Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.108 no.11 Pretoria Nov. 2018
http://dx.doi.org/10.7196/samj.2018.v108i11.13168
RESEARCH
Anorectal malformations and the impact of HIV on surgical outcome
T D GablerI; J LovelandII, III; A TheronIV; C Westgarth-TaylorV
IMB BCh, BSc; Division of Paediatric Surgery, Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB BCh, FCS (SA), Cert Paed Surg (SA); Division of Paediatric Surgery, Department of Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMB BCh, FCS (SA), Cert Paed Surg (SA); Department of Paediatric Surgery, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IVMB ChB, MMed (Paediatric Surgery), FC Paed Surg (SA); Department of Paediatric Surgery, Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
VBSc Hons, MB ChB, MRCS (Eng), FC Paed Surg (SA); Department of Paediatric Surgery, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Anorectal malformations (ARMs) represent a significant surgical load in South African (SA) paediatric surgical centres. Surgical treatment of ARMs may be associated with postoperative complications owing to the nature of surgical procedures necessary in the neonatal and infant period. HIV and its effect on the immune response compound postoperative surgical complications. The impact of HIV exposure and its effect on the child's immune status, independent of the child's HIV status, has yet to be studied in the surgical population.
OBJECTIVES. To assess the incidence of complications in our population of ARM patients and to explore whether these were increased in HIV-exposed but serologically negative children compared with HIV-unexposed children.
METHODS. This was a prospective study of all patients presenting with ARMs to the paediatric surgery units attached to the University of the Witwatersrand, Johannesburg, SA. Specifically, exposure to an HIV-positive mother, patient HIV status and presence of surgical complications were documented. Data were analysed for the period August 2016 - September 2017.
RESULTS. A total of 50 children were included (none were excluded); 19 (38%) were HIV-exposed but none were HIV-positive, and 28 (56%) were male and 22 (44%) female. Seventy-six operative procedures were performed, with 27 operative complications. In the HIV-exposed group, 68% of patients experienced operative complications, compared with 45% in the unexposed group (p=0.1); 50% of the HIV-exposed patients who had stoma formation experienced complications, compared with 20% in the unexposed group (p=0.08).
CONCLUSIONS. The incidence of postoperative infectious complications in HIV-exposed patients was higher than in HIV-unexposed patients. The incidence of postoperative complications in HIV-unexposed patients parallels that in the international literature, except in the posterior sagittal anorectoplasty groups. It remains critically important to follow stringent perioperative protocols for infection prevention and aggressively treat any infection that arises, particularly in patients born to HIV-positive mothers, regardless of the patient's HIV status.
Anorectal malformations (ARMs) represent a large clinical and surgical load, especially in low- and middle-income countries (LMICs).[1] HIV adds to this burden by increasing infectious surgical complications and medical morbidity.[2] ARMs are a spectrum of congenital anomalies leading to an imperforate anus with or without a fistula that opens into the male urinary tract or the female genital tract, or onto the perineum. The aetiology is multifactorial. The incidence of ARMs in the referral area for the teaching hospitals of the University of the Witwatersrand in Johannesburg, South Africa (SA), is 1:4 000.[3]Management of ARMs typically consists of three operations, an initial diverting colostomy, definitive repair by means of a posterior sagittal anorectoplasty (PSARP), and finally colostomy reversal. This traditional approach is especially applicable in LMICs, where patients often present late with established obstruction of the gastrointestinal tract, systemic sepsis and dehydration.[4-7]Some lesions are amenable to a primary PSARP without formation of a colostomy if the anatomical defect is favourable, the child is clinically stable and has no associated life-threatening congenital anomalies, and there is no gross abdominal distension.[8]In the paediatric population, intra-abdominal surgery is associated with a 20% surgical site infection rate and an 11% anastomotic leak rate.[9] In terms of stoma formation and closure, this equates to high morbidity; as Oda et al.[10]stated in 2014, creating a colostomy is a minor surgical procedure but with potentially significant morbidity'.
SA is a country heavily burdened by HIV. In 2014, 6.8 million people were living with HIV, of whom 3.9 million were females aged >15 years and 340 000 were children.[11] Much of the burden of HIV infection in children comes from mother-to-child transmission (MTCT) of the virus. The prevention of MTCT programme improved roll-out of antiretroviral (ARV) medicines to >90% of HIV-positive pregnant women.[12] This effective use of ARVs in SA decreased MTCT of HIV to 2% in 2015.[12] However, in 2011, Venkatesh et al [13]showed that infants born to HIV-positive mothers had a two-fold greater risk of hospitalisation and a six-fold greater risk of mortality, independent of infant HIV status. This illustrated the impact of maternal immunodeficiency on childhood morbidity and mortality, regardless of the child's HIV status.
Objectives
It is known that children undergoing major surgical procedures are at a higher risk of surgical complications than their adult counterparts.[9] It is also known that HIV-infected children have a higher rate of post-surgical complications than HIV-unexposed children.[2] The objectives of this study were to assess the incidence of complications in our own practice and to explore whether these complications are increased in HIVexposed but serologically negative children compared with HIV-unexposed children.
Methods
After approval from the Human Research Ethics Committee, University of the Wit-watersrand (ref. no. M160.513), data were prospectively collected from patients presenting to either of the two paediatric surgery units linked to the University of the Witwatersrand, these being at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) and Chris Hani Baragwanath Academic Hospital (CHBAH). All patients aged <6 years with ARMs requiring surgical intervention were included. This was a prospective cross-sectional descriptive study, and data collection occurred over 13 months between 1 August 2016 and 30 September 2017. Data collected included mother's age and HIV test results, child's HIV test results (if HIV-exposed), type of ARM, presence of associated anomalies, type of surgical intervention, perioperative antibiotic use, and presence and type of surgical complication if any.
Statistical analysis
The X2 test was used to assess the relationships between categorical variables and HIV exposure group. Fisher's exact test was used where the requirements for the X2 test could not be met. One-way analysis of variance assessed the relationship between continuous variables and HIV exposure group. Where the data did not meet the assumptions of this test, a non-parametric Kruskal-Wallis test was used. Data analysis was carried out using SAS version 9.4 for Windows (SAS Institute, USA). A 5% significance level was used.
Results
Entire cohort
Data were collected prospectively from 50 mother-child dyads. The majority (84%) of the children were referred to our units from surrounding hospitals. Thirty-two of the children (64%) were operated on at CHBAH, where there is a dedicated colorectal unit. None of the children in this cohort were antenatally diagnosed.
Mothers
The mean age of mothers in the cohort was 28 years; 19 (38%) were HIV-positive. All the mothers were diagnosed antenatally and had been on ARVs for an average of 30 months prior to delivery. As such, the median CD4+ count was 537 cells/uL (interquartile range (IQR) 363 - 730) and the median viral load (VL) was 147 IU/mL (IQR 0 - 1 075, with a high of 14 500).
Children
The median birth weight was 2 970 g (IQR 2 700 - 3 300) and the median gestational age 40 weeks (IQR 37 - 40). Thirty-eight percent of the children were HIV-exposed, but notably there had been no seroconversions at the time of writing. All the exposed children had received post-exposure prophylaxis for a minimum of 6 weeks after birth.
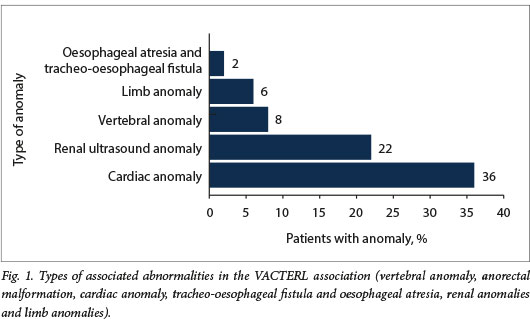
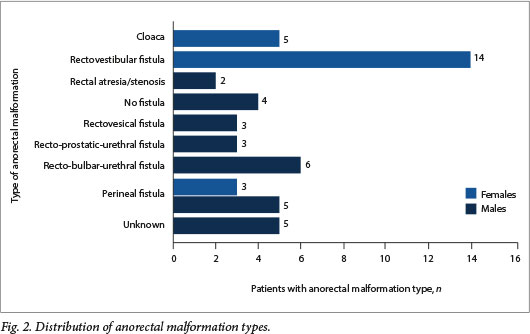
In total, 28 of the 50 children (56%) had at least one associated anomaly (Fig. 1). Ten participants (20%) had three or more systemic anomalies and therefore the VACTERL association (vertebral anomaly anorectal malformation, cardiac anomaly tracheo-oesophageal fistula and oesophageal atresia, renal anomalies and limb anomalies). One child had a chromosomal anomaly (trisomy 21). The types of malformation encountered are shown in Fig. 2.
Management and complications
CHBAH performed 46 surgical procedures on 32 patients with 15 complications. CMJAH performed 31 surgical procedures on 18 patients with 15 complications.
Thirty-eight stomas were performed with 12 complications in 12 participants, giving a 31.6% complication rate (Fig. 3). Seven of the 14 participants who were HIV-exposed had complications (50.0%), whereas only 5 of the 24 participants who were unexposed had complications (20.8%) (Fig. 4). Perioperative antibiotic use was erratic across all surgical interventions and was surgeon specific rather than protocol driven (Fig. 5).
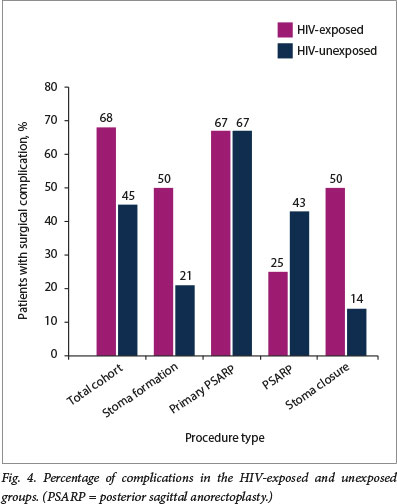
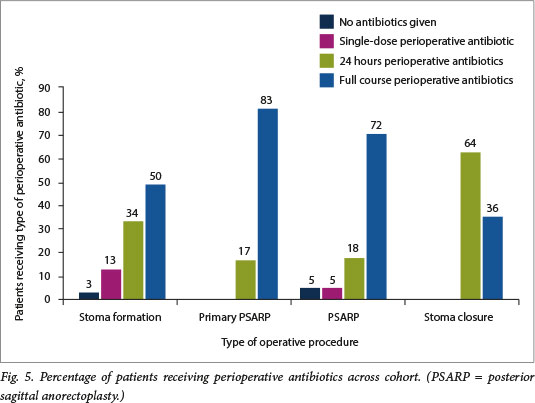
Six primary PSARPs were performed. There were 6 complications in 4 patients (Fig. 3). The total incidence of complications in this group was 66.7%. Of the 3 patients in this group who were HIV-exposed, 2 (66.7%) had complications, and 2 (66.7%) of the 3 participants who were HIV-unexposed had complications (Fig. 4).
The majority of these patients received a full course of antibiotic therapy (Fig. 5).
Twenty-two PSARPs were performed. There were 9 complications in 8 patients (Fig. 3). The total incidence of complications in this group was 40.9%. Of the 8 patients who were HIV-exposed, 2 (25.0%) had complications, and 6 (42.9%) of the 14 patients who were HIV-unexposed had complications (Fig. 4). The majority of these patients received a full course of antibiotic therapy (Fig. 5).
Eleven stoma closures were performed. There were 3 complications (Fig. 3), giving a total incidence of complications in this group of 27.3%. Of the 4 patients who were HIV-exposed in this group, 2 (50.0%) had complications, and 1 (14.3%) of the 7 patients who were HIV-unexposed had complications (Fig. 4). Most patients in this group received 24 hours of perioperative antibiotics (Fig. 5).
Four deaths occurred in this cohort, 3 after stoma formation and 1 after PSARP. One death occurred within 48 hours of surgery. In this case, an infant with an ARM with a rectovestibular fistula was only referred for management on day 16 after birth. On arrival, the abdomen was markedly distended, with established peritonitis. At laparotomy it was noted that the entire small and large bowel was necrotic, secondary to abdominal compartment syndrome. The child received palliative treatment. The other 3 deaths occurred in hospital but were unrelated to the initial surgery. Two patients died due to overwhelming sepsis, one secondary to meningitis and the other to aspiration pneumonia. The 4th patient died due to complications related to perforation of the distal rectal segment during an augmented pressure distal colostogram. Of the 4 deaths, 2 (50.0%) occurred in HIV-exposed infants and 2 (50.0%) in unexposed infants.
Discussion
We have gained significant insights into our own practice as a result of the outcomes of this 1-year collection of data. As expected, the vast majority of patients were referred to our institutions from surrounding referral hospitals (a large area is drained by only two centres that offer paediatric surgical services). None of the patients were diagnosed antenatally. This is very common, as ARMs are difficult to detect with antenatal screening. In additionally, SA practises basic antenatal care, where ultrasound imaging in pregnancy and referral to higher-level antenatal care centres is reserved for patients with suspected multiple pregnancies or suspected congenital anomalies on clinical evaluation of the gravid uterus.[14] The delay in diagnosis and referral can have devastating consequences, as noted in one of our patients in whom the entire bowel was necrotic as a result of untreated abdominal compartment syndrome from a missed ARM that was only diagnosed on day 16 after birth. This highlights one of the many areas for improvement in the SA setting, not just related to the treatment of ARMs but to the diagnosis, referral and treatment of all paediatric surgical patients in the country. Of note, when assessing the types of ARMs presenting to our institutions, we found that there was a very low incidence of perineal fistulas in females compared with the international literature[5](p=o.006). We propose that this is not because fewer cases of perineal fistula occur in our referral area, but because the majority of cases of this anatomical variant of ARM are missed on examination, so that the patient is not referred for treatment at all, or is only referred when she is much older and presents with constipation or overflow incontinence.
In 2013, the prevalence of HIV among women presenting to antenatal clinics was 28.6%, compared with 29.8% in 2009.[15] Inour cohort, the prevalence of HIV-infected mothers, and therefore HIV-exposed children, was 38%. This is 10% higher than the previously documented prevalence in Gauteng Province, and although not statistically significant (p=0.14), the prevalence is still substantially higher than in the general population. HIV-positive mothers were significantly older than mothers in the HIV-negative group (30.7 years v. 26.6 years; p=0.026). This may be because the decreasing prevalence of HIV is occurring in the younger population, secondary to the improved rollout of ARVs, or because this younger population has had fewer opportunities to contract the virus compared with their older counterparts. Another positive finding was that all mothers who were HIV-positive at the time of delivery had been diagnosed antenatally, and all had been on ARVs for an average of 30 months prior to delivery. The result of this treatment is that the median VL of our mothers (147 IU/mL) was lower than that quoted by Venkatesh et al.[13] (>100 000 IU/ mL), which was shown to increase the risk of hospitalisation and mortality in HIV-exposed infants independent of their HIV status.
At the time of writing, none of the HIV-exposed children in this cohort had seroconverted. This should be considered with caution, as children require a polymerase chain reaction test for HIV at birth and at 10 weeks and 18 weeks of age, as well as an HIV enzyme-linked immunosorbent assay at 18 months of age, in order to be confirmed HIV-negative after antenatal HIV exposure.[16] Many of the patients in the above cohort were aged <19 months and had therefore not completed all these tests. There were no significant differences in birth weight or gestational age between the participants who were HIV-exposed and those who were unexposed (p=0.7 and p=0.28, respectively).
Complications in the colostomy formation and colostomy closure groups were mainly related to sepsis (superficial wound sepsis and dehiscence of the entire wound secondary to sepsis). Rates of these complications are high (32% and 27%, respectively) when compared with the international literature (20% surgical site infections).[9] However, it is important to consider the complication rates in patients who were HIV-exposed compared with those who were not. In both the stoma formation and stoma closure groups, 50% of the patients in each group who were HIV-exposed experienced postoperative complications compared with only 20% and 14%, respectively, in the groups of patients who were not exposed. The complication rates in the HIV-unexposed patients are similar to and lower than the international literature.[9] Although not statistically significant, because of small numbers, children who were HIV-exposed tended to have far more infection-related postoperative complications than those who were unexposed (entire cohort: p=0.11, stoma formation group:p=0.081). Collection of higher numbers of patients may confirm this. We believe that this increased propensity to infection-related complication in the HIV-exposed group is secondary to inherent immune compromise in all HIV-exposed infants, regardless of whether they seroconvert or not.
Although it is difficult to infer any significance from only 6 patients in the primary PSARP group, there was a 66.7% complication rate. All complications were related to sepsis, namely wound sepsis and wound dehiscence secondary to sepsis (with 2 patients requiring subsequent stoma formation). This is exceptionally high even when compared with other countries including LMICs, for example Nigeria, where a complication rate of 30% was found in a cohort of primary PSARPs.[17] Although this number of patients is small, it has changed our approach to performing primary PSARPs and our institution is now far more judicious with the use of colostomies in all patients regardless of the type of malformation, particularly in delayed presentations. Why the rate of complications is so high in this particular group is unknown, and it does not follow any trend towards HIV exposure in this small number of patients. This is an area for further research in our department to help elucidate the reasons for this trend and, we hope, lead to improvement strategies to decrease the complication rate in this subset of patients.
The complication rate in the PSARP group was 40.9%. However, the complications in this group were predominantly technical and not infectious in nature (strictures, prolapse, fistula formation, vaginal injury and post-PSARP rectal necrosis). These complications therefore showed no predilection for the HIV-exposed group, as they were surgeon dependent rather than patient dependent. Further collection of data will clarify whether complications thought to be technical in nature (stricture and fistula formation) are increased in the HIV-exposed group owing to differences in wound healing compared with HIV-unexposed patients. One reason for technical complications in this group is related to the number of surgeons of different experience levels operating on this cohort of patients, some of whom are still on the upward slope of the learning curve related to these procedures.
If one considers all complications in terms of procedures done at CHBAH (with its dedicated colorectal unit) and at CMJAH (a general surgical unit) separately, it becomes clear that centralisation of services may indeed be the way to improve outcomes on a broader scale. The incidence of complications in the colorectal unit was 32.6% compared with 48.3% in the general surgical unit, although this difference is not statistically significant owing to small numbers (p=0.23). The difference cannot be explained on the basis of HIV exposure, and in fact there was a higher incidence of HIV exposure (43.7%) in the colorectal unit than in the general surgical unit (33.3%). As such, the relative risk of developing a complication from a colorectal procedure in the general surgical unit compared with the colorectal unit was 1.5.
There was no significant association between the presence or absence of complications and associated malformations and the VACTERL association (p=0.37).
We did note that choice of antibiotic prophylaxis across all surgeries was not standardised, with different surgeons using different antibiotic protocols, and this may have implications related to further investigation of septic complications in HIV-exposed patients. Where numbers permitted, for example in the stoma formation group, there was no statistical difference between the type of antibiotic prophylaxis and the septic complication rate between the HIV-exposed and unexposed groups (p=0.22).
The data suggest an increase in infection-related complications in HIV-exposed patients compared with those who were not exposed. We therefore need to define antibiotic protocols better to ensure that we are working to prevent sepsis-related complications in all patients, but especially in those who were HIV-exposed. We also need to elucidate nutritional status better in patients coming for elective procedures (PSARP and colostomy closure), as this may also help improve outcomes, although it is normal practice in our institutions not to do an elective operation on any child unless they are nutritionally replete.
Further research needs to done on this subject. Once patient numbers permit better statistical analysis, interventions such as antibiotic protocols and nutritional analysis and support may be instituted to see if they help improve our outcomes. In the interim, we need to carefully evaluate all patients who were HIV-exposed and optimise management as far as possible preoperatively to assist in preventing postoperative septic complications.
Study limitations
The major limitation of this study was the small number of enrolled participants, although data collection is ongoing and we hope to report statistically significant results in the next few years. There was also great difficulty in contacting enrolled participants after discharge to follow them up appropriately.
Conclusions
ARMs are not uncommon in SA and present a significant surgical load. HIV is also very common, and although antiretroviral rollout is adequate, there is still a higher rate of HIV infection among the mothers of children presenting with ARMs compared with the general antenatal population. Our prevalence of postoperative complications in HIV-unexposed patients parallels rates reported in the international literature (except for PSARPs), in contrast to the much higher prevalence of complications, primarily infection related, in the HIV-exposed group. HIV exposure tends to increase the rate of postoperative infectious complications, even when the exposed child has tested HIV-negative. Children who were HIV-exposed therefore need to be optimised as much as possible prior to operation, with every attempt made to decrease the risk of postoperative infection-related complication. Further research is needed in this area to adequately quantify the burden of HI V exposure on paediatric surgical units across SA.
Declaration. This prospective study was completed in accordance with the requirements of TDG's MMed (Paediatric Surgery) degree.
Acknowledgements. We thank Petra Gaylard and Giulia Brisighelli for their invaluable help with statistical analysis, and all the staff in the departments of paediatric surgery at CHBAH and CMJAH for their help with collection of data.
Author contributions. TDG: conceptualising the research question, data collection and writing of the article; CW-T and AT: conceptualising the research question, writing advice and editing of the final article; JL: writing advice and editing of the final article.
Funding. None.
Conflicts of interest. None.
References
1. Beudeker N, Broadls E, Borgstein E, Heij HA. The hidden mortality of imperforate anus. Afr J Pediatr Surg 2013-,10(4).302-306. https://doi.org/10.4103/0189-6725.125417 [ Links ]
2. Karpelowsky JS, Zar HJ, van Bogerijen G, van de Graaf N, Millar AJW. Predictors of postoperative complications in HIV-infected children undergoing surgery. J Pediatr Surg 2011,46(4).674-678. https://doi.org/10.1016/j.jpedsurg.2010.11.026 [ Links ]
3. Tneron A, Loveland J. Birth prevalence of anorectal malformations in the referral area for the University of the Witwatersrand tertiary hospitals, South Africa. Eur J Pediatr Surg 2015-,25(02).220-225. https://doi.org/10.1055/s-0033-1360456 [ Links ]
4. Gangopadhyay AN, Pandey V. Anorectal malformations. J Indian Assoc Pediatr Surg 201520(1).10-15. https://doi.org/10.4103/0971-9261.145438 [ Links ]
5. Tneron AP, Brisighelli G, Tneron AE, Leva E, Numanoglu A. Comparison in the incidence of anorectal malformations between a first- and a third-world referral centre. Pediatr Surg Int 2015-,31(8).759-764. https://doi.org/10.1007/s00383-015-3740-x [ Links ]
6. Poenary D, Borgstein E, Numanoglu A, Azzie G. Caring for children with colorectal disease in the context of limited resources. Semin Pediatr Surg 2010,19(2).118-127. https://doi.org/10.1053/j.sempedsurg.2009.11.017 [ Links ]
7. Lawai TA, Olulana DI, Ogundoyin OO. Spectrum of colorectal surgery operations performed in a single pediatric surgery unit in sub-Saharan Africa. Afr J Pediatr Surg 201411(2).128-131. https://doi.org/10.4103/0189-6725.132802 [ Links ]
8. Osifo OD, Osagie TO, Udefiagbon EO. Outcome of primary posterior sagittal anorectoplasty of high anorectal malformations in well selected neonates. Niger J Clin Pract 2014,17(1).1-5. https://doi.org/10.4103/1119-3077.122821 [ Links ]
9. Mattioli G, Avanzini S, Pini-Prato A, et al. Risk management in pediatric surgery Pediatr Surg Int 2009,25(8).683-690. https://doi.org/10.1007/s00383-009-2407-x [ Links ]
10. Oda O, Davies D, Colapinto K, Gerstle JT. Loop versus divided colostomy for the management of anorectal malformations. J Pediatr Int 2014,49(1)87-90. https://doi.org/10.1016/j.jpedsurg.2013.09.032 [ Links ]
11. Joint United Nations Programme on HIV/AIDS (UNAIDS). South Africa. HIV and AIDS estimates. http://www.unaids.org/en/regionscountries/countries/southafrica/ (accessed 18 April 2016). [ Links ]
12. Joint United Nations Programme on HIV/AIDS (UNAIDS). On the fast-track to an AIDS-free generation. http://www.unalds.org/sites/default/mes/medla_asset/GlobalPlan2016_en.pdf (accessed 11 September 2016). [ Links ]
13. Venkatesh KK, de Bmyn G, Marinda E, et al. Morbidity and mortality among infants born to HIV-infected women in South Africa. Implications for child health in resource-limited settings. J Trop Pediatr 2011,57(2).109-119. https://doi.org/10.1093/tropej/fmq061 [ Links ]
14. National Department of Health, South Africa. Guidelines for Maternity Care in South Africa. A Manual for Clinics, Community Health Centres and District Hospitals. Pretoria. NDoH, 2015. https://www.health-e.org.za/wp-content/uploads/2015/ll/Maternal-Care-Guidelines-2015_FINAL-21.7.15.pdf (accessed 2 December 2017). [ Links ]
15. National Department of Health, South Africa. The National Antenatal Sentinel HIV Prevalence Survey South Africa. Pretoria. NDoH, 2013. https://www.health-e.org.za/wp-content/uploads/2016/03/Dept-Health-HIV-High-Res-7102015.pdf (accessed 2 December 2017). [ Links ]
16. National Department of Health, South Africa. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Pretoria: NDoH, 2015. http://wwwsahivsoc.org/FUes/ART%20Guidelines%2015052015.pdf (accessed 2 December 2017). [ Links ]
17. Amanollahi O, Ketabchian S. One-stage vs. three-stage repair in anorectal malformation with rectovestibular fistula. Afr J Paediatric Surg 2016,13(1).20-25. https://doi.org/10.4103/0189-6725.181702 [ Links ]
 Correspondence:
Correspondence:
TD Gabler
tarryn.gabler@gmail.com
Accepted 7 May 2018














