Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 no.2 Pretoria Fev. 2019
http://dx.doi.org/10.7196/samj.2019.v109i2.00010
CME
Mechanical thrombectomy for acute ischaemic stroke
R Harrichandparsad
MB ChB, MMed (Neurosurg), FCNeurosurg (SA); Department of Neurosurgery, College of Health Sciences, University of KwaZulu-Natal and Inkosi Albert Luthuli Central Hospital, Durban, South Africa
ABSTRACT
Rapid, safe and effective arterial recanalisation to restore blood flow and improve functional outcome is the primary goal of hyperacute management of acute ischaemic stroke. This is possible either through thrombolysis or direct mechanical removal of clot from the blocked artery. Current evidence from randomised controlled trials shows that, for correctly selected patients, functional independence can be achieved in 32 - 71% of those who undergo clot removal. It is estimated that 10 - 15% of all ischaemic stroke patients have large-vessel occlusion and qualify for mechanical thrombectomy. It is important to have systems in place to identify and treat these patients.
History of intervention for acute ischaemic stroke
In 1995, the National Institute of Neurological Disorders and Stroke (NINDS) demonstrated that treatment with intravenous recombinant tissue plasminogen activator (IV r-tPA) within 3 hours of onset of ischaemic stroke improved clinical outcome at 3 months.[1] However, there was less benefit to patients with proximal or large-vessel occlusion, possibly because the clot burden and nature of the lesion meant that thrombolysis was less effective than in smaller-vessel occlusions. In 1998, ECASS (European Cooperative Acute Stroke Study) extended the benefit of IV r-tPA to 4.5 hours.[2] In 1999, the PROACT II (Prolyse in Acute Cerebral Thromboembolism II) trial demonstrated the efficacy of intra-arterial pro-urokinase for proven middle cerebral artery occlusion.[3] These early endovascular approaches ultimately led to the development of mechanical thrombectomy.
The first mechanical intra-arterial clot-retrieval endovascular device, the MERCI Retriever (UCLA, USA), used in the MERCI (Mechanical Embolus Removal in Cerebral Ischemia) trial in 2005, showed a recanalisation rate of 46% by the MERCI device alone and 60.8% when combined with IV r-tPA.[4] However, intracranial haemorrhage occurred in 7.8% of patients. In the Multi MERCI trial a later-generation MERCI device was used, which demonstrated a 69.5% recanalisation rate with favourable clinical outcomes in 34%; however, the trial did not include a control therapy group.[5] Optimism about mechanical thrombectomy diminished when 3 early randomised controlled trials (RCTs) published in 2013 (IMS III (Interventional Management of Stroke III), MR RESCUE (Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy) and SYNTHESIS (Local Versus Systemic Thrombolysis for Acute Ischemic Stroke)) failed to show improved efficacy of endovascular clot retrieval compared with IV r-tPA alone.[6] Limitations of these early trials were as follows: patient selection (proven large-vessel occlusion was not a requirement for inclusion), use of older technology (mainly first-generation clot retrieval devices) and a long delay from stroke onset to intervention. However, a post hoc subgroup analysis still showed benefit in patients with proven large-vessel occlusion who underwent endovascular clot retrieval within 90 minutes of IV r-tPA.[7]
Current evidence for mechanical thrombectomy
A paradigm shift occurred in 2015 with the publication, in rapid succession, of 5 landmark RCTs that tested new-generation stent retriever devices. These trials showed consistent superiority of endovascular clot retrieval over standard medical therapy in reducing disability at 90 days, as measured by the modified Rankin scale (mRS) (Table 1) in patients with acute ischaemic stroke (AIS) due to large-vessel occlusion in the anterior circulation.[8]
The first study was MR CLEAN (Multicenter Randomised Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands), with all subsequent studies discontinued early owing to efficacy, loss of equipoise or both. Functional independence (mRS 0 - 2) was achieved in up to 60% of patients. There was an absolute benefit of 13.5 - 31% for patients who had undergone mechanical thrombectomy compared with those who received best medical treatment alone. Some key features of these trials are summarised in Table 2. In contrast to the earlier RCTs, selection was confined to patients with proven large-vessel occlusion diagnosed on computed tomography (CT) angiography, randomisation of patients within 6 hours of stroke onset and mandatory use of newer-generation stent retrievers in the later trials.
HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke) trials pooled data from the abovementioned 5 trials in a meta-analysis to investigate outcomes in subgroups that were too small to investigate in the individual trials.[9] A total of 1 287 patients (n=634 endovascular therapy patients and n=653 controls) were included. There was significantly reduced disability at 90 days in the endovascular group, with good (independent) functional outcome (mRS 0 - 2) in 46% compared with 26.5% in the medical control group. Subgroup analysis further showed that benefit was seen in patients >80 years of age, those randomised >300 minutes after onset and those not eligible for IV r-tPA. There was no difference in risk of symptomatic intracerebral haemorrhage at 90 days. The number needed to treat for patients to achieve an improvement of >1 points on mRS was 2.6. This provided robust evidence for the safety and efficacy of mechanical thrombectomy for most patients with AIS caused by occlusion of proximal anterior circulation, irrespective of patient characteristics or geographical location. Subsequent RCTs (THRACE (mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke), THERAPY (The Randomized, Concurrent Controlled Trial to Assess the Penumbra System's Safety and Effectiveness in the Treatment of Acute Stroke), PISTE (Pragmatic Ischaemic Stroke Thrombectomy Evaluation) and EASI (Endovascular Acute Stroke Intervention)) confirmed the benefit of mechanical thrombectomy. Based on evidence from these trials, updated practice guidelines were rapidly published in the USA, Canada, Europe and the UK, recommending that mechanical thrombectomy be provided to patients with occlusion of the internal carotid artery or proximal middle cerebral artery who had received treatment with IV r-tPA within 4.5 hours of onset and who could undergo the procedure (arterial puncture) within 6 hours of symptom onset.[7]
Patient selection
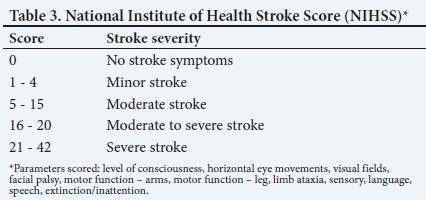
The decision for mechanical thrombectomy should be made by a physician trained in the diagnosis and treatment of stroke, in conjunction with a neurointerventionalist who has the relevant images (non-contrast CT of the brain and a CT angiogram) available for review. Prompt assessment of stroke severity using the National Institute of Health Stroke Score (NIHSS (Table 3)), baseline functional status (mRS) and consideration of comorbidities is essential. To achieve optimal clinical outcomes, patient selection criteria should parallel those of the successful mechanical thrombectomy trials:
• Documented anterior circulation large-vessel occlusion (proximal middle cerebral artery, anterior cerebral or internal carotid artery).
• Significant clinical deficit at the time of assessment (NIHSS >5).
• Lack of extensive early ischaemic change (ASPECTS (Alberta Stroke Program Early CT Score) >5) as assessed on non-contrast CT of the brain. ASPECTS is a 10-point quantitative score (Fig. 1). Each area of the middle cerebral artery territory is allocated 1 point. For each region showing ischaemic change, 1 point is deducted. A normal CT scan receives an ASPECT score of 10 points; a score of <7 is equal to involvement of one-third of the middle cerebral artery territory. A score of 0 indicates diffuse ischaemic involvement throughout the middle cerebral artery territory.
• Reasonable pre-stroke functional status and lack of serious comorbidities, indicating potential to benefit from treatment (age >80 years alone is not a contraindication for treatment).
• Treatment with IV r-tPA within 4.5 hours (patients ineligible for IV r-tPA owing to bleeding risk can be considered for treatment).
• The occluded vessel can be opened by mechanical thrombectomy within 6 hours.
The speed of delivery of mechanical thrombectomy is key to achieving the best possible outcomes. Therefore, the question of benefit in patients with unwitnessed stroke, wake-up stroke (a patient goes to bed well, but wakes up with symptoms of a stroke) or delayed presentation after 6 - 8 hours, requires assessment with more advanced imaging. The DAWN trial (Diffusion Weighted Imaging (DWI) or Computerized Tomography Perfusion (CTP) Assessment with Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention) was a prospective, multicentre RCT comparing mechanical thrombectomy and medical treatment with medical treatment alone for AIS. The therapeutic window was extended to 24 hours. Patients had to have proven large-vessel occlusion and an NIHSS >10. Advanced imaging (DWI or CT perfusion) was used to assess core infarct volume relative to volume of salvageable penumbra. A total of 206 patients were enrolled (enrolment discontinued before the target of 500 after interim analysis). Results showed significantly reduced post-stroke disability and improved functional independence at 90 days (48.6% v. 13.1%) in the endovascular group. There was a relative reduction in disability of 73%, with 1 in 2.8 patients saved from severe disability.[10] The DEFUSE-3 trial (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) also showed improved functional outcomes with mechanical thrombectomy for ischaemic stroke 6 - 16 hours after onset compared with medical therapy alone for patients with anterior circulation large-vessel occlusion and a region of tissue that was ischaemic but not yet infarcted.[11]
Practical aspects
Devices and techniques
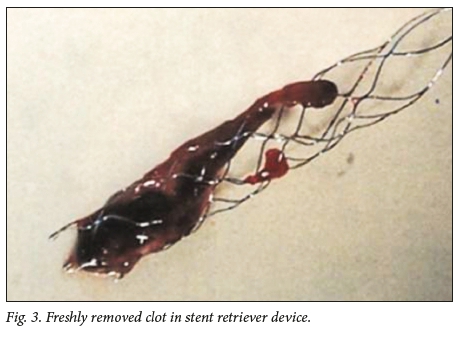
After successful RCTs, the Solitaire FR (Medtronic, USA) stent retriever became the benchmark for mechanical thrombectomy. However, rapid and safe recanalisation and reperfusion of the occluded territory is the key factor, rather than any specific device or technique. Multiple options are available, including the use of supplementary devices, such as balloon-guide catheters, intermediate catheters and suction pumps v. manual aspiration.[7] After femoral artery puncture, an 8 Fr guide catheter is navigated into the internal carotid artery, where an intermediate catheter (5 - 6 Fr) is directed to the circle of Willis. Through this, a microcatheter is navigated under fluoroscopic guidance distal to the clot over a microwire. The microwire is removed and a run is done through the microcatheter to confirm position past the clot, filling the distal occluded territory. The stent retriever is navigated through the microcatheter, positioned at the level of the clot and opened by unsheathing, i.e. pulling the microcatheter back while keeping the stent in place. This exposes the struts of the stent to the clot. Once the stent is integrated into the clot, the device is pulled back into the intermediate catheter, which has suction applied simultaneously. A balloon guide, forming a cuff around the guide catheter, may be used to stop the forward flow and reduce the chance of distal emboli. When using such a guide, the intermediate catheter may be omitted. Fig. 2 shows angiographic images of mechanical thrombectomy in a 59-year-old man who presented with acute right hemiparesis and dysphasia. The freshly removed clot in the stent retriever device is shown in Fig. 3. The patient's functional status improved to mRS 1 after mechanical thrombectomy.
An increasingly popular approach is to attempt to aspirate the clot directly into the intermediate catheter, which has become feasible since the advent of large-lumen soft catheters that can be safely navigated into the M1 segment of the middle cerebral artery and beyond. It is critical to choose a catheter with a lumen approaching the size of the vessel where the clot is lodged, allowing the clot to be suctioned completely. If this fails, a stent retriever may be easily deployed through the initial system.
Anaesthesia
The use of general anaesthesia (GA) v. local anaesthesia (conscious sedation) currently varies, with each strategy having potential advantages. GA associated with systolic blood pressure <140 mmHg was also associated with poor functional outcome (mRS >2) at 90 days. However, more recent studies suggest that GA and conscious sedation are equally safe; therefore, the choice should be individualised, based on patient and institutional factors.[12,13]
Complications
The complications of endovascular procedures can be device related, access related or contrast related. Device-related complications include vessel perforation, symptomatic intracranial haemorrhage, subarachnoid haemorrhage, arterial dissection, emboli to new vascular territories and vasospasm. Access-related complications include femoral artery dissection, pseudoaneurysm, retro-peritoneal haematoma and infection. The overall procedural complication rate from recent RCTs is ~15%; however, many of these complications do not adversely affect clinical outcome.
Who should perform mechanical thrombectomy?
To minimise complications, the key strategy is for mechanical thrombectomy to be performed only in high-volume centres by trained physicians competent in intra-cranial endovascular procedures and who undertake these regularly to maintain skills. Mechanical thrombectomy should only be performed in the context of a multidiscipli-nary team, operating in comprehensive stroke centres with adequate neurointerventional procedural volumes (e.g. >200 per year), of which a reasonable proportion are mechanical thrombectomy, and where regular assessment/audit of technical and clinical results, process times and complications is undertaken.[7] When complications do occur, the immediate availability of neuro-critical care and/or neurosurgical support is mandatory and may be lifesaving.
Challenges and future questions
The practical implementation of mechanical thrombectomy in South Africa (SA) presents many challenges. For the trial results to be replicated, identification and support of comprehensive stroke units with appropriate referral pathways of selected patients from primary stroke centres are needed.[14] A comprehensive stroke unit should include qualified neurointerventionalists who are able to offer mechanical thrombectomy 24 hours a day, 7 days a week. To get the right patient to the right place within the right time frame requires reorganisation of SA stroke services that involves substantial investment, great attention to care pathways and extensive co-operation between services, including ambulances and hospitals.
The value of mechanical thrombectomy for posterior circulation strokes, distal middle cerebral artery strokes, mild stroke symptoms (NIHSS <5) and the role of direct thrombectomy without IV r-tPA remain to be defined. Ongoing RCTs should provide these answers in the next 3 - 5 years.
Declaration. None.
Acknowledgements. None.
Author contributions. Sole author.
Funding. None.
Conflicts of interest. None.
References
1. Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome The NINDS rt-PA stroke study. Neurology 2000;55(11):1649-1655. https://doi.org/10.1212/wnl.55.11.1649 [ Links ]
2. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998;352(9136):1245-1251. https://doi.org/10.1016/s0140-6736(98)08020-9 [ Links ]
3. Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. JAMA 1999;282(21):2003-2011. https://doi.org/10.1001/jama.282.21.2003 [ Links ]
4. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke 2005;36(7):1432-1438. https://doi.org/10.1161/01.str.0000171066.25248.1d [ Links ]
5. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the Multi MERCI trial. Stroke 2008;39(4):1205-1212. https://doi.org/10.1161/strokeaha.107.497115 [ Links ]
6. Pierot L, Gralla J, Cognard C, White P. Mechanical thrombectomy after IMS III, synthesis, and MR-RESCUE. Am J Neuroradiol 2013;34(9):1671-1673. https://doi.org/10.3174/ajnr.a3654 [ Links ]
7. Evans M, White P, Cowley P, Werring D. Revolution in acute ischaemic stroke care: A practical guide to mechanical thrombectomy. Pract Neurol 2017;17(4):252-265. https://doi.org/10.1136/practneurol-2017-001685 [ Links ]
8. Campbell BC, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015;14(8):846-854. https://doi.org/10.1016/s1474-4422(15)00140-4 [ Links ]
9. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723-1731. https://doi.org/10.1016/s0140-6736(16)00163-x [ Links ]
10. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378(1):11-21. https://doi.org/10.1016/j.jemermed.2018.02.029 [ Links ]
11. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378(8):708-718. https://doi.org/10.1056/NEJMoa1713973 [ Links ]
12. Löwhagen Hendén PL, Rentzos A, Karlsson JE, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: The AnStroke trial (anesthesia during stroke). Stroke 2017;48(6):1601-1607. https://doi.org/10.1161/strokeaha.117.016554 [ Links ]
13. Simonsen CZ, S0rensen LH, Juul N, et al. Anesthetic strategy during endovascular therapy: General anesthesia or conscious sedation? (GOLIATH - general or local anesthesia in intra arterial therapy) A single-center randomized trial. Int J Stroke 2016;11(9):1045-1052. https://doi.org/10.1177/1747493016660103 [ Links ]
14. Taylor A, le Feuvre D, Mngomezulu V, et al. Advances in stroke treatment are within reach. S Afr Med J 2016;106(5):454-455. https://doi.org/10.7196/SAMJ.2016.v106i5.10355 [ Links ]
 Correspondence:
Correspondence:
R Harrichandparsad
harrichandparsad@ukzn.ac.za
Accepted 27 December 2018














