient ratio. Bolus insulin administration offers the benefit of not requiring flow regulators, which can save costs, and limits constant monitoring of the infusion, which would be required in an ICU setting. Blood glucose could be checked hourly when insulin boluses are administered and decreases work load for nursing staff. Despite assumptions that bolus insulin would result in a rapid peak and effect and subsequent absence of insulin during the remainder of the hour, clinically this does not seem to be the case.[8]
The Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) guidelines, 2017, for the management of DKA, state that insulin therapy should ideally be provided as a CII in an ICU setting. Where an ICU is not available or there is delayed admission, insulin should be administered by hourly IV or IM boluses to decrease the incidence of hypoglycaemia.[9] The literature to support bolus IV administration of insulin includes small trials done >40 years ago, as well as studies done on the pharmacokinetic properties of insulin administered by different routes.[810-13] The results of these studies are difficult to apply in our current setting, as in some much higher doses of insulin were administered hourly and all contained very small sample sizes. This audit aims to fill a gap in the literature, especially in the SA setting, where bolus low-dose IV insulin can be used to safely treat patients with mild, moderate and severe DKA in a setting outside of the ICU.
Methods
Definitions
DKA is diagnosed according to the American Diabetes Association and SEMDSA guidelines:[9] pH <7.3 or bicarbonate <18 mmol/L and presence of ketonaemia. As serum ketones are not readily available, the presence of urine ketones are used as a surrogate marker.
Severity is classified by pH: mild (7.25 - 7.3), moderate (7.0 - 7.24), severe (<7.0).
Resolution of DKA is classified as resolution of acidosis (pH >7.3, bicarbonate >18 mmol/L) and ketonaemia (<1 mmol/L). Similarly, urine ketones (1+ or 0) are used owing to resource constraints.
Objectives
The primary objective of this study was to determine if the bolus IV insulin method was effective in treating DKA. Secondly, we evaluated patient, disease and management characteristics in our cohort.
Setting
This audit was done at Chris Hani Baragwanath Academic Hospital (CHBAH), a tertiary hospital in Soweto, Johannesburg, South Africa (SA), serving a wide catchment area (urban, semi-rural and rural). Patients with DKA are initially seen in the emergency department, where emergency measures are implemented when DKA is diagnosed (fluid bolus, insulin therapy and correction of electrolyte disturbances). The internal medicine department is then consulted and patients are transferred to either the medical acute-care unit (MACU) or ICU, as deemed appropriate by the admitting registrar. The MACU was established in 2011 as a higher-care unit with a nurse-patient ratio of 1:2 - 1:3, where acutely ill, non-ventilated patients can be managed until stable for transfer to a general medical ward. It is a 12-bed unit with 4 - 5 nurses and covered by 1 medical registrar, who is supported by his/her medical intern. Patients with DKA are a large portion of those admitted to the MACU, with an average of 26.67 per month (Table 1).

DKA is managed by 10 IU bolus IV insulin hourly, with blood gases checked every 2 - 4 hours until resolution is achieved. Insulin is not administered if hypoglycaemia is present. IV fluids (crystalloids) are administered and adjusted according to hourly finger-prick glucose readings and electrolytes, as monitored on serial blood gases.
All patients aged >14 years admitted to CHBAH's internal medicine department with a diagnosis of DKA during September - December 2017 were evaluated. Patients diagnosed with a hyperosmolar hyperglycaemic state (HHS) were not included. Seventy patients were willing to participate in the study, and signed individual consent forms. In the case of participants aged 14 - 17 years, an assent form was signed. One patient was readmitted twice during the study period. All these patients were admitted to the MACU. Three patients relapsed after initial resolution and transfer to the general medical ward. They were then transferred back to the MACU, and time to resolution of the relapsed episode of DKA was recorded.
Demographic data (age and gender) of participants were obtained. Patients were interviewed about past management of their diabetes: facility accessed for care, historical DKA rate and recent treatment regimens, where known. Initial biochemical parameters were recorded on first medical contact, often in the emergency department, prior to the initiation of DKA management. The initial blood glucose level was ascertained from the first blood gas determination before initiation of DKA treatment. Where the latter method was not calibrated, the finger-prick glucose value was recorded. The On Call Plus glucometer (ACON, USA) was used in the MACU, which records blood glucose readings up to 33.3 mmol/L - thereafter recording as 'HI' (high). For statistical purposes, these readings were excluded from the data set, as we could not ascertain the correct value above 33.3 mmol/L. Where possible, we tried to identify the precipitant as sepsis, non-compliance or other. Patients were asked where they would prefer to attend follow-up examination after treatment, which was recorded. Patients were classified as type 1 or type 2 DM based on clinical judgement of the investigator regarding several factors (previously known, age of onset, body mass index (BMI), prior antibody testing, acanthosis nigricans or polycystic ovary syndrome and family history of first-degree relatives with DM). HbA1c was included if measured during the current admission or if available from LABTRAK (NHLS, SA) within the preceding 3 months. LABTRAK is the only laboratory information system that is used by medical staff to access patients' results from any government hospital or clinic in SA.
Time to resolution of DKA was measured in hours as time from start of DKA treatment to cessation of insulin therapy, as guided by the abovementioned SEMDSA criteria. Any complication or death was recorded.
Ethical approval
The study was approved by the University of the Witwatersrand Human Research Ethics Committee (ref. no. M170551).
Results
We audited 69 of 71 patients admitted during the study period. One patient aged 13 years was excluded from the study. The primary outcome was achieved in all, i.e. DKA was resolved in each patient, using only insulin in the form of hourly IV boluses. There were no deaths; therefore, early survival in our cohort was 100%.
The average age of participants was 36 (range 27 - 52) years, with 60.56% male patients. Initial biochemistry is detailed in Table 2.
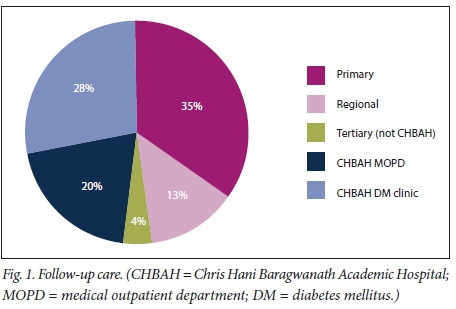
There were 46.48% and 53.52% type 1 and type 2 DM patients, respectively. Some patients (38.03%) reported previous admissions owing to DKA, with an average of 2 episodes per participant within this group. The mean HbA1c measurement was 13.87% (standard deviation (SD) 2.5), with values available from 62 participants. Severity was as follows: mild (19.72%), moderate (50.7%) and severe (29.58%). The average time to resolution was 21 (13.5 - 29) hours. Excluding severe DKA, mild and moderate DKA had an average time to resolution of 20 hours. There were 32.39% patients who were newly diagnosed with DM, with this episode of DKA as their index presentation. Of the patients known to have DM, previous follow-up visits for medication and monitoring are depicted in Fig. 1. Some patients (47.85%) were previously followed up at CHBAH, with the second largest proportion from primary healthcare clinics (34.78%). Of the 3 patients with DKA who relapsed, complete resolution was achieved in 8, 12 and 24 hours of further DKA treatment, respectively. No specific factors were identified to explain why these patients relapsed. One patient was readmitted twice after initial presentation with moderate and severe DKA, non-compliance being the precipitant to each episode. This patient was extensively counselled and the social services department was consulted to assist him. At his last visit, the HbAlc had declined (from 16.6% to 9.1%, measured after 3 months).
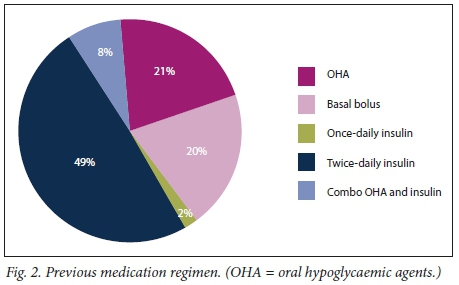
Forty-nine patients could account for their previous treatment regimens, most receiving insulin twice daily (Fig. 2). The majority of patients (74.65%) were issued follow-up dates at CHBAH after discharge. Precipitants were identified as sepsis (15.71%), noncompliance (47.14%) and other/unknown (37.14%). Complications occurred in 9 patients (12.68%). Most of these were related to initial septic precipitants, i.e. surgical procedures aimed at source control, including incision and drainage of a thumb abscess, axillary abscess and below-the-knee amputation of a septic diabetic foot. Two patients had upper gastrointestinal bleeding during their admission. One patient who presented with severe DKA and a low Glascow coma scale developed aspiration pneumonia that resolved with IV administration of antibiotics. Two patients, one with a pyogenic liver abscess and the other with a pancreatic pseudocyst, needed drainage of intra-abdominal collections. Both lesions were drained using percutaneous pigtail catheters. Ali patients recovered and were subsequently discharged.
Time to resolution was divided into <24 h and >24 h to determine which factors influenced a longer time to recovery. These time periods were chosen, as the median time was 21 h; therefore, it was evaluated in which patients duration to recovery was >24 h. Logistic regression analysis showed an association between severity of DKA and time to resolution, with an odds ratio of 4.89 (confidence interval 1.04 - 22.84; p=0.044). No other factors significantly influenced duration of treatment. No correlation was found between the inflammatory markers C-reactive protein and white cell count. Both were above the normal range in 64.79% of patients, despite no identifiable sepsis in the majority of admissions (90.14%); elevation probably resulted from acidosis. Most patients presented with renal impairment, urea and creatinine being deranged in 60.56% and 64.79% of cases, respectively. No patients required dialysis.
Discussion
Managing DKA by bolus IV insulin as opposed to CII has been incorporated into protocols in SA and abroad, even though there is no strong evidence[8,14] This audit shows that IV boluses of low-dose insulin can be successfully used to treat DKA, regardless of severity. Our primary objective was to prove that this method is effective; therefore, there is a need to define the meaning of efficacy in this context. There were no deaths in our cohort, as opposed to other DKA studies done in Africa. SA studies quote a mortality rate of 7.5% and 6.8% over a 2- and 12-month period, respectively, for hyperglycaemic emergencies (DKA and HHS).[15,16] Mortality statistics for the rest of the African subcontinent are estimated at >25%.[17] Managing severe DKA with reduced mortality out of the ICU would be a great advantage and cost benefit to any hospital.
Time to resolution could be evaluated as a function of efficacy. Our average time to resolution was 21 h, slightly longer than that in most other studies in which different insulin treatment regimens were used. Studies with similar biochemical inclusion criteria showed a mean time to resolution of 17 h, 18.7 h and 19.85 h, respectively. CII was employed for the first 2 studies and SC bolus injections for the last.[18, 20] A Cochrane review evaluating SC bolus IV insulin reported a time to resolution of 11 h in 5 RCTs.[21] This study, however, only evaluated participants with mild to moderate DKA. Analysis of our data showed that severe DKA was correlated with a longer time to resolution, which could be an explanation for the shorter time to resolution observed in the previous study.[21] There are notable differences between our sample and the abovementioned studies: they were performed in developed-world settings, demographics were different, children were included as participants, insulin analogues were sometimes used as opposed to human insulin and patients were mostly managed in an ICU setting. It is, however, not clear whether time to resolution has any impact on improved clinical outcomes; therefore, this cannot necessarily be used to prove efficacy until further studies are performed.[5]
The number of DKA admissions of 26.67 per month is historically similar to that in previous SA studies, i.e. Groote Schuur Hospital in 1991 and CHBAH in 1986.[22,23] Zouvanis et al.[16] evaluated patients with severe DKA (pH <7.25, which equates to moderate and severe DKA in our study) at an academic hospital in Johannesburg. The majority of patients were aged >40 years, as opposed to our average of 36 years. The percentage of newly diagnosed patients with DM (31%) was similar to that in our data, as was the proportion of type 1 to type 2 DM. An identifiable precipitant was present in two-thirds of participants, which was in keeping with our data; however, infection was more common than non-compliance. There were no deaths in the DKA group.
The majority of patients in our sample presented with moderate DKA and some degree of renal impairment, while a large majority showed an initial potassium reading at the upper limit of normal or high (National Health Laboratory Service (NHLS) 3.5 - 5.1 mmol/L). This increased potassium reading does not represent total body potassium, but instead diminished renal tubular secretion in addition to hydrogen-potassium shifts across the cellular membrane.[24]Inflammatory markers were raised in the majority of patients, not always correlating with the presence of sepsis, which was possibly caused by acidosis.[25]
Low-dose insulin given as a hourly IV bolus has been found to be just as effective as a pharmacological high-dose in a study with 9 parti-cipants.[26] The main clinical benefit observed was the reduced rates of hypokalaemia and hypoglycaemia. Fisher et al.[11] randomised 15 patients to an hourly IV, SC or IM bolus of 7 IU insulin, observing no difference after 8 hours in rates of decline of glucose or serum ketones. Similarly, Clumeck et al.[8] administered hourly low-dose insulin at 5 IU IV in 19 patients with DKA. Qnce glucose had reached 13.9 mmol/L, insulin was changed to SC 6-hourly doses, regardless of whether ketonaemia had resolved. Very small numbers were used in these landmark studies.
IM insulin has an unpredictable absorption and may accumulate in tissues, resulting in delayed hypoglycaemia[9] It may also be unacceptable to the patient, as injections are painful and need to be administered several times. Umpierrez et al.[27,28] proved that CII or SC insulin analogues are as effective as regular human insulin, which must be balanced against their increased cost and lesser availability. SC insulin is a reasonable alternative to treat patients with mild to moderate DKA. Human insulin, used effectively in this audit, has the advantage over insulin analogues in a lower-income country such as SA, as it is readily available and has a cost advantage.
Study limitations
There were several limitations to this study. A large portion of data was self-reported by patients, which could lead to recall bias. Determination of the type of diabetes did not include type 3 (pancreatic) DM, as in many cases this was not clearly evident from patient history, past clinical diagnoses or notes. CHBAH is a very busy hospital and complete adherence to DKA protocol by doctors and nurses could not be ensured (an audit in this regard might be necessary). Blood gas determinations were sometimes not performed at precise 2 - 4-hourly intervals as recommended, which could affect time to resolution. Excluded blood glucose values (7 of 71) resulted in a minimally underestimated mean initial blood glucose value. DKA was successfully treated in all patients; therefore, Kaplan-Meier survival analysis could not be performed, as stated in the study protocol.
Conclusions
This audit is the first of its kind in SA that shows that bolus IV insulin management of DKA is a safe and feasible way to manage the condition. This method can be used in patients with severe DKA who are not in an ICU. It is effective, as the condition of all patients with DKA resolved and there were no deaths in our cohort. No complications related to bolus insulin administration were observed. There was a longer time to resolution, but this does not necessarily correlate with increased complications or efficacy of the treatment regimen.
This study could serve as a platform to influence other SA health centres to employ this route of insulin administration. Further audits could strengthen evidence for its use. Large RCTs are needed to compare all routes of administration of insulin and provide clear data on the preferred method.
Declaration. Article based on a study done by ST as part of her FCP (Internal Medicine) qualification.
Acknowledgements. A special thank you to Dr D Mpanya, Prof. C Menezes and Prof. N Tsabedze for their guidance with regard to this research.
Author contributions. ST: design, data collection, analysis, writing; SB: conceptualisation of project, review of manuscript; and NAM: conceptualisation of project, review of manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633 [ Links ]
2. Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med 2010;38(4):422-427. https://doi.org/10.1016/j.jemermed.2007.11.033 [ Links ]
3. Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a priming dose of insulin necessary in a low-dose insulin protocol for the treatment of diabetic ketoacidosis? Diabet Care 2008;31(11):2081-2085. https://doi.org/10.2337/dc08-0509 [ Links ]
4. Gosmanov AR, Gosmanova EQ Dillard-Cannon E. Management of adult diabetic ketoacidosis. Diabet Metabol Syndr Qbes Targ Ther 2014;7:255-264. https://doi.org/10.2147/dmso.s50516 [ Links ]
5. Tran TT, Pease A, Wood AJ, et al. Review of evidence for adult diabetic ketoacidosis management protocols. Front Endocrinol 2017;8:106. https://doi.org/10.3389/fendo.2017.00106 [ Links ]
6. Dhatariya KK, Vellanki P. Treatment of diabetic ketoacidosis (DKA)/hyperglycemic hyperosmolar state (HHS): Novel advances in the management of hyperglycemic crises (UK versus USA). Curr Diabet Report 2017;17(5):33. https://doi.org/10.1007/s11892-017-0857-4 [ Links ]
7. Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Gonzalez-Padilla DA. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Database Syst Rev 2016;(1):CD011281. https://doi.org/10.1002/14651858.cd011281.pub2 [ Links ]
8. Clumeck N, de Troyer A, Naeije R, Somers G, Smekens L, Balasse EQ Treatment of diabetic coma with small intravenous insulin boluses. BMJ 1976;2(6032):394-396. https://doi.org/10.1136/bmj.26032.394 [ Links ]
9. Society for Endocrinology, Metabolism and Diabetes of South Africa Type 2 Diabetes Guidelines Expert Committee. Hyperglycaemic emergencies in SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus. J Endocrinol Metab Diabet 2017;21(1)(Suppl 1):S64-S67. [ Links ]
10. Butkiewicz EK, Leibson CL, O'Brien PC, Palumbo PJ, Rizza RA. Insulin therapy for diabetic ketoacidosis: Bolus insulin injection versus continuous insulin infusion. Diabet Care 1995;18(8):1187-1190. https://doi.org/10.2337/diacare.18.8.1187 [ Links ]
11. Fisher JN, Shahshahani MN, Kitabchi AE. Diabetic ketoacidosis: Low-dose insulin therapy by various routes. N Engl J Med 1977;297(5):238-241. https://doi.org/10.1056/nejm197708042970502 [ Links ]
12. Felig P. Diabetic ketoacidosis. N Engl J Med 1974;290(24):1360-1363. https://doi.org/10.1056/nejm197406132902405 [ Links ]
13. Hayton WL, Grisafe JA. Pharmacokinetic evaluation of dosing regimens for insulin in diabetic ketoacidosis. Diabetes 1976;25(9):771-775. https://doi.org/10.2337/diabetes.25.9.771 [ Links ]
14. Henriksen QM, Prahl JB, Roder ME, Svendsen QL. Treatment of diabetic ketoacidosis in adults in Denmark: A national survey. Diabet Res Clin Pract 2007;77(1):113-119. https://doi.org/10.1016/j.diabres.2006.10.013 [ Links ]
15. Pepper DJ, Burch VC, Levitt NS, Cleary S. Hyperglycaemic emergency admissions to a secondary-level hospital - an unnecessary financial burden. J Endocrinol Metab Diabet 2007;12(2):56-60. https://doi.org/10.1080/22201009.2007.10872157 [ Links ]
16. Zouvanis M, Pieterse AC, Seftel HC, Joffe BI. Clinical characteristics and outcome of hyperglycaemic emergencies in Johannesburg Africans. Diabet Med 1997;14(7):603-606. [ Links ]
17. Qtieno CF, Kayima JK, Qmonge EQ, Qyoo GQ. Diabetic ketoacidosis: Risk factors, mechanisms and management strategies in sub-Saharan Africa: A review. East Afr Med J 2005;82(12 Suppl):S197-S203. [ Links ]
18. Dhatariya KK, Nunney I, Higgins K, Sampson MJ, Iceton G. National survey of the management of diabetic ketoacidosis (DKA) in the UK in 2014. Diabet Med 2015;33(2):252-260. https://doi.org/10.1111/dme.12875 [ Links ]
19. Stefanadis G, Schad J, McAllister K, Smigiel B. Evaluation of the diabetic ketoacidosis protocol at Carilion Roanoke Memorial Hospital, Virginia. 2015. http://www.easternstates.org/global_engine/download_custom.asp?fileid=38edb7eb-6e71-447f-a69d-lefb28230318.pdf&filename=Stefanadis_ ESRC_Abstract.pdf&blnIsPublic=2 (accessed 22 March 2018). [ Links ]
20. Teevan C. Evaluation of a diabetic ketoacidosis treatment protocol using subcutaneous insulin aspart. Crit Care Med 2012;40(12):1-328. https://doi.org/10.1097/01.ccm.0000425167.26876.c7 [ Links ]
21. Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Gonzalez-Padilla DA. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Database Syst Rev 2016;(1):CD011281. https://doi.org/10.1002/14651858.cd011281.pub2 [ Links ]
22. Levetan BN, Levitt NS, Bonnici F. Hyperglycaemic emergencies are a common problem. S Afr Med J 1997;87(3 Suppl):368-370. [ Links ]
23. Huddle KR, Gill GV. Reducing acute hyperglycaemic mortality in African diabetic patients. Diabet Med 1989;6(1):64-66. https://doi.org/10.1111/j.1464-5491.1989.tb01141.x [ Links ]
24. West ML, Magner PO, Richardson RM, Halperin ML. A renal mechanism limiting the degree of potassium loss in severely hyperglycemic patients. Am J Nephrol 1988;8(5):373-378. https://doi.org/10.1159/000167620 [ Links ]
25. Xu W, Wu HF, Ma SG, et al Correlation between peripheral white blood cell counts and hyperglycaemic emergencies. Int J Med Sci 2013;10(6):758-765. https://doi.org/10.7150/ijms.6155 [ Links ]
26. Maruyama H, Kato M, Suzuki A, et al Comparative study of small-dose intravenous insulin boluses and conventional large-dose insulin therapy in the treatment of diabetic coma. J Japan Diabet Soc 1981;24(7):721-728. [ Links ]
27. Umpierrez GE, Jones S, Smiley D, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: A randomized controlled trial. Diabet Care 2009;32(7):1164-1169.https://doi.org/10.2337/dc09-0169 [ Links ]
28. Umpierrez GE, Latif K, Stoever J, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med 2004;117(5):291-296. https://doi.org/10.1016/j.amjmed.2004.05.010 [ Links ]
 Correspondence:
Correspondence:
S Thomas
sumythomas9061@gmail.com
Accepted 8 November 2018














