Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.108 no.8 supl.1 Pretoria Ago. 2018
http://dx.doi.org/10.7196/samj.2018.v108i8.13501
RESEARCH
Prevention of hepatocellular carcinoma with antiviral therapy
A M Di Bisceglie
MD, FCP (SA), FACP, FAASLD; Department of Internal Medicine, Saint Louis University School of Medicine, Missouri, USA
ABSTRACT
Chronic viral hepatitis types B and C may eventually lead to the development of hepatocellular carcinoma. Although hepatitis B is readily preventable by vaccination, there is growing evidence that antiviral therapy directed against hepatitis B may reduce the risk of liver cancer among those already infected. There is no vaccine against hepatitis C, but the evidence is now strong that antiviral therapy with sustained virological response (viral cure) reduces, but does not eliminate, the risk of hepatocellular carcinoma.
Hepatocellular carcinoma (HCC) is one of the very few major cancers in which the aetiology can be identified in individual patients. Thus, in contrast to some cancers in which risk factors (diet, smoking, genetic factors) increase the risk of developing a particular cancer, the presence of an identifiable underlying liver disease in the majority of patients with HCC is readily apparent. Globally, chronic viral hepatitis (B and C) with their associated hepatitis and/or cirrhosis account for nearly 75% of all cases of HCC.1 One of the important consequences of this observation is that HCC may be more readily preventable than other major cancers.
It has already been well established that vaccination against hepatitis B virus (HBV) is an effective strategy in preventing HBV-related HCC. Thus the introduction of universal infant vaccination against HBV infection in Taiwan in the early 1980s has been shown to be associated with a dramatic and statistically significant decrease in childhood HCC.2 These cohorts of individuals vaccinated in the 1980s continue to be monitored and it is expected that their lower incidences of HCC will continue on into their adulthood and their protection is expected to be lifelong. Unfortunately, there is not yet an effective vaccine against the hepatitis C virus (HCV), although public health efforts, such as screening of donated blood and education about the risks of blood-borne infection have been associated with steadily decreasing rates of new HCV infections in the developed Western world.
However, a challenge remains for those persons who are already chronically infected with HBV or HCV, as they are already at risk of developing HCC at some point. Over the last 3 decades enormous strides have been made in developing effective antiviral therapies against these agents, which raises the question of whether antiviral therapy may decrease their risk of HCC.
Hepatitis B
Antiviral therapy for chronic HBV infection has evolved over the last several decades, from interferon and a variety of other less effective agents, to now include nucleoside or nucleotide analogues with high potency and barriers to resistance. The use of alpha interferons (initially standard interferon-alpha (IFNcx), and more recently pegylated IFNcx (PEG-IFNcx)) appears to be associated with achieving immune control of HBV in about 30 - 40% of cases.3 This is followed by viral cure or loss of hepatitis B surface antigen (HBsAg) in ~10% of treated individuals. However, the use of interferons has been limited because of their substantial side-effect profile and the need for administration by subcutaneous injection. The first nucleoside analogue to be introduced was lamivudine, an agent initially developed for use against HIV infection. Whereas lamivudine is moderately potent against HBV, viral resistance emerges quite rapidly and limits the prolonged use of this agent as monotherapy. The next drug to be developed against HBV was adefovir, which was somewhat less potent than lamivudine, but also somewhat less likely to result in emergence of treatment-resistant viral variants. Telbivudine is widely used in some countries but has a resistance profile similar to lamivudine. The current mainstream agents used to control HBV infection are tenofovir and entecavir, which have now been available for 5 - 10 years.
There is currently very little evidence to suggest that the use of interferon is associated with a reduced rate of HBV-related HCC.4 However, it is not clear that appropriate studies have been done on patients with chronic hepatitis B treated with interferon, focusing on those who have cleared HBsAg or maintained viral suppression after therapy.
Lamivudine is the nucleoside analogue agent that has been in use for the longest time, and has therefore permitted the most comprehensive study of its putative benefits in preventing HCC. The best evidence suggesting that antiviral therapy may be of benefit is a large prospective, randomised, placebo-controlled trial of lamivudine therapy in patients with advanced liver disease associated with HBV infection.5 The study enrolled 651 patients with chronic HBV infection and advanced hepatic fibrosis. The patients were randomised to receive either lamivudine or an oral placebo. The planned outcomes of the study were clinically apparent events such as hepatic decompensation, death, and the development of HCC. The study was halted early upon the recommendation of a data-safety monitoring board, because of an early and readily apparent difference in morbidity and mortality rates among patients in the different treatment groups. While an analysis of the data showed a statistically significant reduction in rates of hepatic decompensation and death, the difference in HCC rates was of borderline statistical significance at the time that the study was stopped. Thus, 3.9% of patients receiving lamivudine developed HCC compared with 7.4% on placebo (p=0.047). It is certainly possible that if this study had been continued this trend towards a significantly lower rate of HCC may have continued and become more significant. One of the challenges in showing that lamivudine reduces the risk of HCC is the relatively high rate of development of viral resistance associated with loss of viral control, making results difficult to interpret.5,6
Cohort studies of patients receiving lamivudine therapy have had mixed results. Thus, Kurokawa et al.7 reported on their experience of 293 patients receiving lamivudine for an average time of 67.6 months. Although this study did not include an untreated control group for comparison, the authors did find that the incidence of HCC was reduced in those patients achieving a maintained virological response, compared with those in whom HBV was not well controlled due to the emergence of viral resistance. A similar result was achieved in a study from Korea, reported by Eun et al.8 Among patients with HBV-induced compensated cirrhosis, HCC occurred in 4.9% of cases with maintained viral response, 11.8% in those with viral breakthrough, and 19.4% of those with a suboptimal response to lamivudine. This study included an untreated control group that had an HCC rate of 25.0% after a mean follow-up of 6.1 years.
More recently, entecavir and tenofovir have become widely used as the main antiviral agents to control HBV infection.30 Both agents appear to have a much higher barrier to resistance than lamivudine, and seem to be reasonably well tolerated. Randomised controlled trials of these agents have focused on their ability to control HBV infection and not so much on clinical outcomes or HCC development.
Hosaka et al.9 reported on their experience with longterm entecavir treatment in Japan. They assembled a cohort of 1 615 patients with chronic HBV infection. Of these, 472 mono-infected patients with HBV received entecavir. Their outcomes were compared with 1 143 mono-infected patients who did not receive antiviral therapy. The cumulative HCC incidence rates at 5 years were 3.7% and 13.7% for the entecavir group and the controls, respectively (p<0.001). They found this protective effect to be even greater in subsets of patients at higher risk of HCC, based on calculation of a propensity score, including risk factors such as age, gender, and the presence of cirrhosis, HBeAg and HBV DNA levels. Papatheodoridis et al.10 reported on the outcomes of 1 666 patients recruited from multiple sites in Europe. They were treated with entecavir or tenofovir, and essentially all patients achieved a maintained virological response (i.e. undetectable serum HBV DNA) by year 5. Despite this excellent viral control, HCC still occurred at a frequency of 8.7% after the 5 years of treatment. Again, there was no untreated comparison group with which to compare the frequency of HCC, but it is clear that development of liver cancer is not completely suppressed by antiviral therapy.
A similar experience with tenofovir has not yet been reported, perhaps because it has not yet been used against HBV for as long as lamivudine or entecavir. Marcellin et al. 11 reported on a long-term follow-up study of 641 patients initially randomised into one of the pivotal studies done to achieve licensure of tenofovir for HBV. The main finding of this study was that almost all patients achieved a maintained virological response with dramatic improvements in their liver disease, including the apparent regression of cirrhosis in 74% of those who had biopsy-proven cirrhosis at baseline. It was noted in this report that seven patients (1.0%) died from malignant disease ,which included three with HCC, one with cholangiocarcinoma, and three with cancers at other sites.
Most of the data related to risk reduction of HCC have been gathered in Asia, although a recent cohort study reported from the USA indicated some benefit of antiviral therapy. Thus, Gordon et al.12recently described their analysis of electronic health records from 2 671 adult individuals (49% of whom were Asian) being tracked in the Chronic Hepatitis Cohort Study (CHeCS).13 Of these, 820 had received some form of antiviral therapy, including possibly IFN-α, while the remaining 1851 were untreated. Patients who received antiviral therapy had a lower risk of HCC than those who did not (AHR 0.39; 95% confidence interval 0.27 - 0.56; p<0.001).
In a review article summarising data on prevention of HBV-related HCC, Lai and Yuen14 noted that, while long-term follow-up studies of interferon-treated patients show inconsistent results, beneficial effects could be observed in those treated with nucleoside analogues, particularly among those with pre-existing cirrhosis and those who achieved substantial viral control on therapy.
At least two meta-analyses have been done of the anti-cancer benefits of antiviral therapy in chronic hepatitis B.15,16 Interestingly, one of these found a greater reduction in HCC in patients treated with lamivudine compared with those who were not treated, and even more substantial benefits in patients receiving the newer nucleoside analogues, whereas the second meta-analysis showed only a marginal benefit, limited to those patients with cirrhosis, possibly because the latter study only included controlled trials, whereas other analyses have also included cohort studies.
Hepatitis D
The hepatitis D virus (HDV) is an RNA virus that requires HBsAg to complete its replicative cycle. Therefore, it only infects individuals who are already infected with HBV.17 HDV appears to aggravate HBV liver disease, with a higher occurrence of cirrhosis. Because of this higher frequency of cirrhosis, HCC also appears to be more common.18 The mainstay of therapy for chronic HDV infection remains IF^, often given for very prolonged periods or even indefinitely. There have, so far, been no reliable studies of HDV therapy aimed at preventing the development of HCC.
Hepatitis A and hepatitis E
There is currently no evidence that infection with either of these viral agents increases the risk of subsequent development of HCC. This is likely to be because both agents are predominantly associated with acute, self-limited infection. Indeed, hepatitis A viral infection never becomes chronic and, while hepatitis E viral infection may be chronic, this is a rare occurrence and tends to occur predominantly in immunosuppressed persons.
Hepatitis C
Chronic HCV infection appears to be associated with about 30% of HCCs worldwide, although in some regions, including Europe and the United States, it is the leading underlying cause of HCC.19 Measures to prevent HCV-related HCC include prevention of HCV infection in the first place (primary prevention), control of elimination of HCV infection (secondary prevention), and treatment of HCV after HCC has already developed and been treated, with the hope of preventing new tumours from developing (tertiary prevention).
Public health efforts to reduce the incidence of HCV infection have been remarkably successful. Post-transfusion HCV is now an extremely rare event in the developed world, where transfusions of blood and blood products was a major source of hepatitis C infection as recently as three decades ago.20 In the USA, the overall number of acute hepatitis C cases has been declining steadily since 1992, presumably because of greater awareness of the risk of blood-borne infections among injection drug users. Interestingly, however, there may be a recent spike in incidence of acute hepatitis C infection related to the ongoing epidemic of heroin use in the USA but incidence rates are still way below those seen in previous decades.21 Unfortunately, little progress has been made in developing a vaccine against HCV, but tremendous strides have been taken in developing effective antiviral therapy, as described below.
In contrast to hepatitis B, the evidence is substantial that effective treatment of hepatitis C can reduce the risk of HCC. It has been estimated that about 20% of patients with chronic HCV infection will go on to develop cirrhosis, and it is these patients with advanced liver disease who are at greatest risk of hepatic decompensation and HCC. The first suggestion that antiviral therapy might be effective in preventing HCC came from a study of interferon, conducted in Japan and reported in 1995,'221 which showed a lower rate of HCC among patients with HCV-related cirrhosis who were treated with IFNa compared with those who were not, in a randomised controlled trial, although this study did not really differentiate outcomes in virological responders and non-responders. Over the next decade or so, there followed a series of reports based on retrospective studies showing that successful treatment of HCV with interferon, achieving a sustained virological response (SVR), was associated with substantially lower rates of HCC than among those who were untreated or those who were treated but did not achieve an SVR.23
The only other randomised controlled trials to be done on this topic were trials of long-term maintenance therapy using alphainterferon in patients who had previously not responded virologically to a course of interferon treatment. The HALT-C Trial (Hepatitis C Antiviral Long-term Therapy against Cirrhosis) was a large randomised controlled trial carried out at multiple sites within the USA. More than 1 000 patients were enrolled in the trial24 and it was one of only a few prospective randomised controlled trials aimed at trying to reduce the incidence of HCC with alpha-interferon as antiviral therapy.
This NIH-funded study randomised patients with HCV infection and advanced fibrosis (bridging hepatic fibrosis or cirrhosis) to receive long-term maintenance therapy with a low dose of PEG-IFNa or to remain untreated for a period of 4 years. The initial report from this study showed that antiviral therapy did not reduce the rate of either hepatic decompensation or HCC.24 A more prolonged follow-up of these cohorts showed that a slightly lower rate of HCC occurred in the interferon-treated patients - interestingly, this difference appeared several years after stopping therapy.25 Perhaps the best evidence to emerge from the HALT-C Trial in this regard was a comparison of liver-related outcomes after 7-8 years of follow-up among patients who did not respond to therapy. Morgan et al.26 found that patients treated in HALT-C, but who achieved an SVR, had significantly lower rates of hepatic decompensation, liver transplantation, or HCC than patients who remained non-responders to interferon after a follow up period of 7 - 8 years.
George et al27 reported on a 5-year follow-up of 150 patients achieving SVR. Among them, 49 had liver biopsies done prior to therapy and again after the 5-year period of follow-up. The degree of hepatic fibrosis declined substantially in almost all patients. Interestingly the only two patients in whom fibrosis was not reduced went on to develop HCC.
Other large cohort studies were reported by Aleman et al.,28 based on patients in Sweden, and by Van der Meer et al.,29 based on patients in several countries in Europe and Canada. The rates of HCC are summarised in Tables 1 and 2 , but were significantly lower in patients achieving SVR compared with those who were either untreated on did not respond to therapy.
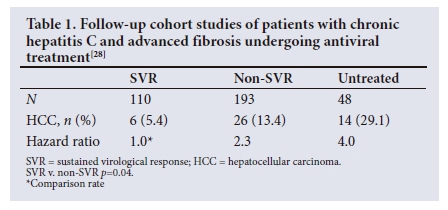
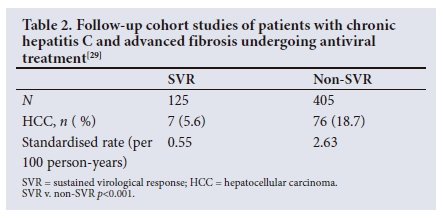
As with hepatitis B, meta-analyses have been conducted to assess the impact of antiviral therapy in preventing HCV-related HCC.
One of these used Cochrane methodology, focused on randomised controlled trials, and found statistically significant overall effects (p=0.007) when comparing cirrhotic patients achieving SVR with controls.30 A second meta-analysis looked at patients with advanced fibrosis or cirrhosis, and included both randomised controlled trials and cohort studies, and similarly found a statistically significant overall effect both in cirrhotics and patients will all degrees of fibrosis (p<0.001).31
Most recently, concerns have been raised about higher than estimated rates of occurrence and recurrence of HCC after viral clearance with the new direct-acting antiviral agents, particularly in patients with cirrhosis.32 These preliminary observations require further study in order to be validated.
Conclusion
In summary, antiviral treatment for viral hepatitis appears to have benefits in decreasing the risk of HCC in chronically infected patients. This benefit is most pronounced in patients with chronic hepatitis C and advanced hepatic fibrosis or cirrhosis in patients achieving viral clearance, while it seems much more marginal in patients with chronic hepatitis B, where antiviral therapy usually results in viral suppression rather than elimination.
Acknowledgements. None.
Author contributions. Sole author.
Funding. None.
Conflicts of interest. The author's institution has received research support from Gilead Sciences, Bristol-Myers Squibb and AbbVie. The author serves as a paid advisor to Gilead Sciences, Bristol-Myers Squibb, Tekmira, Editas and Target Pharmasolutions.
References
1. Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030-3044. https://doi.org/10.1002/ijc.21731 [ Links ]
2. Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013;310(9):974-976. https://doi.org/10.1001/jama.2013.276701 [ Links ]
3. Lok ASF, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology 2009;50(3):661-662. https://doi.org/10.1002/hep.23190 [ Links ]
4. Bonino F, Oliveri F, Colombatto P, Brunetto MR. Impact of interferon-alpha therapy on the development of hepatocellular carcinoma in patients with liver cirrhosis: Results of an international survey. J Viral Hepat 1997;4(Suppl 2):79-82. [ Links ]
5. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351(15):1521-1531. https://doi.org/10.1056/NEJMoa033364 [ Links ]
6. Poordad F, Chee GM. Viral resistance in hepatitis B: Prevalence and management. Curr Gastroenterol Rep 2010;12(1):62-69. https://doi.org/10.1007%2Fs11894-009-0088-1 [ Links ]
7. Kurokawa M, Hiramatsu N, Oze T, Takehara T. Long-term effect of lamivudine treatment on the incidence of hepatocellular carcinoma in patients with hepatitis B virus infection. J Gastroenterol 2012;47:577-585. https://doi.org/10.1007/s00535-011-0522-7 [ Links ]
8. Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: According to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol 2010;53(1):118-125. https://doi.org/10.1016/j.jhep.2010.02.026 [ Links ]
9. Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013;58(1):98-107. https://doi.org/10.1002/hep.26180 [ Links ]
10. Papatheodoridis GV, Dalekos GN, Yurdaydin C, et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol 2015;62(2):363-370. https://doi.org/10.1016/j.jhep.2014.08.045 [ Links ]
11. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013;381(9865):468-475. https://doi.org/10.1016/S0140-6736(12)61425-1 [ Links ]
12. Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol 2014;12(5):885-893. https://doi.org/10.1016/j.cgh.2013.09.062 [ Links ]
13. Di Bisceglie AM. Prevention of hepatocellular carcinoma resulting from hepatitis B: Are we there yet; Clin Gastroenterol Hepatol 2014;12(5):894-896. https://doi.org/10.1016/j.cgh.2013.11.027 [ Links ]
14. Lai C-L, Yuen M-F. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology 2013;57(1):399-408. https://doi.org/10.1002/hep.25937 [ Links ]
15. Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: The impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther 2013;38(2):98-106. https://doi.org/10.1111/apt.12344 [ Links ]
16. Thiele M, Gluud LL, Dahl EK, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma and mortality in chronic hepatitis B: Systematic review and meta-analysis. BMJ Open 2013;2(5):e003265. https://doi.org/10.1136/bmjopen-2013-003265 [ Links ]
17. Negro F. Hepatitis D virus coinfection and superinfection. Cold Spring Harb Perspect Med 2014;4(11):a021550. https://doi.org/10.1101%2Fcshperspect.a021550 [ Links ]
18. Oliveri F, Brunetto MR, Baldi M, et al. Hepatitis delta virus (HDV) infection and hepatocellular carcinoma (HCC). Prog Clin Biol Res1991;364:217-222. https://doi.org/10.4254%2Fwjh.v7.i5.777 [ Links ]
19. El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we; Where do we go; Hepatology 2014;60(5):1767-1775. https://doi.org/10.1002/hep.27222 [ Links ]
20. Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 2010;50(7):1495-1504. https://doi.org/10.1111/j.1537-2995.2010.02622.x [ Links ]
21. Strathdee SA, Beyrer C. Threading the needle - how to stop the HIV outbreak in rural Indiana. N Engl J Med 2015;373(5):397-399. https://doi.org/10.1056/NEJMp1507252 [ Links ]
22. Nishiguchi S, Kuroki T, Nakatani S, et al Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995;346(8982):1051-1055. [ Links ]
23. Imai Y, Kawata S, Tamura S, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Ann Intern Med 1998;129(2):94-99. [ Links ]
24. Di Bisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med 2008;359(23):2429-2441. https://doi.org/10.1056/NEJMoa0707615 [ Links ]
25. Lok AS, Seeff LB, Morgan TR, et al Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136(1):138-148. [ Links ]
26. Morgan TR, Ghany MG, Kim H, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52(3):833-844. https://doi.org/10.1002/hep.23744 [ Links ]
27. George SL, Bacon BR, Brunt EM, et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: A 5 year follow-up of 150 patients. Hepatology 2009;49(3):729-738. https://doi.org/10.1002/hep.22694 [ Links ]
28. Aleman S, Rahbin N, Weiland O, et al A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis 2013;57(2):230-236. https://doi.org/10.1093/cid/cit234 [ Links ]
29. Van der Meer AJ, Veldt BJ, Feld JJ, et al Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308(24):2584-2593. https://doi.org/10.1001/jama.2012.144878 [ Links ]
30. Kimer N, Dahl EK, Gluud LL, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: Systematic review and meta-analysis of randomised controlled trials. BMJ Open 2012;2(5):e001313 https://doi.org/10.1136/bmjopen-2012-001313 [ Links ]
31. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann Intern Med 2013;158(5 Pt 1):329-337. https://doi.org/10.7326/0003-4819-158-5-201303050-00005 [ Links ]
32. Conti F, Buonfiglioli F, Scuteri A, et al Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65(4):727-733. https://doi.org/10.1016/j.jhep.2016.06.015 [ Links ]
 Correspondence:
Correspondence:
A M Di Bisceglie
adrian.dibisceglie@health.slu.edu














