Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.108 n.9 Pretoria Sep. 2018
http://dx.doi.org/10.7196/samj.2018.v108i9.13435
RESEARCH
Clinical and pathological features of acral melanoma in a South African population: A retrospective study
J de WetI; B TodII; W I VisserIII; H F JordaanIV; J W SchneiderV
IMB ChB, MMed; Division of Dermatology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Academic Hospital, Cape Town, South Africa
IIMB BCh, MMed, FCDerm; Division of Dermatology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Academic Hospital, Cape Town, South Africa
IIIMB ChB, MFamMed, MMed; Division of Dermatology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Academic Hospital, Cape Town, South Africa
IVMB ChB, MMed, M Akad SA; Division of Dermatology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Academic Hospital, Cape Town, South Africa
VMB ChB, MMed, FCPath; Division of Anatomical Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University and National Health Laboratory Service, Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND. Acral melanoma (AM) is a rare subtype of cutaneous melanoma (CM) that disproportionately affects skin of colour and carries a poorer prognosis than other melanoma subtypes. The poor prognosis is attributed to late diagnosis and subsequent relatively high Breslow thickness, but also to an intrinsic biological aggressiveness. Scientific data on AM from the developing world are limited and a need exists to characterise the disease further in the South African (SA) population.
OBJECTIVES. To describe the clinical and pathological features of AM in an SA population.
METHODOLOGY. A retrospective chart review characterised the demographics, clinical features and histological data of 66 patients diagnosed with AM between January 2010 and June 2016 at Tygerberg Academic Hospital, Cape Town, SA.
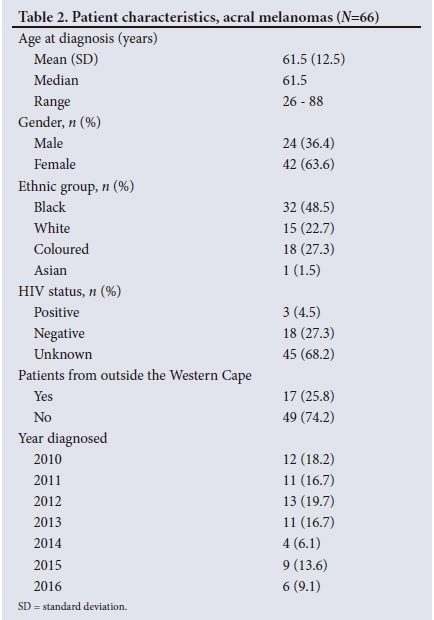
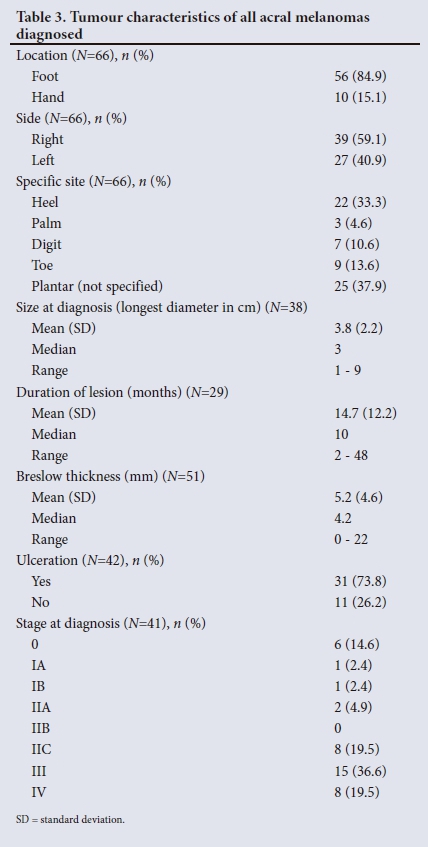
RESULTS. Sixty-six patients with AM were identified from 335 patients diagnosed with CM during the set time frame. The mean age (standard deviation (SD)) was 61.5 (12.5) years. Forty-two (63.6%) of the patients were female (male/female ratio 1:1.75). The majority of patients diagnosed with AM were black (48.5%), and the proportion of AM in black patients with CM was 80.0%. Fifty-six AMs (84.8%) were located on the foot and 10 (15.2%) on the hand. The median duration of the lesion before diagnosis was 10 months (range 2 - 84) and the mean (SD) tumour size was 3.8 (2.2) cm at diagnosis. The mean Breslow thickness of all AMs at diagnosis was 5.2 mm (median 4.2 mm, range 0 - 22). Stage of disease was known in 41 patients, 23 (56.1%) of whom had at least stage III disease at diagnosis. Mean Breslow thickness for foot and hand melanomas was 4.9 mm (range 0 - 22) and 6.9 mm (range 0 - 13.3), respectively (p=0.2552). The mean Breslow thickness in the black population was 6.3 mm compared with 4.2 mm and 4.3 mm, respectively, in the white and coloured populations (p=0.178). Patients from outside the Western Cape Province (WC) presented with a mean Breslow thickness of 6.6 mm (range 0 - 14.5) and patients from the WC with a mean Breslow thickness of 4.9 mm (range 0 - 22) (p=0.3602).
CONCLUSIONS. AMs accounted for a significant proportion of all CMs diagnosed. Patients presented with an advanced stage of disease at diagnosis, and further studies are needed to further investigate the reasons for delayed diagnosis.
Cutaneous melanoma (CM) is the leading cause of skin cancer mortality worldwide despite constituting only 4% of skin cancers. [1] The incidence of and mortality from invasive melanoma has risen steadily for at least the past two decades in most developed countries.[2] Acral melanoma (AM) is a rare distinct variant of CM that arises from the palms, soles and nail apparatus. AMs account for only 1 -7% of all CMs diagnosed, and although the incidence of AM is the same for all racial and ethnic groups, it represents a disproportionate percentage of melanomas diagnosed in black, Hispanic and Asian individuals.[3-5]
The term AM includes all histopathological subtypes of melanoma that occur at acral sites, including acral lentiginous melanoma (ALM), which comprises up to 80% of all AMs.[4,6] First described by Reed in 1976, ALM occurs preferentially at acral sites (i.e. palms, soles and nail apparatus) and demonstrates a radial lentiginous growth phase.[7] Recent studies suggest that ALMs have unique patterns of genetic mutations compared with other forms of CM.[8]
Soudry et al.[9] studied the clinicopathological features of AM in 45 patients and found no difference between the ALM and non-ALM groups with reference to tumour characteristics, lymph node status and survival.[9] AM, regardless of histological subtype, is generally associated with a poorer prognosis than CM at other sites.[6,9,10] In a large population-based study of ALM in the USA, 5- and 10-year melanoma-specific survival rates were 80.3% and 67.5%, respectively, with significantly lower rates in individuals with darker skin types.[3] Prognostic data from the African continent are scarce, but 5-year survival rates as low as 26% for plantar melanomas in the black population have been reported.[11] Studies from Asia and Mexico also showed significantly lower survival rates in Hispanic and Asian populations.[1,12,13]
The poor prognosis associated with AM is generally attributed to late diagnosis and relatively higher Breslow thickness, but also to an intrinsic biological aggressiveness.[10]
Current knowledge on AM originates mainly from population-based studies from North America, Europe and Asia,[1,12-16] with few data from the developing world.[4] Studies from Africa on this topic are limited and dated.[11,17,18] Hudson and Krige[17] investigated plantar CM in patients in Cape Town, South Africa (SA), between 1972 and 1985 and reported that plantar CM accounted for 73% of all CMs in black African patients. In 71% of these cases ALM was identified as the histological subtype. A more recent report by York et al.[19]on primary cutaneous malignancies in the Northern Cape Province of SA showed that ALM was the subtype most commonly observed in all CMs diagnosed.
Objectives
To address deficiencies in the current literature by reviewing the clinical and pathological features of AM in an SA population.
Methods
This retrospective study included all patients diagnosed with AM at Tygerberg Academic Hospital (TAH), Cape Town, between 1 January 2010 and 30 June 2016. AM was defined as all melanomas involving the palms, soles and nail apparatus, regardless of histological subtype.
All adult patients (>18 years old, male or female) diagnosed with AM were included in the study. Exclusion criteria were: (i) patients aged <18 years; and (ii) inadequate clinical and demographic data available.
The National Health Laboratory Service (NHLS) information system at TAH was used to identify all patients diagnosed with CM within the set time frame. From this group all AM patients were identified. Epidemiological, clinical, radiological and histological data for AM patients were collected using the NHLS database, Picture Archiving and Communication System (PACS) and Enterprise Content Management (ECM) system at TAH.
A hot-deck imputation method was used for subjects where race/ethnicity was not indicated. Subjects were assigned to a group by comparing their surnames with a reference database of approximately 1.4 million surnames of known race/ethnicity. This method was construe ted for the National Cancer Registry as a Statistical Analysis Software (SAS) program by the Data Management and Statistical Analysis Unit of the University of the Witwatersrand. [20]
Stata version 14 (StataCorp, USA) was used for data analysis. Descriptive statistics consisting of summary statistics (i.e. mean, range) fo r numerical data an d frequencie s for categori cal data were used.
The study was carried out with the approval of the Stellenbosch University Human Subjects Research Ethics Committee (ref. no. S16/10/192). The need to obtain informed consent was waived, as this was a retrospective study and no identifying details were included.
Results
Using appropriate SNOMED codes to search the NHLS database yielded 335 patients diagnosed with CM at TAH between January 2010 and June 2016. This group was further characterised in terms of demographics and clinical features, which are summarised in Table 1.
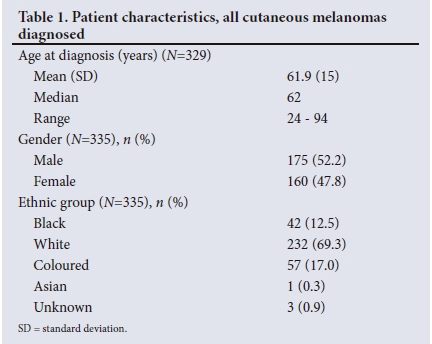
The location of the lesion was known in 293 patients, of whom 66 (22.5%) had AM. Fig. 1 illustrates the distribution of all CMs diagnosed. AMs accounted for 80.0% of all CMs diagnosed in the black population compared with 8.0% and 34.0% in the white and coloured populations, respectively.
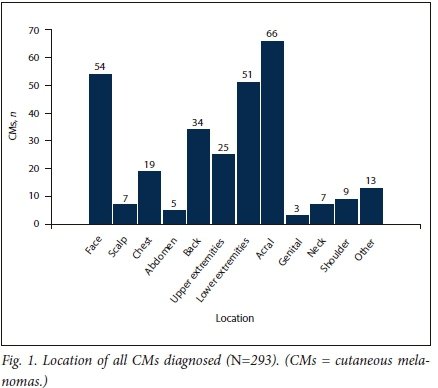
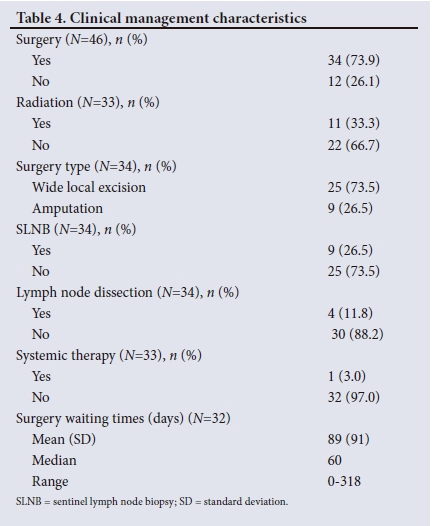
The 66 patients with AM were further analysed for patient, tumour and clinical management characteristics (Tables 2, 3 and 4).
The mean Breslow thickness of AM in male and female patients was 5.3 mm and 5.2 mm, respectively.
HIV status was known in 21 of the AM patients, and three of these patients were HIV-positive and presented with at least stage III metastatic disease (Table 5).
When comparing Breslow thickness of AM among ethnic groups, black patients presented with a mean Breslow thickness of 6.3 mm, white patients with 4.2 mm and coloured patients with 4.3 mm (p=0.1762).
Patients from outside the Western Cape Province (WC) (initial biopsy performed in another province) presented with a mean Breslow thickness of 6.6 mm (range 0 - 14.5) and patients from within the WC with a mean of 4.9 mm (range 0 - 22) (p= 0.3602).
Mean Breslow thickness for foot and hand AM was 4.9 mm (range 0 - 22) and 6.9 mm (range 0 - 13.3), respectively (p=0.2552).
Clinical management in the study cohort involved surgery, radiation and systemic therapy. Information regarding surgery was only available for 46 patients. Of the 34 patients (73.9%) who underwent surgery, 25 (73.5%) had wide local excision and 9 (26.5%) had an amputation.
Discussion
This study provides valuable scientific data on the demographics, clinical features and histopathology of patients diagnosed with AM in an SA population.
AMs accounted for 22.5% of all CMs diagnosed. This proportion is significantly higher than previously reported rates of AM, which range from 1% to 7%.[4,5] The problem with most large population-based studies on AM is that they include mostly Caucasian patients. In contrast, studies from Hawaii, India and the French West Indies, where the populations largely consist of people with darker skin types, show AMs to represent up to 50% of all CMs diagnosed.[4] This supports the hypothesis of Durbec et al.[4] that, although rare, AM could represent an underestimated public health problem in countries mostly populated by people with darker skin types.
Data from this study on the male/female ratio were consistent with previous reports and showed that AM has a predilection for the female gender in this SA population.[5,6,15] The mean (standard deviation (SD)) age at diagnosis in the AM cohort was 61.5 (12.5) years. This was not significantly different from the mean (SD) age of all patients diagnosed with CM, which was 61.9 (15) years. Previous studies suggested that the median age at onset of AM is higher than that observed generally in other forms of CM, but this was not the case in the current study.[15]
It is well established in the literature that AM disproportionately afflicts darker-coloured skin, and our data support this finding.[3] In a large US-based study, Bradford et al.[3] reported the proportion of ALM in the USA to be 36% in the black population, 18% in Asian/ Pacific Islanders, 9% in Hispanic whites and only 1% in non-Hispanic whites. Previous studies from SA estimated the proportion of ALM in black individuals to be 60 - 70%,[15] and studies from Asia reported proportion rates between 47% and 86.6%.[5]
In this study, black patients accounted for 48.5% of all patients diagnosed with AM and constituted the largest ethnic group, followed by coloured and white patients, respectively.
The proportion of AMs among all CMs diagnosed in the black population was 80.0%, even higher than previously estimated. AMs accounted for 34.0% and 8.0% of CMs diagnosed in the coloured and white populations, respectively, and these figures are in keeping with previous reports.[5,11,21,22] The current study included only one Asian patient.
HIV status was known in 21 patients, of whom only three were HIVpositive. Studies on the link between HIV infection and increased rates of melanoma are conflicting, with most studies not demonstrating an association.[23] All three HIV-positive patients presented at an advanced stage of disease that is generally associated with poor prognosis (Table 5). Two studies have investigated CM in HIV and showed that patients with HIV suffer increased melanoma mortality.[24,25]
With regard to tumour characteristics, more than 80% of AMs were located on the feet. These data are supported by previous reports that showed a lower prevalence of AM on the hands than on the feet.[3,5,10] When considering foot melanomas in the current study, 47 (83.9%) were on the plantar aspect of the foot and the remainder on the toes and subungual areas. In 22 of the plantar AMs the heel was indicated as the exact location of the lesion. In the remainder, exact location was not specified. A study in Japan showed more AMs in the weight-bearing areas than in the non-weight-bearing areas of the plantar foot, suggesting that mechanical stress and shear force may play a role in the pathogenesis of these lesions.[26] This finding was recently challenged by a study from the USA that reported no significant difference in the distribution of AMs with regard to weight-bearing and non-weight-bearing regions.[27] In both the US and Japanese populations, AMs occurred more frequently on the heel than in the other areas of the plantar surface.
Tumour characteristics in the current study were consistent with a delay in diagnosis and subsequent advanced disease at presentation. This was evident in tumour size, duration of lesion, tumour thickness and stage of disease at presentation.
Previous studies on AM reported a mean (SD) size at diagnosis of 1.65 (1.1) cm.[10] In contrast, patients in the current study presented with a mean (SD) tumour size (longest diameter) at presentation of 3.8 (2.2) cm.
A lengthy delay in the diagnosis of AM is well documented in the literature, ranging from 1 to 3.7 years. Factors that contribute to the delay in diagnosis include the hidden location, poor access to healthcare, disregard of the lesion by patients and misdiagnosis by healthcare providers.[15] The median duration of lesions at presentation in the current study was 10 months (range 2 - 48), which confirms delayed diagnosis of AM.
Tumour thickness, ulceration and an advanced stage of disease at presentation are considered major negative prognostic factors and relate to increased melanoma-specific mortality.[10] Several studies have also shown that AM typically presents with higher Breslow thickness compared with other forms of CM and therefore a poorer prognosis.[3,5,6] In a large German cohort (2 050 ALM patients) the mean Breslow thickness was 3.08 mm (median 2.2 mm),[5] which was in keeping with other studies on ALM from developed countries that reported a mean Breslow thickness between 2.51 mm and 2.8 mm at presentation.[6,15] York et al.[19] reported a mean Breslow depth of 4.13 mm in ALMs diagnosed in the Northern Cape Province of SA. In the current study, mean Breslow thickness at diagnosis was 5.2 mm (median 4.2, range 0 - 22), suggesting that patients present much later than the norm in developed countries.
Complete histological data were only available for 42 of the 66 patients. Ulceration was reported in 31 cases (73.8%). Again, this figure is significantly higher compared with other AM studies, where ulceration was reported in 33 - 47.9% of cases.[5,28]
The delay in diagnosis was further demonstrated in the study cohort by the advanced stage of disease at presentation. The majority of patients (56.1%) had at least stage III disease at diagnosis with metastatic disease. These data are in stark contrast to results from studies in other parts of the world. The German study showed that 77.4% of ALM patients had stage I or II disease and only 20% presented with some form of metastasis at diagnosis.[5] A recent study from South Korea showed similar results, with most patients presenting with stage I and II disease (80%).[28]
Although previous reports showed a greater Breslow thickness at diagnosis in male patients compared with female patients, no significant difference was demonstrated in this study.[3,15]
The poor prognosis of AM has in the past been attributed to the hidden location of foot AMs.[7] In the current study, the mean Breslow thickness for foot and hand melanomas was 4.9 mm (range 0 - 22) and 6.9 mm (range 0 - 13.3), respectively (p=0.2552), suggesting an equally poor prognosis for both groups. Although not statistically significant, this finding may support the hypothesis of Paolino et al.[7]that the poor prognosis associated with AM is not related to the hidden location of the lesion but rather to site-specific clinicopathological features. In their study they reported no differences in terms of median Breslow thickness and survival between hand and foot AM.[7]
In the past race/ethnicity was also reported to be an independent prognostic factor,[29] but this point has been challenged by more recent studies.
Bradford et al.[3]used Surveillance, Epidemiology, and End Results (SEER) data to show that when controlled for thickness or stage, there were no statistical differences between 5- and 10-year melanoma-specific survival rates in the different racial groups. The authors cited access to care and lack of awareness by patient and provider, resulting in delayed diagnosis, as contributing factors for worse AM survival in certain ethnic groups.[3]
This hypothesis was further supported by a recent US study that investigated a cohort with ALM with equal access to healthcare. Results from this study showed melanoma-specific mortality among patients with ALM not to be associated with race/ethnicity.[10] The poor prognosis generally observed among non-white patients was again attributed to the advanced tumour stage at presentation and limited access to clinical care.
Unequal access to healthcare is an unfortunate reality in SA and may explain our findings that showed AM in black patients to present with greater Breslow thickness. Mean Breslow thickness in the black population was 6.3 mm compared with 4.1 mm in the non-black groups (p=0.074).
Access to care as a factor contributing to more advanced disease at presentation was also demonstrated by the fact that patients from outside the WC presented with thicker tumours. Seventeen patients (25.8%) in this cohort were from outside the WC, mostly from the Eastern Cape (EC). The mean Breslow thickness for these patients was 6.6 mm compared with 4.9 mm for the patients who were from within the WC. According to the 2011 SA census,[30] the EC is predominantly a rural province with almost two-thirds of the population living in rural areas, which may limit access to healthcare.
Lack of awareness of AM on the part of patients and the general public and misdiagnosis by healthcare providers may also be factors contributing to a delayed diagnosis that need further investigation. Although it was not specifically measured in the current study, rates of clinical misdiagnosis in other studies ranged from 20% to 50%.[15] Education of patients and healthcare providers, especially in countries with large populations of black patients, is therefore crucial to improve clinical diagnosis.
The median surgical waiting time in our study was 60 days (range 0 - 318) compared with the median surgical interval for melanoma in the existing literature of 30 days.[31,32] A study from Scotland, however, showed no association between surgical intervals (<14 days, 15 - 28 days, 29 - 42 days and 43 - 91 days v. >92 days) and overall survival, disease-free survival or recurrence-free survival.[32] A study from the USA also found no association between overall survival or disease-free survival and surgical intervals of <28 days v. >28 days.[26-No formal international guidelines for surgical interval for melanoma exist, but informal guidelines recommend treatment within 4 - 6 weeks.[33] The waiting time in this study was therefore reasonable and not a significant contributor to the advanced stage of disease.
Another reason for the poor prognosis of AM may be biological differences between AM and non-AM. Molecular genetic research has demonstrated that BRAF and NRAS mutations occur less frequently in ALM compared with other forms of CM and that mutations of the KIT gene have been observed more frequently in AM.[34,35] The current study did not involve genetic analysis, but this is an area that needs further investigation to better understand mutational variation in AM in the SA population.
Study limitations
This is a retrospective descriptive study with a small sample size. Quality and completeness of medical records were limiting factors. Treatment and follow-up data were also lacking. Complete histological data, including histological subtypes, were not available for all patients. Because of poor record keeping, outcomes could not be measured and survival data were therefore not included.
Conclusions
AM is a rare subtype of CM that carries a poor prognosis. The frequency of AM among all diagnoses of CM in SA appears higher than international published rates. The present study highlights the delay in diagnosis of AM in an SA population served by a large tertiary hospital, as illustrated by the size of the tumours at presentation, greater Breslow thickness and advanced stage of disease at diagnosis. Limited access to clinical care and delayed clinical diagnosis by healthcare providers probably contributed to delayed diagnosis. Further studies are needed to further delineate the reasons for the delayed diagnosis and to develop strategies to create public awareness and to educate healthcare workers in the early detection of AM. The paucity of information on genetic mutations specific to AM in the SA population justifies further study.
Acknowledgements. A consultant at the Biostatistics Unit in the Centre for Evidence-Based Health Care at Stellenbosch University assisted with the analysis of this study through support from the Faculty of Medicine and Health Science's Dean's Fund.
Author contributions. All authors contributed.
Funding. None.
Conflicts of interest. None.
References
1. Lino-Silva LS, Domínguez-Rodríguez JA, Aguilar-Romero JM, et al. Melanoma in Mexico: Clinicopathologic features in a population with predominance of acral lentiginous subtype. Ann Surg Oncol 2016;23(13):4189-4194. https://doi.org/10.1245/s10434-016-5394-x [ Links ]
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7-30. https://doi.org/10.3322/caac.21332 [ Links ]
3. Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986 - 2005. Arch Dermatol 2009;145(4):427-434. https://doi.org/10.1001/archdermatol.2008.609 [ Links ]
4. Durbec F, Martin L, Derancourt C, Grange F. Melanoma of the hand and foot: Epidemiological, prognostic and genetic features: A systematic review. Br J Dermatol 2012;166(4):727-739. https://doi.org/10.1111/j.1365-2133.2011.10772.x [ Links ]
5. Teramoto Y, Keim U, Gesierich A, et al. Acral lentiginous melanoma - a skin cancer with unfavourable prognostic features: A study of the German Central Malignant Melanoma Registry (CMMR) in 2050 patients. Br J Dermatol 2018;178(2):443-451. https://doi.org/10.1111/bjd.15803 [ Links ]
6. Rex J, Paradelo C, Mangas C, Hilari JM, Fernández-Figueras MT, Ferrándiz C. Management of primary cutaneous melanoma of the hands and feet: A clinicoprognostic study. Dermatol Surg 2009;35(10):1505-1513. https://doi.org/10.1111/j.1524-4725.2009.01265.x [ Links ]
7. Paolino G, Bekkenk MW, Didona D, et al. Is the prognosis and course of acral melanoma related to site-specific clinicopathological features? Eur Rev Med Pharmacol Sci 2016;20(5):842-848. [ Links ]
8. Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: Relevance of spreading pattern and physical stress. JAMA Dermatol 2013;149(11):1281-1288. https://doi.org/10.1001/jamadermatol.2013.5853 [ Links ]
9. Soudry E, Gutman H, Feinmesser M, Gutman R, Schachter J. 'Gloves-and-socks' melanoma: Does histology make a difference? Dermatol Surg 2008;34(10):1372-1378. https://doi.org/10.1111/j.1524-4725.2008.34290.x [ Links ]
10. Asgari MM, Shen L, Sokil MM, Yeh I, Jorgenson E. Prognostic factors and survival in acral lentiginous melanoma. Br J Dermatol 2017;177(2):428-435. https://doi.org/10.1111/bjd.15600 [ Links ]
11. Hudson DA, Krige JE, Stubbings H. Plantar melanoma: Results of treatment in three population groups. Surgery 1998;124(5):877-882. https://doi.org/10.1016/s0039-6060(98)70012-1 [ Links ]
12. Lv J, Dai B, Kong Y, Shen X, Kong J. Acral melanoma in Chinese: A clinicopathological and prognostic study of 142 cases. Sci Rep 2016;22(6):31432. https://doi.org/10.1038/srep31432 [ Links ]
13. Chang JW. Acral melanoma: A unique disease in Asia. JAMA Dermatol 2013;149(11):1272-1273. https://doi.org/10.1001/jamadermatol.2013.5941 [ Links ]
14. Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in Caucasians: Clinical features, histopathology and prognosis in 112 patients. Br J Dermatol 2000;143(2):275-280. https://doi.org/10.1046/j.1365-2133.2000.03651.x [ Links ]
15. Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: A clinicoprognostic study of 126 cases. Br J Dermatol 2006;155(3):561-569. https://doi.org/10.1111/j.1365-2133.2006.07368.x [ Links ]
16. Pereda C, Traves V, Requena C, et al. Clinical presentation of acral lentiginous melanoma: A descriptive study. Actas Dermosifiliogr 2013;104(3):220-226. https://doi.org/10.1016/j.adengl.2012.06.024 [ Links ]
17. Hudson D, Krige J. Melanoma in black South Africans. J Am Coll Surg 1995;180(8):65-71. https://doi.org/10.1002/bjs.1800800818 [ Links ]
18. Norval M, Kellett P, Wright CY. The incidence and body site of skin cancers in the population groups of South Africa. Photodermatol Photoimmunol Photomed 2014;30(5):262-265. https://doi.org/10.1111/phpp.12106 [ Links ]
19. York K, Dlova NC, Wright CY, et al. Primary cutaneous malignancies in the Northern Cape Province of South Africa: A retrospective histopathological review. S Afr Med J 2017;107(1):83-88. https://doi.org/10.7196/SAMJ.2016.v107.i1.10924 [ Links ]
20. Little RJ, Rubin DB. The analysis of social science data with missing values. In: Fox J, Long JS, eds. Modern Methods of Data Analysis. London: Sage Publications, 1990:292-326. https://doi.org/10.1177/0049124189018002004 [ Links ]
21. Desai A, Ugorji R, Khachemoune A. Acral melanoma foot lesions. Part 1: Epidemiology, aetiology, and molecular pathology. Clin Exp Dermatol 2017;42(8):845-848. https://doi.org/10.1111/ced.13243 [ Links ]
22. Swan MC, Hudson DA. Malignant melanoma in South Africans of mixed ancestry: A retrospective analysis. Melanoma Res 2003;13(4):415-419. https://doi.org/10.1097/00008390-200308000-00012 [ Links ]
23. Chang A, Doiron P, Maurer T. Cutaneous malignancies in HIV. Curr Opin HIV AIDS 2017;12(1):57-62. https://doi.org/10.1097/coh.0000000000000338 [ Links ]
24. Zucchetto A, Virdone S, Taborelli M, et al. Non-AIDS defining cancer mortality: Emerging patterns in the late HAART era. J Acquir Immune Defic Syndr 2016;73(2):190-196. https://doi.org/10.1097/qai.0000000000001033 [ Links ]
25. Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer specific mortality among HIV-infected patients in the United States. J Clin Oncol 2015;33(21):2376-2383. https://doi.org/10.1200/jco.2014.59.5967 [ Links ]
26. Minagawa A, Omodaka T, Okuyama R. Melanomas and mechanical stress points on the plantar surface of the foot. N Engl J Med 2016;374(24):2404-2406. https://doi.org/10.1056/nejmc1512354 [ Links ]
27. Costello CM, Pittelkow MR, Mangold AR. Acral melanoma and mechanical stress on the plantar surface of the foot. N Engl J Med 2017;377(4):395-396. https://doi.org/10.1056/nejmc1706162 [ Links ]
28. Moon HR, Kang HJ, Won CH, et al. Heterogeneous spectrum of acral melanoma: A clinicoprognostic study of 213 acral melanomas according to tumor site. J Am Acad Dermatol 2018;78(1):179-182.e3. https://doi.org/10.1016/j.jaad.2017.07.029 [ Links ]
29. Slingluff CL, Vollmer R, Seigler HF. Acral melanoma: A review of 185 patients with identification of prognostic variables. J Surg Oncol 1990;45(2):91-98. https://doi.org/10.1002/jso.2930450207 [ Links ]
30. Census 2011: Statistical release. http://www.statssa.gov.za/publications/P03014/P030142011.pdf (accessed 18 January 2018). [ Links ]
31. Carpenter S, Pockaj B, Dueck A, et al. Factors influencing time between biopsy and definitive surgery for malignant melanoma: Do they impact clinical outcome? Am J Surg 2008;196(6):834-843. https://doi.org/10.1016/j.amjsurg.2008.07.044 [ Links ]
32. McKenna D, Lee R, Prescott R, Doherty V The time from diagnostic excision biopsy to wide local excision for primary cutaneous malignant melanoma may not affect patient survival. Br J Dermatol 2002;147(1):48-54. https://doi.org/10.1046/j.1365-2133.2002.04815.x [ Links ]
33. Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Rep 2012;4(1):e2. https://doi.org/10.4081/dr.2012.e2 [ Links ]
34. Fernandes JD, Hsieh R, de Freitas LA, et al. MAP kinase pathways: Molecular roads to primary acral lentiginous melanoma. Am J Dermatopathol 2015;37(12):892-897. https://doi.org/10.1097/dad.0000000000000317 [ Links ]
35. Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci 2013;72(3):284-289. https://doi.org/10.1016/j.jdermsci.2013.07.013 [ Links ]
 Correspondence:
Correspondence:
J de Wet
dewetjohann@yahoo.com
Accepted 3 July 2018














