Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 no.2 Pretoria Fev. 2019
http://dx.doi.org/10.7196/samj.2019.v109i2.13438
RESEARCH
The association between preterm labour, perinatal mortality and infant death (during the first year) in Bishop Lavis, Cape Town, South Africa
L T BrinkI; G S GebhardtII; D MasonIII; C A GroenewaldIV; H J OdendaalV
IMSc; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIMB ChB, MMed, FCOG (SA), MSc (Med Sci), PhD; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIMB ChB; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IVMB ChB, MMed, FCOG (SA), MCom; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VMB ChB, MMed, FCOG (SA), MD, FRCOG; Department of Obstetrics and Gynaecology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: We present further analyses from the Safe Passage Study, where the effect of alcohol exposure during pregnancy on sudden infant death syndrome and stillbirth was investigated
OBJECTIVES: To describe pregnancy and neonatal outcome in a large prospective study where information on the outcome of pregnancy was known in >98.3% of participants and ultrasound was used to determine gestational age (GA).
METHODS: As part of the Safe Passage Study of the PASS Network in Cape Town, South Africa, the outcomes of 6 866 singleton pregnancies were prospectively followed from recruitment in early pregnancy until the infant was 12 months old to assess pregnancy outcome. Fetal growth was assessed by z-scores of the birth weight, and GA at birth was derived from early ultrasound assessments. The effects of fetal growth restriction and preterm delivery on pregnancy outcome were determined.
RESULTS: There were 66 miscarriages, 107 stillbirths at >22 weeks' gestation, 66 stillbirths at >28 weeks' gestation, 29 and 18 neonatal deaths at >22 and >28 weeks' gestation, respectively, and 54 post-neonatal deaths (28 days - 12 months). The miscarriage rate was 9.6/1 000 and the infant mortality rate 12.4/1 000. Of the births, 13.8% were preterm. For deliveries at >22 and >28 weeks, the stillbirth rates were 15.7 and 9.8/1 000 deliveries, respectively. For deliveries at >22 and >28 weeks, the neonatal death rates were 4.3 and 2.7/1 000 live births, respectively. For these pregnancies the perinatal mortality rates were 20.0/1 000 (>22 weeks) and 12.5/1 000 (>28 weeks), respectively. Only 15.9% of stillbirths occurred during labour (in 15.9% of cases it was uncertain whether death had occurred during labour). In the majority of cases (68.2%) fetal death occurred before labour, and 82.2% of stillbirths and 62.1% of neonatal deaths occurred in deliveries before 37 weeks. Including the miscarriages, stillbirths and infant deaths, there were 256 pregnancy losses; 77.3% were associated with deliveries before 37 weeks. Only 1.8% of all the women were HIV-positive, whereas the HIV-positive rate was 3.7% among those who had stillbirths. Birth weight was below the 10th centile in 25.6% of neonatal and post-neonatal deaths compared with 17.7% of survivors.
CONCLUSIONS: Preterm birth and fetal growth restriction play significant roles in fetal, neonatal and infant losses
To the best of our knowledge, no information is available on perinatal, neonatal and infant mortality rates based on gestational age (GA) at delivery for any community-based cohort in South Africa (SA). In addition, birth weight is commonly used to define the lower borders of fetal viability. To distinguish between a late miscarriage and an early stillbirth, a birth weight of 500 g rather than GA of 22 weeks is often used. However, in defining stillbirth, GA is the preferred criterion with regard to the lower cut-off point, as using birth weight may exclude growth-restricted fetuses.[1]
Furthermore, patients are often discharged from the delivery unit very early, and it is therefore unlikely that all neonatal deaths after discharge are accurately reported. There is also no information on the rates of late miscarriages, as this information is not prospectively collected.
The true incidence of mid-trimester miscarriage is unknown.[2] Information on the incidence and causes of miscarriages may provide valuable information for programmes to reduce stillbirths. It is also necessary to analyse stillbirth and perinatal mortality rates (PNMRs) regularly to predict whether the Sustainable Development Goals for 2030 will be reached.[3]
The Safe Passage Study (SPS) was undertaken in three populations, of which the 'coloured' (mixed race) population in Bishop Lavis, a suburb of Cape Town, SA, was one, to determine the role of alcohol consumption in stillbirths and sudden infant deaths.[4] Robust information on all pregnancies, from the first antenatal visit until the infant was 1 year old, allowed accurate calculation of perinatal and infant mortality. It also created an opportunity to calculate late miscarriage rates for participants who booked early and to study the role of preterm delivery in perinatal mortality and infant deaths during the first year.
Objectives
To determine the true population-based risk of miscarriage, late stillbirth, neonatal death and preterm delivery in otherwise low-risk coloured pregnant women in a suburban area of Cape Town.
Methods
This prospective cohort study was conducted in Bishop Lavis. Recruitment was done between August 2007 and January 2015. A woman was eligible if all the following criteria were met: (i) able to provide informed consent; (ii) pregnant with one or two fetuses; (iii) >16 years of age; (iv) GA of at least 6 weeks and 0 days and not at the delivery admission unit; and (v) able to speak English or Afrikaans. A woman was excluded if any of the following criteria were met: (i) planned abortion; (ii) planned relocation from catchment area prior to delivery; or (iii) advice against participation by a healthcare provider (e.g. additional medical care required).
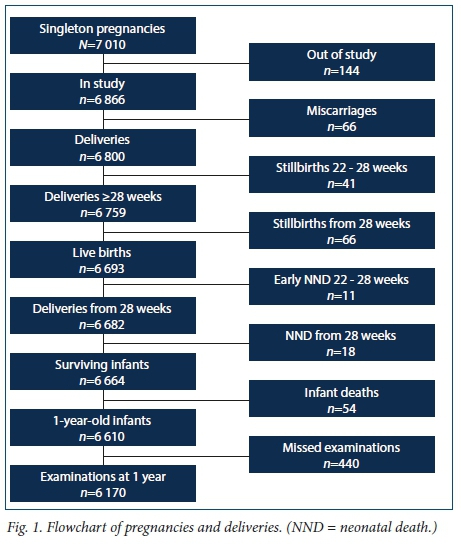
A total of 7 060 pregnant women were recruited at the antenatal clinics. Determination of GA was done by ultrasound at the first or before the second antenatal visit. A trained midwife or experienced ultrasonographer performed all the ultrasound examinations. For this analysis we selected only the 7 010 singleton pregnancies (Fig. 1). There were no exclusions for any medical conditions.
At the recruitment visit, participants provided informed consent, which included consent for collection of the placenta for histological examination after delivery. Alcohol use and cigarette smoking were determined in detail at up to four occasions during pregnancy as described by Dukes et al.[4]
After a fetal death, separate informed consent was obtained for autopsy.[5] Research midwives checked labour ward admissions and deliveries daily to determine whether a study participant had delivered. In cases of fetal death, the social worker and/or senior study personnel were alerted to provide support and bereavement counselling, and discussed consent for autopsy at an appropriate time.
All deaths were presented and discussed at the weekly perinatal mortality meetings of the hospital, which were attended by obstetricians, neonatologists, pathologists, geneticists, fetal and maternal subspecialists and midwives. A primary cause of death was assigned and coded using the classification system of the Perinatal Problem Identification Programme (PPIP).[6]
The World Health Organization's definition of stillbirth was used for the study (a baby born with no signs of life at or after 28 weeks' gestation).[7] The definition used for stillbirth in high-income countries (death at or after 22 weeks) was used to classify an early stillbirth.[8] There were therefore three groups of different outcomes: miscarriage (pregnancy loss from recruitment to <22 weeks), early stillbirth (fetal death 22 weeks 0 days - 27 weeks 6 days), and late stillbirth (fetal death at >28 weeks). As death sometimes occurred before 22 (or 28) weeks but delivery only after these GAs, the deaths before 22 (or 28) weeks were excluded from the respective study group but used for the earlier gestation group. GA in all stillbirths was assessed individually by comparing GA at delivery (as determined by ultrasound at booking), date of last fetal heart rate (FHR) recording, and measurement of the foot length at autopsy.[9] Any death where a FHR was recorded at or after 22/28 weeks was regarded as a stillbirth, irrespective of the birth weight. To establish whether the fetal death occurred before or during labour, clinical information such as detection of a fetal heartbeat at admission for labour or during labour and autopsy findings such as maceration of the fetal skin were used.[10] Clinical judgement was used in cases where it was difficult to determine whether death had occurred during early labour before the participant was admitted to hospital.
In the case of live births, contact with the mother and infant was maintained after discharge as the infant was brought back to the research unit within the first 5 days of life, at ~1 month of age, and finally at the age of 1 year. If any of these visits were missed, the mother was contacted to enquire about the condition of the infant. Deaths up to 28 days after birth were regarded as neonatal deaths. Post-neonatal (infant) death was defined as death between the age of 28 days and 366 days.[11] Reference values on the Intergrowth-21 study were used to determine whether the newborns weighed below the 10th percentile.[12]
Statistical analysis
Data were entered in Excel 365 (Microsoft, USA) and then coded and exported for analysis in Stata 14 (StataCorp, USA). Analyses were performed using SAS/STAT software, version 9.3 (SAS Institute, USA). Descriptive statistics were used to describe continuous variables. As some of the findings were not normally distributed, both the mean and median values were calculated. The χ2 test was used to determine significance in categorical data.
Ethics approval
Permission to conduct the study was obtained from the Health Research Ethics Committee of Stellenbosch University (ref. no. N06/10/210).
Results
The recruited cohort consisted of 6 866 women with singleton pregnancies (caesarean section rate 13.0%), of whom 6 170 (89.8%) brought their infants for the final follow-up visit at the age of 1 year (Fig. 1).
There were 66 miscarriages that occurred after the diagnosis of pregnancy was made, giving a cumulative incidence of 9.6/1 000 deliveries over the study period (Table 1). Of the 2 724 women with singleton pregnancies who enrolled between 14 weeks 0 days and 21 weeks 6 days, 20 had miscarriages, giving a mid-trimester miscarriage rate of 7.3/1 000 pregnancies.
There were 107 stillbirths at or after a GA of 22 weeks, presenting a risk (or cumulative incidence) of 15.7/1 000; of these stillbirths, 66 occurred at or after a GA of 28 weeks, for a risk (cumulative incidence) of late stillbirths of 9.8/1 000 (Table 1).
Of the 107 stillbirths, 88 (82.2%) were before 37 weeks and 73 (68.2%) before 34 weeks. In 17 (15.9%) of the stillbirths, death occurred during labour. The mean GA at enrolment for the 107 stillbirths was 18 weeks and 5 days, compared with a mean GA of 20 weeks and 1 day for all births in the study. The mean GA at delivery for the stillbirths was 30 weeks 4 days, compared with 38 weeks 4 days for all births in the study (Table 2).
Intrapartum deaths occurred in 17 (15.9%) of the 107 stillbirths; in 17 (15.9%) it was uncertain when the death had occurred and in 73 (68.2%) it was before labour.
There were 29 neonatal deaths before 28 days after delivery, giving a neonatal death rate of 4.3/1 000 live births for deliveries at >22 weeks and 2.7/1 000 live births for deliveries at >28 weeks. The PNMR was therefore 20.0/ 1 000 for deliveries from 22 weeks and 12.5/1 000 for deliveries from 28 weeks (Table 1). Sixteen (55.2%) of these neonates were delivered before 34 weeks. Twenty-five (86.2%) of the neonates died before discharge from hospital.
Fifty-three (98.2%) of the 54 infant deaths from 28 days to the age of 1 year occurred after discharge from hospital. Twenty-six (48.2%) of the post-neonates were born before 37 weeks and 17 (31.5%) before 34 weeks. The mean GA at enrolment for the neonates was 20 weeks 1 day (the same as for all subjects in the study), compared with 21 weeks 5 days for infant deaths. The mean GA at delivery for neonatal deaths was 32 weeks 3 days, compared with 36 weeks 0 days for infants who died at 28 days or later (Table 2).
HIV status was known for all women whose fetuses or infants died. Three (4.6%) of the 66 women who had miscarriages were HIVpositive. Four (3.7%) of the 107 women who had stillbirths were HIV-positive. None of the 29 women who had neonatal deaths was HIVpositive, in contrast to 3 (5.6%) of the 54 women who had post-neonatal deaths. In total, only 122 (1.8%) of the 6 866 women with singleton pregnancies were HIV-positive.
There were 256 pregnancy losses, consisting of 66 miscarriages, 107 stillbirths and 83 infant (neonatal and post-neonatal) deaths (Fig. 1); 132 (69.5%) fetal and infant deaths were associated with deliveries before 37 weeks, and this percentage rose to 77.3% when miscarriages were included.
Preterm delivery (before 37 weeks) occurred in 13.8% of pregnancies, and the mean (median) GA was 240 (248) days. Delivery before 34 weeks occurred in 271 cases (4.0%); the mean (median) GA was 212 (220) days. The mean (median) GA for the 5 865 term pregnancies (>37 weeks) was 276 (276) days (39 weeks 3 days) (Table 2).
Birth weight was below the 10th centile in 25.6% of infants who died, as opposed to 17.7% of survivors. Birth weight was below the 10th centile in 18.1% (1 210/6 691) of newborns. Newborns with a birth weight below the 90th centile accounted for 5.5% of deliveries.
At recruitment, a body mass index (BMI) of <18 kg/m2 was observed in 3.3% of pregnant women. Their mean BMI was 17.1 kg/m2 (range 13.7 - 17.99). Fifty-four (25.5%) of these women delivered small-for-gestational-age (SGA) babies, 72.2% delivered appropriate-for-gestational-age (AGA) babies, and 2.4% delivered large-for-gestational-age (LGA) babies. Only 52.8% of women had a normal BMI (18 - 25 kg/m2), of whom 21.6% had SGA, 75.7% AGA and 2.7% LGA babies. A BMI >25 kg/m2 was observed in 44.0% of women, of whom 13.1% delivered SGA, 77.6% AGA and 9.3% LGA babies. These frequencies differed significantly (Pearson χ2 = 199.0650, df = 4; p<0.0001).
Discussion
Longitudinal studies are essential to examine the effects of environmental conditions on birth outcome.[13] Such studies would be of great value to investigate the late complications of preterm delivery or address late abortion, as this information is not routinely collected. Depending on the prevalence of the condition studied, the size of longitudinal cohorts can vary from 800 to 100 000.[14] Our large sample size (6 866 participants) is sufficient to allow meaningful analysis of pregnancy complications.
We found a miscarriage rate of 9.6/1 000, but for mid-trimester miscarriages, it was 7.3/1 000 in the 2 724 pregnancies followed from 14 weeks' gestation. The miscarriage rates of 7.3 - 9.6/1 000 are very similar to the stillbirth rate of 9.8/1 000 we have found for pregnancies at or after 28 weeks' gestation. The burden of late miscarriage is therefore considerable.
To reduce the number of stillbirths, conditions associated with late mid-trimester miscarriages should also be addressed. Sending the placenta for histological examination after late mid-trimester miscarriage should provide valuable additional information. As there are few publications on the causes of mid-trimester miscarriages,[15-17] more studies investigating their causes should be done.
We found a stillbirth rate of 9.8/1 000 for deliveries at or after 28 weeks. The estimated worldwide stillbirth rate for deliveries at or after 28 weeks is 18.4/1 000. The highest rates reported were in south-eastern Asia and sub-Saharan Africa (SSA), at 25.5 and 28.7/1 000, respectively.[3] For SA the rate was 17.6/1 000 in 2012 and 2013, according to the Saving Babies Report.[18] For the Western Cape Province the most recent rate was 12.3/1 000.[18] For the coloured population served by Tygerberg Hospital the latest available rate was 12.6/1 000 in 2011.[19] The rate of 9.8/1 000 in Bishop Lavis therefore compares very favourably with other rates in low-income countries and other rates in the Western Cape. It also compares favourably with the goals of the Every Newborn Action Plan, where the target is <12 stillbirths per 1 000 deliveries.[20] In addition, it compares favourably with the highest stillbirth rates in high-income countries, such as the rates of 7.9 and 8.8 in Moldova and Ukraine, respectively.[21]
In SSA, it is estimated that 51% of stillbirths occur during labour. [3] In a large study consisting of >200 000 women from 106 communities in seven sites in six low-income countries, ~70% of stillbirths were probably intrapartum.[22] The rate of 15.9% in the present study is therefore indicative of good care during labour and delivery.
Nutrition and potentially modifiable lifestyle conditions, each contributing 10%, were associated with stillbirths.[3] Gray et al.[23] examined the records of >500 000 singleton live births and 2 699 stillbirths in Scotland from 1994 to 2003. Smoking during pregnancy accounted for 38% of the social inequality in stillbirths and 31% of inequalities in infant deaths. As smoking and drinking alcohol were associated with stillbirths in the Bishop Lavis cohort,[2] efforts to address these unhealthy lifestyle factors should reduce the stillbirth rate further.
We found a stillbirth/neonatal death ratio of 3.7 for deliveries at >22 weeks or >28 weeks. This does not compare favourably with reported ratios. In a study in 24 countries, addressing 1 134 stillbirths at >28 weeks' gestation and 1 465 neonatal deaths at >24 weeks, a ratio of 0.8 was found.[24] This is very similar to the ratio of 1.2 in a study in 106 communities in seven sites in six low- and middle-income countries where 97.2% of the 220 235 enrolled women completed follow-up.[23] A ratio of 1.03 was reported in a study of 8 230 women in four rural health districts in the Democratic Republic of the Congo.[25] In the assessment of 2 656 000 stillbirths and 3 072 000 neonatal deaths in 193 countries, all United Nations members, it was found that the stillbirth/neonatal death ratio for 2010 was 0.86.[26] A high ratio was found in a hospital in Uganda, where there were 430 stillbirths and 80 neonatal deaths, producing a ratio of 5.4.[27]
Only 1.8% of our participants were HIV-positive. The estimated overall HIV prevalence rate is 12.6% for the total SA population; the total number of people living with HIV was estimated at 7.06 million in 2017. For females aged 15 - 49 years, an estimated 21.2% of the population is HIV-positive.[28] According to another study, the prevalence for women aged 15 - 49 years is 23.3%.[29] The reasons for the low prevalence rate in our study are unclear. It is most likely that exposure to HIV among pregnant women in this community is low, but it is also possible that a higher resistance to HIV could have played a role. [30,31]
When compared with international standards,[12] the 18.1% prevalence of SGA in our local community-based cohort is high, but it is lower than the rate of 25.5% reported for SSA.[32] Of the liveborn babies who died after delivery, 25.6% had a birth weight below the 10th centile, in contrast to 17.7% for newborns who were alive at 1 year. The odds ratio for dying after delivery for SGA infants was 1.87 (95% confidence interval (CI) 1.144 - 3.066; p<0.05). This is in keeping with the finding of Aiken[33] that SGA infants have increased perinatal morbidity and mortality. Compared with AGA babies, SGA infants are also at risk for long-term sequelae that include poor neurodevelopmental scores such as motor skills, vision and hearing, low educational attainment and neurological morbidity (including cerebral palsy).[33] Long-term follow-up of SGA infants is therefore essential.
When suboptimal growth is suspected, umbilical artery Doppler assessment is recommended to differentiate between fetuses thought to be 'constitutionally small' and those with fetal growth restriction due to placental insufficiency, which is associated with many risks.[34] However, diagnosing fetal growth restriction, even in well-resourced countries, remains difficulty[34] Various strategies have been investigated to increase the yield of prenatal diagnosis, all with only limited success. It seems that the use of flow velocity in the middle cerebral artery may be helpful to identify at-risk fetuses not identified by other methods.[35] Our study highlights the importance of identifying SGA in order to prevent perinatal morbidity and mortality. However, the way forward in a low-income country is difficult. There is a high prevalence of many social confounders such as poverty, smoking, alcohol and illicit drug use and poor nutrition that contribute to a suboptimal intrauterine environment, and all need to be addressed.
Preterm delivery is a worldwide problem. According to 20 cohorts, providing data for >2 million live births from Asia, Africa and Latin America, the pooled overall relative risk for neonatal mortality from preterm delivery was 6.82 (95% CI 3.56 - 13.07). Although preterm birth affects fewer neonates than SGA does, it is associated with a higher mortality risk.[36] We found a preterm delivery rate of 13.8%, which is higher than the 2010 rate of 12.3% for SSA.[32] The high preterm delivery rate is probably one of the reasons for a decrease of only 1.5% per year in neonatal mortality for SSA from 2000 to 2010.[26] The high preterm delivery rate for SSA is in sharp contrast to the low rate of 5.4 - 8.9% for 24 European countries in 2010.[24]
Conclusion
Key findings in our study were the high rates of mid-trimester abortions, preterm deliveries and SGA infants, as well as the high stillbirth/neonatal death ratios. As only 15.9% of stillbirths occurred during labour and the maceration rate was 52%,[9] the underlying causes are more likely to be related to placental and environmental conditions than to poor care during and after delivery. Environmental conditions such as cigarette smoking and excessive use of alcohol associated with the increased risks of mid-trimester abortion, stillbirth and preterm birth should therefore be addressed in the pursuit of reducing perinatal mortality.
Declaration. This publication was one of the requirements for a PhD for the first author (LTB).
Acknowledgements. We are grateful to all the other members of the SPS for collection of data.
Author contributions. LTB extracted the required clinical information from the database, designed the tables and figure, provided information to calculate the z-scores, performed quality control of the data, contributed to the writing, and checked the data at the end. GSG co-ordinated perinatal mortality meetings, helped with the statistical analysis, contributed to the writing, and proofread the manuscript several times. DM co-ordinated perinatal mortality meetings, contributed to the writing, and proofread the manuscript several times. CAG was manager of the study, performed quality control of the data, and contributed to editing of the manuscript. HJO was principal investigator of the study, designed the study, initiated the manuscript, wrote the initial draft, and contributed to editing.
Funding. The study was funded by the National Institute on Alcohol Abuse and Alcoholism, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders (U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991 and U01 AA016501).
Conflicts of interest. None.
References
1. Lawn JE, Gravettt MG, Nunes TM, Rubens CE, Stanton C. Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 2010;10(1):S1. https://doi.org/10.1186/1471-2393-10-S1-S1 [ Links ]
2. McNamee KM, Dawood F, Farquharson RG. Mid-trimester pregnancy loss. Obstet Gynecol Clin North Am 2014;41(1):87-102. https://doi.org/10.1016/j.ogc.2013.10.007 [ Links ]
3. Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: Rates, risk factors, and acceleration towards 2030. Lancet 2016;387(10018):587-603. https://doi.org/10.1016/S0140-6736(15)00837-5 [ Links ]
4. Dukes KA, Burd L, Elliott AJ, et al. The Safe Passage Study: Design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol 2014;28(5):455-465. https://doi.org/10.1111/ppe.12136 [ Links ]
5. Odendaal HJ, Elliott A, Kinney HC, et al. Consent for autopsy research for unexpected death in early life. Obstet Gynecol 2011;117(1):167-171. https://doi.org/10.1097/AOG.0b013e318200cb17 [ Links ]
6. Perinatal Problem Identification Program. https://www.ppip.co.za/ (accessed 31 December 2018). [ Links ]
7. World Health Organization 2016. Stillbirths. 2016. http://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/ (accessed 3 September 2017). [ Links ]
8. Hure AJ, Powers JR, Mishra GD, Herbert DL, Byles JE, Loxton D. Miscarriage, preterm delivery, and stillbirth: Large variations in rates within a cohort of Australian women. PLoS One 2012;7(5). https://doi.org/10.1371/journal.pone.0037109 [ Links ]
9. Geldenhuys E, Coldrey J, Wright C, et al. Fetal foot length at delivery as a tool for determining gestation length in non-macerated stillbirths. Int J Gynecol Obstet 2017;138(1):107-112. https://doi.org/10.1002/ijgo.12177 [ Links ]
10. Genest DR, Singer DB. Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstet Gynecol 1992;80(4):593-600. [ Links ]
11. Kamath-Rayne BD, Defranco EA, Chung E, Chen A. Subtypes of preterm birth and the risk of postneonatal death. J Pediatr 2013;162(1):28-34e2. https://doi.org/10.1016/j.jpeds.2012.06.051 [ Links ]
12. Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: The Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014;384(9946):869-879. https://doi.org/10.1016/S0140-6736(14)61490-2 [ Links ]
13. Golding J, Jones R, Preece A, Bruné M, Pronczuk J. Choice of environmental components for a longitudinal birth cohort study. Paediatr Perinat Epidemiol 2009;23(s1):134-153. https://doi.org/10.1111/j.1365-3016.2009.01014.x [ Links ]
14. Golding J, Steer C. How many subjects are needed in a longitudinal birth cohort study? Paediatr Perinat Epidemiol 2009;23(s1):31-38. https://doi.org/10.1111/j.1365-3016.2008.00997.x [ Links ]
15. Greenwood DC, Alwan N, Boylan S, et al. Caffeine intake during pregnancy, late miscarriage and stillbirth. Eur J Epidemiol 2010;25(4):275-280. https://doi.org/10.1007/s10654-010-9443-7 [ Links ]
16. Mazzucconi MG, de Sanctis V, Alfo M, et al. Maternal thrombophilia and adverse pregnancy outcome: A case-control study. Acta Haematol 2015;133(2):242-248. https://doi.org/10.1159/000363048 [ Links ]
17. Ball E, Bulmer J, Ayis S, Lyall F, Robson S. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol 2006;208(4):535-542. https://doi.org/10.1002/path.1927 [ Links ]
18. Perinatal Problem Identification Program. http://www.ppip.co.za/wp-content/uploads/Saving-Babies-2012-2013 (accessed 31 December 2018). [ Links ]
19. Odendaal HJ, Gebhardt GS, Theron GB. Stillbirth rates in singleton pregnancies in a stable population at Karl Bremer and Tygerberg hospitals over 50 years. S Afr J Obstet Gynaecol 2013;19(3):67-70. http://www.sajog.org.za/index.php/SAJOG/article/view/662/406 (accessed 4 January 2019). [ Links ]
20. Qureshi ZU, Millum J, Blencowe H, et al. Stillbirth should be given greater priority on the global health agenda. BMJ 2015;351:h4620. https://doi.org/10.1136/bmj.h4620 [ Links ]
21. Flenady V, Wojcieszek AM, Middleton P, et al. Stillbirths: Recall to action in high-income countries. Lancet 2016;387(10019):691-702. https://doi.org/10.1016/S0140-6736(15)01020-X [ Links ]
22. Saleem S, McClure EM, Goudar SS, et al. A prospective study of maternal, fetal and neonatal deaths in low-and middle-income countries. Bull World Health Organ 2014;92(8):605-612. https://doi.org/10.2471/BLT.13.127464 [ Links ]
23. Gray R, Bonellie SR, Chalmers J, et al. Contribution of smoking during pregnancy to inequalities in stillbirth and infant death in Scotland 1994 - 2003: Retrospective population based study using hospital maternity records. BMJ 2009;339:b3754. https://doi.org/10.1136/bmj.b3754 [ Links ]
24. Zeitlin J, Mortensen L, Cuttini M, et al. Declines in stillbirth and neonatal mortality rates in Europe between 2004 and 2010: Results from the Euro-Peristat project. J Epidemiol Community Health 2016;70(6):609-615. https://doi.org/10.1136/jech-2015-207013 [ Links ]
25. Engmann C, Matendo R, Kinoshita R, et al. Stillbirth and early neonatal mortality in rural Central Africa. Int J Gynecol Obstet 2009;105(2):112-117. https://doi.org/10.1016/j.ijgo.2008.12.012 [ Links ]
26. Lawn JE, Kinney MV, Black RE, et al. Newborn survival: A multi-country analysis of a decade of change. Health Policy Plan 2012;27(Suppl_3):iii6-iii28. https://doi.org/10.1093/heapol/czs053 [ Links ]
27. Moyer CA, Kolars CK, Oppong SA, Bakari A, Bell A, Busingye P. Predictors of stillbirths and neonatal deaths in rural western Uganda. Int J Gynecol Obstet 2016;134(2):190-193. https://doi.org/10.1016/j.ijgo.2016.01.009 [ Links ]
28. TBFACTS.ORG. HIV statistics for South Africa - prevalence, incidence, ARVs, deaths. https://www.tbfacts.org/hiv-statistics-south-africa/ (accessed 15 January 2019). [ Links ]
29. Harling G, Moyo S, McGovern ME, et al. National South African HIV prevalence estimates robust despite substantial test non-participation. S Afr Med J 2017;107(7):590-594. https://doi.org/10.7196/SAMJ.2017.v107i7.11207 [ Links ]
30. Fowke KR, Nagelkerke NJ, Kimani J, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet 1996;348(9038):1347-1351. https://doi.org/10.1016/S0140-6736(95)12269-2 [ Links ]
31. Pancino G, Saez-Cirion A, Scott-Algara D, Paul P. Natural resistance to HIV infection: Lessons learned from HIV-exposed uninfected individuals. J Infect Dis 2010;202(Suppl 3):S345-S350. https://doi.org/10.1086/655973 [ Links ]
32. Lee AC, Katz J, Blencowe H, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health 2013;1(1):e26-e36. https://doi.org/10.1016/S2214-109X(13)70006-8 [ Links ]
33. Aiken C. Long-term neurodevelopmental outcomes in small babies. Obstet Gynaecol Reprod Med 2017;27(8):235-238. https://doi.org/10.1016/j.ogrm.2017.06.001 [ Links ]
34. Figueras F, Eixarch E, Gratacos E, Gardosi J. Predictiveness of antenatal umbilical artery Doppler for adverse pregnancy outcome in small-for-gestational-age babies according to customised birthweight centiles: Population-based study. BJOG 2008;115(5):590-594. https://doi.org/10.1111/j.1471-0528.2008.01670.x [ Links ]
35. Triunfo S, Crispi F, Gratacos E, Figueras F. Prediction of delivery of small-for-gestational-age neonates and adverse perinatal outcome by fetoplacental Doppler at 37 weeks' gestation. Ultrasound Obstet Gynecol 2017;49(3):364-371. https://doi.org/10.1002/uog.15979 [ Links ]
36. Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. Lancet 2013;382(9890):417-425. https://doi.org/10.1016/S0140-6736(13)60993-9 [ Links ]
 Correspondence:
Correspondence:
H J Odendaal
hjo@sun.ac.za
Accepted 10 July 2018














