Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.109 n.2 Pretoria Feb. 2019
http://dx.doi.org/10.7196/samj.2019.v109i2.00008
CME
Medical management of acute ischaemic stroke
K Bateman
MB ChB, FCNeurol (SA); Division of Neurology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
This article provides a practical overview of current medical treatments for acute ischaemic stroke, particularly for those in a busy family or general practice. Stroke is defined as an acute neurological deficit lasting >24 hours and caused by cerebrovascular disease. It may be ischaemic, caused by vessel stenosis or occlusion, or haemorrhagic, caused by rupture of vessels, resulting in intraparenchymal and/or subarachnoid haemorrhage. Transient ischaemic attack is defined as a transient episode of neurological dysfunction caused by focal brain, spinal cord or retinal ischaemia without acute infarction, replacing the old time-based definition. The goals of management of patients with an acute stroke are as follows: make an accurate assessment and diagnosis, limit the extent of the brain injury, avoid and treat stroke-related complications, evaluate the underlying aetiology that is closely linked to the prognosis for recurrent stroke, institute appropriate secondary prevention and facilitate post-stroke recovery.
Initial evaluation
The emphasis of the initial screening of a suspected acute stroke patient is on rapid evaluation and diagnosis that adequately excludes stroke mimics. Mimics such as seizures, migraine, hypoglycaemia and psychogenic weakness may account for 20 - 25% of suspected stroke or transient ischaemic attack (TIA) presentations.[1] Stroke should be suspected in all patients with abrupt onset of neurological symptoms, particularly in those with risk factors for stroke. Facial drooping, arm drift, speech difficulties and time (FAST) score (Table 1) is a useful emergency unit screening test to improve stroke recognition with acceptable sensitivity and specificity. Although it may miss atypical presentations, the presence of >1 of these signs raises the likelihood of stroke by >5 times.[2]

Initial management of an acute stroke (Fig. 1) includes assessment and correction of disordered airway, breathing and circulation. The next step is to determine whether brain reperfusion can be achieved. Brain imaging should be performed without delay to exclude haemorrhage and mimics. An uncontrasted computed tomography (CT) brain scan detects fresh intracranial haemorrhage very well, but has a poor sensitivity for infarction, especially small infarcts, posterior fossae involvement, and when performed too soon after the event. A normal CT brain scan therefore does not exclude an acute ischaemic stroke (AIS).
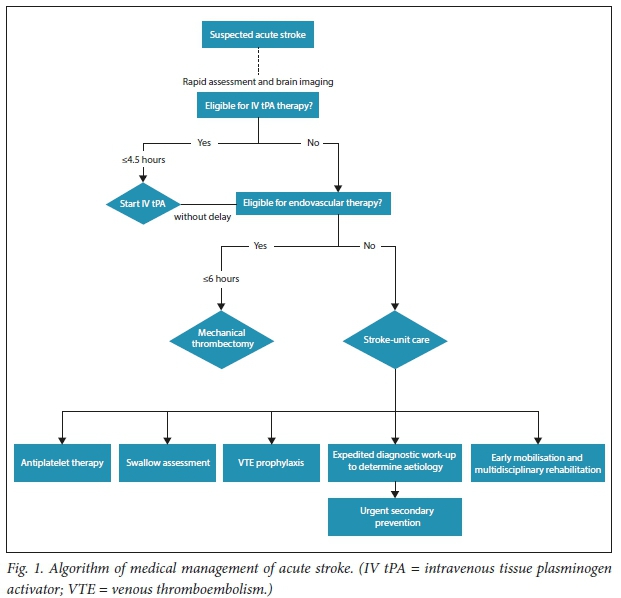
Diffusion-weighted magnetic resonance imaging (MRI) performs better and detects approximately one-third of transient attacks lasting <24 hours and ~90% of acute brain infarctions. However, obtaining an MRI scan may delay the start of emergency reperfusion therapies; an uncontrasted CT brain scan, which is frequently more accessible, provides the necessary information in most instances.
A targeted neurological (National Institute of Health Stroke Score (NIHSS)) and cardiovascular examination and rapid blood glucose test should be performed before the start of reperfusion therapy. Further work-up, including blood investigations, an electrocardiogram (ECG) and a chest radiograph, should not delay treatment.
Acute reperfusion therapies
Intravenous thrombolysis with alteplase
Whether a given area of ischaemic brain tissue becomes irreversibly damaged, i.e. infarcted, depends on both the degree and duration of ischaemia, and therefore specific treatments for AIS focus on achieving early reperfusion of ischaemic brain tissue. Alteplase, a recombinant tissue plasminogen activator (r-tPA), stimulates thrombolysis and thereby recanalisation and reperfusion. Intravenous alteplase (0.9 mg/kg) given within 4.5 hours of onset of AIS improves the odds of having no significant disability at 3 - 6 months by about one-third.[3] The benefits of alteplase are proportionately larger and the risk of symptomatic brain haemorrhage is lower the earlier the treatment is administered after the onset of stroke symptoms. Approximately 4.5 patients need to be treated within 90 minutes, 9 patients between 90 and 180 minutes, and 15 patients between 180 and 270 minutes after onset of stroke for one of them to have an excellent outcome (minimal signs, if any; no disability).[4] Although alteplase for AIS does increase the odds of having a symptomatic intracranial haemorrhage with an overall risk of ~6%, it does not affect overall mortality at 3 months after stroke.[3] Bleeding risk increases with elevated serum glucose, evidence of early ischaemic changes or a hyperdense middle cerebral artery sign on CT brain imaging - higher NIHSS indicating a more severe stroke. However, within 4.5 hours of stroke, the likelihood of achieving an excellent outcome with r-tPA is greater than the risk of death, with early treatment particularly important in patients with large strokes. Early administration of alteplase to appropriate patients is therefore recommended. This is, however, of particular importance in severe stroke, and is more effective if given sooner than later in all eligible patients.[4]
Candidates for intravenous thrombolysis should meet the criteria for use, which are readily available in stroke guidelines.[5] The benefits and risks of r-tPA treatment should be discussed with the patient, if competent, or with a family member who is able to make decisions on behalf of the patient. Verbal or written consent should be obtained, if possible, but in the event that the patient is unable to make medical decisions and a suitable family member or decision-maker cannot be identified and approached in a timely manner, the judgement of the physician may substitute.
Where patients are eligible for intravenous thrombolysis and the onset is <4.5 hours, they should be treated with alteplase even if they are potential candidates for endovascular therapy with a stent retriever.[5] Aspirin should be given immediately to all patients with AIS, with the exception of those receiving intravenous thrombolysis. In the latter, the initiation of aspirin should be delayed by 24 hours after r-tPA and only administered once a CT brain scan has confirmed the absence of intracranial haemorrhage.[5]
Endovascular thrombectomy
Proximal large-vessel occlusions that are amenable to mechanical thrombectomy occur in ~10% of AIS.[6] Endovascular thrombectomy with stent retrievers (i.e. second-generation devices) performed in addition to early alteplase treatment within 6 hours of AIS in suitable patients doubles the rate of angiographic revascularisation at 24 hours and functional independence at 3 months. This effect is seen across the age spectrum and appears to be consistent in patients who are ineligible for r-tPA.[7] The number needed to treat to achieve one more patient with an independent functional outcome is between 3.2 and 7.1.[8]
General supportive measures in acute stroke care
Homeostasis
Restoring and maintaining bodily homeostasis is a key component of facilitating recovery after stroke. The following aspects should be targeted within the first 72 hours to improve outcomes:
• Airway support and ventilation. This may be acutely necessary in patients who have reduced consciousness or bulbar dysfunction that compromises the airway. Supplemental oxygen is not beneficial in patients with AIS who maintain normal oxygen saturation.
• Blood pressure (BP) management. There is insufficient evidence to advise management in AIS; however, in general, high BP should not be lowered acutely but over the following days to weeks unless the patient is severely hypertensive (>220 mmHg systolic BP) or hypertensive complications are present. Causes of low BP should be sought and corrected.
• Temperature. Fever is associated with worse outcomes; therefore, antipyretics might be of benefit.
• Blood glucose. Hypoglycaemia can mimic a stroke and should be rapidly corrected. Hyperglycaemia is associated with worse outcomes, and judicious lowering of blood glucose levels is recommended.
• Screening and formal swallow assessment. Up to 50% of stroke patients have dysphagia, and early detection and management may prevent aspiration pneumonia and improve outcomes. Swallowing function should be assessed within the first 24 hours after stroke, keeping the patient nil per os on intravenous saline until they have been evaluated for nasogastric tube feeding, which may be necessary if significant dysphagia is present.
• Adequate hydration and nutrition. Decent early nutrition is associated with a lower rate of pressure sores.
Stroke-unit care
Stroke patients have better odds of being alive, independent and at home 1 year after the event when they are treated in a dedicated stroke unit that has a multidisciplinary team, including trained nursing staff, doctors, occupational therapists, physiotherapists, speech and language therapists and dieticians.[9] When the population-level effect of stroke-unit care is considered by weighing the annual number of people in a population who are likely to have a stroke, eligible to receive this intervention and the potential benefit from the intervention (in terms of additional survivors independent in daily activities), several studies suggest that a basic model of stroke-unit care may be the most effective for improving independent survival. Although most of the evidence for the stroke-unit model of care comes from higher-income countries, limited reports from developing countries show similar benefit.[10] Avoidance of complications, improved supportive care, earlier mobilisation and rehabilitation efforts may account for these better outcomes.
Avoiding and treating acute complications
Medical complications after stroke occur frequently and should be avoided where possible and treated effectively when they occur. Venous thromboembolism might be the cause of ~10% of stroke deaths. Therefore, deep vein thrombosis (DVT) prophylaxis and pressure care for patients immobilised by stroke are priorities. While graduated compression stockings are not effective, thigh-length intermittent pneumatic compression prevents DVT and improves survival at 6 months. Low-dose subcutaneous low-molecular-weight heparin has been used for DVT prevention but should be avoided, as the overall increased risk of symptomatic intracerebral haemorrhage is higher than the risk of pulmonary embolism. In selected high-risk patients, such as those with previous venous thromboembolism or morbid obesity, it may still provide some benefit.
Life-threatening cerebral oedema may occur after a large cerebral infarction. This may complicate large strokes, particularly in younger patients who have less age-related atrophy, and hence manifest with critically raised intracranial pressures. Early decompressive hemicraniectomy for malignant middle cerebral artery syndrome may be life-saving, halving the untreated 80% mortality, but careful patient choice and early timing of the procedure are necessary to select those with the best chance of surviving with an acceptable functional outcome.[11]
Secondary prevention and recovery
All patients with AIS or TIA are in need of urgent secondary prevention. Untreated, after AIS and TIA, the risk of recurrent stroke is ~10% at 1 week, 15% at 1 month, and 18% at 3 months[12] In some individuals, e.g. those with recent atherosclerotic symptoms, the risk is even higher. Various scoring systems have been proposed to identify patients at high risk of early stroke recurrence in primary care or emergency unit settings to fast-track them for urgent treatment. The age, blood pressure, clinical features, duration and diabetes (ABCD2) score has been widely used, with a score of >4 denoting high risk (Table 2). However, the ABCD2 score has drawbacks, neither distinguishing between strokes and mimics nor accurately identifying all high-risk patients. One-fifth of all TIA patients who scored <4 (classified as low risk), were found to have either significant carotid stenosis or atrial fibrillation requiring urgent interventions. The age, blood pressure, clinical features, duration and diabetes plus dual TIA and imaging (ABCD3-I) scoring system (Table 2) improves the detection of patients at high risk, incorporating those with 2 TIAs in 1 week, but the score also requires brain and carotid artery vessel MRI scans and therefore is harder to use in primary care settings or secondary level hospitals, where many patients may first be seen.
Evidence clearly shows that most recurrent strokes occur within days after the index event. Timely initiation of appropriate secondary prevention by stroke specialists reduces the rate of stroke recurrence by 80%.[13] Choice of secondary prevention differs, depending on whether the stroke is thought to be of arterial or cardiac origin. Therefore, the aim of a diagnostic work-up after a stroke is to determine the most likely underlying aetiology to optimise treatment choice.
Prevention of recurrent ischaemic strokes due to arterial disease
Antiplatelet therapy
Aspirin commenced immediately after a stroke at doses of 160 - 300 mg per day decreases early recurrent strokes by half within the first 6 -12 weeks.[14] Longer-term antiplatelet treatments that are effective in reducing the risk of stroke include daily aspirin 75 - 150 mg or clopidogrel 75 mg or twice-daily aspirin 25 mg plus extended-release dipyridamole 200 mg. Dual antiplatelet treatment appears to be even more effective than aspirin in preventing early stroke recurrences in minor stroke (NIHSS <4) and TIA. In a large Chinese study,[15] the early use (<3 days) of a combination of aspirin (75 - 300 mg loading dose, then 75 mg) and clopidogrel (300 mg loading dose, then 75 mg) for 3 weeks, followed by clopidogrel alone for 3 months and thereafter monotherapy with either aspirin or clopidogrel, reduced the risk of early recurrent stroke at 90 days by one-third (6% v. 9%) compared with aspirin monotherapy, without increasing the risk of major bleeding. Recently, a similar international study showed that aspirin plus clopidogrel (600 mg loading dose, then 75 mg) for 90 days in minor stroke and high-risk TIA (ABCD2 >4) decreased recurrent stroke by 25%, albeit with an increased risk of major bleeding (0.9% v. 0.4%), compared with aspirin alone.[16] Therefore, early dual antiplatelet therapy appears to be effective in TIA and minor strokes, but its role is not established in cardioembolic or more severe strokes. Moreover, longer-term dual antiplatelet treatment for <1 year after stroke does not reduce stroke recurrence compared with monotherapy, and has a higher risk of major bleeding; it is therefore not recommended.
Carotid artery revascularisation
In patients with recent, symptomatic extracranial atherosclerotic carotid stenosis >70 - 99%, carotid endarterectomy (CEA) halves the 5-year risk of stroke and death compared with best medical treatment of >2 decades ago. For moderate stenoses (50 - 69%), this risk is reduced by a quarter. Carotid stenting has higher rates of periprocedural stroke and death than CEA in patients >70 years of age, lower rates of local complications than CEA, but similar rates of longer-term stroke, death and functional outcome.[17] If carotid revascularisation is to be performed, it should be done within 1 week of stroke or TIA, after which time benefit has not been demonstrated. Intracranial and vertebral artery stenting do not compare favourably with optimal medical treatment, with unacceptably high rates of periprocedural stroke and death.
Prevention of recurrent ischaemic strokes due to cardioembolism
Antithrombotic therapy
Anticoagulation does not reduce the early risk of stroke of arterial origin, even in patients with a high risk of thrombosis; it is also not more effective than antiplatelet agents in cervical arterial dissection. However, anticoagulation can dramatically lower stroke recurrence rates in certain settings. Patients with atrial fibrillation (AF) are frequently at high risk of recurrent stroke. Higher congestive heart failure, hypertension, age, diabetes, and stroke/TIA (CHA2DS2)-vascular disease (VASc) scores are associated with a higher risk of stroke in non-valvular AF and may be used to estimate risk in individual patients and to evaluate when to commence anticoagulation (Table 3). Most major guidelines recommend anticoagulation with an annual stroke risk of >1 - 2%. After stroke or TIA, the use of warfarin, aiming for an international normalised ratio (INR) of 2 - 3, reduces the risk of recurrent stroke by two-thirds. Direct oral anticoagulants, such as dabigatran and rivaroxaban, reduce stroke recurrence in non-valvular AF by a further 15% compared with warfarin, without increasing major bleeding. It is not clear when the best time is to start oral anticoagulation after stroke. For confirmed TIAs, it may be reasonable to start immediately. In AIS, it is most likely between 4 and 14 days, depending on risk of stroke recurrence (which may be estimated by CHA2DS2-VASc scores (Table 3), infarct size and presence of haemorrhagic conversion.
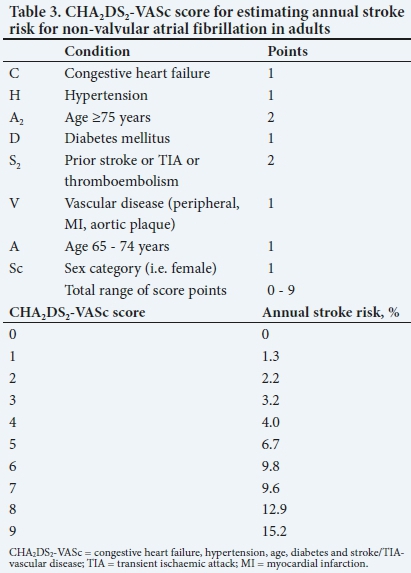
Because of the significant benefits of anticoagulation in preventing stroke in non-valvular AF, an effort should be made to detect paroxysmal AF using prolonged ECG monitoring. Other high-risk cardiac diseases, such as recent myocardial infarction, rheumatic valve disease and dilated cardiomyopathies, may be present and also necessitate anticoagulation or other specific interventions. These conditions should be identified by an adequate cardiac work-up, especially if there are clinical or radiological features suggesting a cardioembolic infarction, such as sudden onset to maximal deficit, aphasia without hemiparesis, a Valsalva manoeuvre at the time of stroke onset, co-occurrence of cerebral and systemic emboli or acute multiple infarcts in multiple cerebral territories.
Lowering of blood pressure
Sustained lowering of BP prevents stroke recurrence, which may be reduced by ~20% if the lowering of BP by ~5 mmHg systolic and 2.5 mmHg diastolic is maintained. Even more strokes are prevented if more intensive BP reductions are achieved.
Lowering of blood cholesterol concentrations
The use of statins to lower low-density lipoprotein (LDL) cholesterol by 1 mmol/L decreases the risk of recurrent stroke by 12%, with more intensive LDL reductions showing even further reduction in stroke risk, although the optimum target is not yet known.
Modification of other vascular risk factors and lifestyle
Lifestyle modifications that are recommended after stroke include weight loss, smoking cessation, low alcohol use, a low-risk diet such as the Mediterranean diet, and regular physical exercise. Hormone therapy in postmenopausal women increases the risk of recurrent stroke by about 25%; it should therefore be discontinued, if possible.
Conclusion
Rapid and accurate assessment and early initiation of suitable therapies are essential in the management of patients with AIS to limit brain injury and increase disability-free survival. Reperfusion therapies, such as intravenous thrombolysis and, more recently, endovascular therapies, improve functional outcomes, often substantially. Because of the time-sensitive nature of AIS and the potential to limit brain injury, it is crucial that stroke services are well organised, including ready access to primary and comprehensive stroke centres with appropriate triage and early management of TIA and stroke. Stroke-unit care may be the most effective population-level intervention for achieving better functional outcomes, as all patients are eligible for this option. Careful avoidance and treatment of complications, such as aspiration, are recommended. A diagnostic work-up to determine the underlying aetiology of stroke should be expedited to inform urgent secondary prevention. Early aspirin and dual antiplatelet therapy for minor atherosclerotic strokes or TIAs are more effective than previously recognised in preventing early recurrent stroke. Newer oral anticoagulants have proved to be more effective than warfarin in cardioembolic stroke prevention and have better safety profiles. Early mobilisation and multidisciplinary team input in acute rehabilitation are recommended to facilitate optimal post-stroke recovery.
Declaration. None.
Acknowledgements. None.
Author contributions. Sole author.
Funding. None.
Conflicts of interest. None.
References
1. Fernandes PM, Whiteley WN, Hart SR, Al-Shahi Salman R. Strokes: Mimics and chameleons. Pract Neurol 2013;13(1):21-28. https://doi.org/10.1136/practneurol-2012-000465 [ Links ]
2. Hankey GJ. Secondary stroke prevention. Lancet Neurol 2014;13(2):178-194. https://doi.org/10.1016/s1474-4422(13)70255-2 [ Links ]
3. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014;384(9958):1929-1935. https://doi.org/10.1016/s0140-6736(14)60584-5 [ Links ]
4. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375(9727):1695-1703. https://doi.org/10.1016/s0140-6736(10)60491-6 [ Links ]
5. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management ofpatients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke 2018;49:e46-e110. https://doi.org/10.1161/STR.0000000000000158 [ Links ]
6. McMeekin P, White P, James MA, Price CI, Flynn D, Ford GA. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J 2017;2(4):319-326. https://doi.org/10.1177/2396987317733343 [ Links ]
7. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723-1731. https://doi.org/10.1016/s0140-6736(16)00163-x [ Links ]
8. Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: The new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015;14(8):846-854. https://doi.org/10.1016/s1474-4422(15)00140-4 [ Links ]
9. Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;(9):CD000197. https://doi.org/10.1002/14651858.CD000197.pub3 [ Links ]
10. Langhorne P, de Villiers L, Pandian JD. Applicability of stroke-unit care to low-income and middle-income countries. Lancet Neurol 2012;11(4):341-348. https://doi.org/10.1016/s1474-4422(12)70024-8 [ Links ]
11. Yang MH, Lin HY, Fu J, Roodrajeetsing G, Shi SL, Xiao SW. Decompressive hemicraniectomy in patients with malignant middle cerebral artery infarction: A systematic review and meta-analysis. Surgeon 2015;13(4):230-240. https://doi.org/10.1016/j.surge.2014.12.002 [ Links ]
12. Coull AJ. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: Implications for public education and organisation of services. BMJ 2004;328(7435):326-330. https://doi.org/10.1136/bmj.37991.635266.44 [ Links ]
13. Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med 2016;374(16):1533-1542. https://doi.org/10.1056/nejmoa1412981 [ Links ]
14. Sandercock-Peter AG, Carl C, Chiun TM, Emanuela C. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev 2014;(3):CD000029. https://doi.org/10.1002/14651858.cd000029.pub3 [ Links ]
15. Wong KSL, Wang Y, Leng X, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: An updated systematic review and meta- analysis. Circulation 2013;128(15):1656-1666. https://doi.org/10.1161/circulationaha.113.003187 [ Links ]
16. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;(epub ahead of print). [ Links ]
17. Bonati H, Lyrer P, Ederle J, Featherstone R, Brown M. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev 2012;(9):CD000515. https://doi.org/10.1002/14651858.cd000515.pub4 [ Links ]
 Correspondence:
Correspondence:
K Bateman
kathleen.bateman@uct.ac.za
Accepted 27 December 2018














