Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.108 no.7 Pretoria Jul. 2018
http://dx.doi.org/10.7196/samj.2018.v108i7.13041
RESEARCH
Hepatitis C prevalence in HIV-infected heterosexual men and men who have sex with men
N A GogelaI; M W SonderupII; K RebeIII, IV; T ChiveseV, VI; C W SpearmanVII
IFCP (SA); Division of Hepatology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
IIFCP (SA), MMed; Division of Hepatology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
IIIFCP (SA); ANOVA Health Institute, Johannesburg and Cape Town, South Africa
IVFCP (SA); Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VMSc; Department of Medicine, Faculty of Health Sciences, University of Cape Town, South Africa
VIMSc; Biostats Unit, Centre for Evidence Based Health Care, Department of Global Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
VIIFCP (SA), PhD; Division of Hepatology, Department of Medicine, Faculty of Health Sciences, University of Cape Town and Groote Schuur Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND. Globally 1% of individuals are infected with hepatitis C virus (HCV). In South Africa (SA) the prevalence ranges between 0.3% and 1%, with few prospective screening data available. Similarly, local data on transmission modes of HCV are limited, but probably include parenteral routes and pre-1992 blood or blood products. The risk of heterosexual transmission of HCV is low but is increased in men who have sex with men (MSM), with co-transmission risk of both HIV and HCV.
OBJECTIVES. Given few local data, we sought to better understand HCV characteristics and prevalence in two groups of HIV-infected men.
METHODS. HIV-positive men in the greater Cape Town metropolitan area were recruited. Sexual orientation was self-identified and demographic and other personal data were obtained via a confidential questionnaire. Participants were screened for HCV after a blood draw. Those with positive HCV tests had further HCV RNA confirmation. Risk factors associated with HCV seropositivity were determined.
RESULTS. Five hundred HIV-positive men were recruited, 285 (57.0%) MSM and 215 (43.0%) non-MSM, median age 36 years (interquartile range (IQR) 20 - 64) and 37 years (IQR 21 - 56), respectively (p=NS). Overall, 3.4% (n=17) screened HCV-positive, 5.6% MSM (n=16) and 0.5% non-MSM (n=1); 82.4% were viraemic for HCV RNA. In respect of genotype distribution, 50.0% were infected with genotype 1a, 14.3% with genotype 4 and 35.7% with genotype 2. In terms of risk, MSM were more likely to have used drugs (54.4% v. 30.2%; p<0.001) and to have used all five modes of drug administration (13.0% MSM v. 0.5% non-MSM for injected drugs, 36.1% v. 2.3% for inhaled, 10.0% v. 0% for rectal, 48.1% v. 28.8% for smoked and 27.4% v. 2.3% for oral). More MSM than non-MSM (46.3% v. 16.7%) reported having sex while using recreational drugs, and similarly more MSM (21.4% v. 14%) reported having sex with a sex worker (SW). Risk factors for HCV seropositivity included drug use history (odds ratio (OR) 6.28, 95% confidence interval (CI) 1.78 - 22.12; p=0.004) and in MSM, sex with an SW (OR 5.5, 95% CI 2.06 - 14.68; p=0.001) or use of recreational drugs with sex (OR 6.88, 95% CI 2.21 - 21.44; p=0.001).
CONCLUSIONS. HCV prevalence in HIV-positive MSM is higher than previously appreciated or documented in SA. Risk factors include injection drug use, use of recreational drugs with sex, and sex with SWs. Targeted interventions are required to address this emerging challenge to achieve the viral hepatitis elimination ideal by 2030.
In 2017 an estimated 71 million people globally were hepatitis C virus (HCV) viraemic, while 2.3 million were HIV co-infected.1HCV is a leading cause of cirrhosis and hepatocellular carcinoma (HCC) worldwide.2 Traditionally HCV transmission has mostly been parenteral, typically in people who inject drugs (PWID), but recent data have demonstrated that HCV prevalence increased by 15 - 20% in HIV-infected men who have sex with men (MSM) between 2007 and 2008.3Supporting these findings are reports from the USA, Australia and Eastern Europe of HCV emerging as a sexually transmitted infection among MSM. Data from the UK Public Health Service in 2012 noted HIV notifications increasing by 24% among MSM, of whom 13% were HCV co-infected.4 In 2015, HCV accounted for almost 38 000 deaths in sub-Saharan Africa.5 However, there are few data for high-risk groups, with accurate data collection complicated by potential cultural bias and laws against PWID and MSM. Approximately 8% of the global PWID population resides in sub-Saharan Africa, and hepatitis C is incompletely characterised in this key population.6
The genotype distribution of HCV in a population is informative, and the finding of predominantly genotypes 1 and 4 in non-PWID MSM and genotype 3a in PWID suggests possible intra-network modes of transmission.7
South Africa (SA) is an epicentre of the HIV pandemic, with an estimated 12.2% HIV prevalence equating to some 7.1 million HIV-infected people.8 There are very few data on HCV and HCV-HIV co-infection prevalence in SA. Data from blood transfusion services indicate donor HCV viraemic rates of <0.3% and random clinic-based screening data reporting 1% seroprevalence rates. Of note, in a 1997 antiretroviral therapy study with mandatory HCV screening that included SA as a site, HCV antibody prevalence in HIV-positive patients was 2%, much higher than would have been anticipated.9Local HIV management guidelines do not include routine screening for HCV. A concern with HIV/HCV co-infection is that it can accelerate the progression of liver disease and HCC risk.10
Objectives
Given the need for the better understanding of our local HCV epidemiology, we elected to determine local HCV prevalence in an at-risk group, viz. HIV-positive men, by comparing heterosexual men and MSM, to better characterise modes of transmission and HCV genotype distribution.
Methods
Study design
Serologically confirmed HIV-positive men aged >18 years were prospectively recruited between 2011 and 2014 from healthcare centres in the greater Cape Town metropolitan area, including a dedicated clinic serving MSM. Following informed consent, participants self-identified their sexual orientation as heterosexual or MSM. A confidential questionnaire was administered during a face-to-face interview. Blood samples were obtained for testing and storage at -80oC within an hour of collection. Serum was tested for the presence of hepatitis C IgG antibody using the ARCHITECT II system (Abbott Diagnostics Division, USA). Samples positive for hepatitis C IgG antibody were analysed for the presence of hepatitis C RNA by means of an in-house polymerase chain reaction (PCR) technique after amplifying the 5'NCR region of the virus. HCV genotype was determined using the Versant HCV Genotype v2.0 Line Probe Assay (Siemens AG, Germany) and viral loads using the COBAS Ampliprep/Cobas TaqMan v2.0 (Roche Diagnostics, Switzerland). All participants identified as viraemic for HCV were referred to the Liver Clinic at Groote Schuur Hospital, Cape Town. The Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town, approved the study (ref. no. 355/ 2009). The study complied with the Declaration of Helsinki (2007).
Data collection
A standardised structured questionnaire that included demographic data was confidentially administered to all participants to capture information on age, self-identified ethnicity/race and sexual orientation, and other risk factors for HCV acquisition, e.g. blood transfusion/blood products received prior to 1992. A detailed history of past or current substance use was obtained, including cannabis, MDMA (methylenedioxymethamphetamine), GHB (gamma-hydroxybutyr-ate), crystal methamphetamine, cocaine, CAT (methcathinone), liquid MDMA (liquid E), heroin and methaqualone. The mode of substance use administration was recorded as oral, sniffing/insufflation, injection, smoking or rectal, and the quantity of alcohol consumed (g/d) was also documented. Information on the use of these substances at times of sexual intercourse and on the use of commercial sex worker (SW) services was also recorded. Additional data including possible needlestick injury (especially for healthcare workers) and known hepatitis B surface antigen status and CD4+ counts were documented.
Statistical analysis
The non-MSM and MSM groups were compared and statistical significance was tested at a level of 0.05 with 95% confidence intervals (CIs). Medians and interquartile ranges (IQRs) were reported for measured data (age and CD4+ count) as they were not normally distributed, while frequencies and percentages were used to describe categorical data. The χ2 test was used to compare categorical outcomes and the Wilcoxon rank-sum test to compare measured data. HCV prevalence as reported by antibody and viraemia was calculated as a simple proportion of HCV-positives divided by the whole sample. Exploratory univariate analysis for factors associated with acquiring HCV infection was performed. The use of individual drugs (cannabis, MDMA, GHB/liquid E, crystal meth, cocaine, CAT, heroin and methaqualone) and drug administration modes (oral, sniffing, injection, smoking and rectal) were also explored for association with acquiring HCV. Other variables considered were previous parenteral injury for former or current healthcare workers, sexual history variables such as whether the individual was in a current relationship or not, current sex partner, MSM v. non-MSM, drug use before or during sex, and sex with a commercial SW. Odds ratios (ORs) and their 95% CIs were reported. Statistical analysis was performed using Stata 15 (StataCorp, USA).
Results
Demographic characteristics
Table 1 provides a summary of the demographics of the study participants. Of the 500 HIV-positive men recruited, 285 (57.0%) were MSM and 215 (43.0%) non-MSM. The median ages of the MSM and the non-MSM were 36 years (IQR 20 - 64) and 37 years (IQR 21 - 56) (p=NS). The median CD4+ T-cell count of the MSM was significantly higher than that of the non-MSM, 413 cells/μL (IQR 78 - 989) and 283 cells/μL (IQR 7 - 727), respectively; p<0.001. Most non-MSM participants (88.8%) were black African, while most MSM (48.1%) were white. There were no differences in pre-1992 blood/blood product exposure between MSM and non-MSM. MSM were more likely to have used drugs (54.4% v. 30.2%, p<0.001). Furthermore, MSM were more likely than non-MSM to have used all five modes of drug administration (p<0.001). MSM were also more likely to have used cannabis, GHB, MDMA, crystal meth, cocaine, CAT or heroin. No participant gave a history of a needlestick injury. Non-MSM were more likely than MSM to be in a monogamous relationship (76.3% v. 42.1%; p<0.001). A small component of MSM (2.3%) reported women as their predominant sexual partners, while 7.8% reported having both males and females as their normal sexual partners. Of the MSM, 46.3% reported having sex under the influence of recreational drugs, compared with 16.7% of non-MSM (p<0.001), while 21.4% of MSM reported having sex with a commercial SW, compared with 14.0% of non-MSM (p=0.033). Significantly more non-MSM than MSM, 26.1% and 18.3%, respectively, reported alcohol consumption of >40 g/day (p=0.036). Hepatitis B surface antigen status did not differ significantly between MSM and non-MSM (p=0.064).
HCV prevalence
In total, 3.4% (n=17) were HCV IgG antibody-positive at screening (Table 2), the majority in the MSM group (5.6% of MSM (n=16) and 0.5% of non-MSM (n=1)). Non-MSM who screened HCV antibody-positive reported only inhaled heroin use and no other drug use. Of the MSM who screened positive, 14 were HCV PCR-positive and 3 negative, yielding a viraemia rate of 2.8%. In respect of genotype distribution, 50.0% were genotype 1a, 14.3% genotype 4 (subtype 4d) and 35.7% genotype 2 (subtypes 2b or 2c). The median HCV viral load was 538 500 IU/mL (range 19 000 - 1 400 000). Of those who screened HCV-positive, a single participant reported using a significant amount of alcohol (>40 g/d).

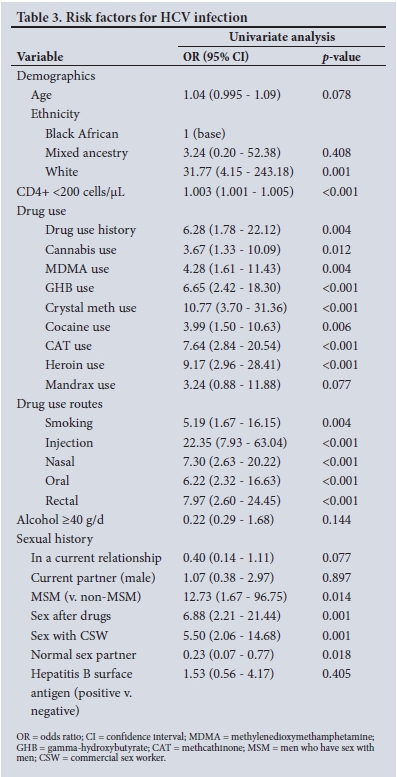
Risk factors for HCV
Factors associated with an increased risk of acquiring HCV infection are listed in Table 3. White race, low CD4+ count and use of drugs were strongly associated with risk. This was irrespective of the type of drug used or route of administration. Use of drugs with sex and sex with an SW were associated with equal risks of being HCV-positive.
Discussion
The overall HCV seroprevalence in HIV-positive men was 3.4%, notably higher than previously reported in SA. However, MSM constituted the overwhelming majority of those who screened HCV-positive. This is in keeping with data supporting MSM as an emerging at-risk population. Our study is the first prospective screening study of its kind in SA specifically looking at this key demographic. A recent systematic review of HCV seroprevalence in the sub-Saharan Africa region suggested a pooled HCV seroprevalence rate of 2.98%. When subcategorised, the HCV seroprevalence rate was 5.7% among HIV-infected individuals.5,11Our findings are not dissimilar. Two studies in Nigeria, based on hepatitis C antibody seroprevalence in different centres, 3 years apart and performed in heterogeneous groups of HIV-positive cohorts, demonstrated even higher seroprevalence rates of 10.8% and 15%, respectively.'12,13 Interestingly, more women tested HCV antibody-positive. In one of the studies there was no significant correlation between injected drug use and HCV infection.'13 This possibly suggests that HIV poses a signficant risk for HCV acquisition regardless of gender. In this cohort, it is unclear whether the women were asked about their sexual risks, or whether they were screened for other sexually transmitted infections. In those who were HCV antibody-positive, 82% confirmed HCV RNA-positive, yielding an overall viraemic rate of 2.8%. All our viraemic patients were in the MSM cohort. We observed that MSM were more likely to use drugs and significantly more used an injection route. Existing data support the fact that injecting drug use is associated with increased risks of both HIV and HCV acquisition.'14 All participants in the MSM group who tested HCV antibody-positive had a history of injecting drug use, among other routes. The single non-MSM participant denied injecting drug use.
An important factor associated with HCV acquisition was high-risk sexual behaviour. There were significant differences in numbers of MSM who engaged in such behaviour, namely having unprotected sex with commercial SWs and having sex with the use of recreational drugs. Several studies have shown that HCV is increasing as a sexually transmitted infection among MSM who do not inject drugs.16,17 Several observational studies comparing injecting with non-injecting MSM have noted that non-injecting patients had different viral phylogenetic profiles compared with injectors.18-20The conclusion from this observation suggested a permucosal route of transmission, especially among HIV-positive MSM who had multiple sexual partners, MSM who tested positive for other sexually transmitted disease, namely syphilis, and MSM who engaged in sexual practices that could cause mucosal trauma, such as fisting and use of objects. Similarly, we observed an association between sex with SWs and HCV seropositivity. We observed that injecting drug users were seven times more likely than non-injecting drug users to acquire HCV infection. It is, however, thought that sexual behaviours that are likely to cause mucosal trauma increase the risk of HCV transmission.21 These behaviours include lack of lubricant use, possibly douching before anal sex, multiple partners and sex in the context of crystal methamphetamine use. We did not record information on specific sexual practices of MSM in our study, other than determination of anal sex. We do, however, note that in our local experience we have observed several HCV-positive MSM without any reported substance use or behaviours likely to cause trauma to anorectal tissue, suggesting ordinary unprotected penile-anal sexual activity as the only risk factor for HCV transmission in often concomitantly HIV-positive MSM.22
An unexpected finding was the genotype distribution in our cohort. Genotype 1a predominated (50.0%), followed by genotype 2 (35.7%) and genotype 4 (14.3%), with no genotype 3 or 5 reported. This contrasts with a study in the general SA population, where even though genotype 1 was a predominant genotype among blood donors (34%), genotype 5 was the most prevalent overall.51 However, a very recent seroprevalence survey of key populations in SA demonstrated genotypes 1, 3 and 4 in MSM, PWID and SWs.23 This suggests that selected genotypes are circulating in SA MSM and other key populations. Furthermore, given that Cape Town is a popular international destination, the possibility of sex tourism by MSM introduces the potential that dominant genotypes, e.g. genotype 2c, from European origins may explain this genotype predominating in our MSM population. Phylogenetic linkage analyses of patients with similar, yet uncommon genotype subtypes would suggest a network spread of virus. Seven of the 14 viraemic patients have been linked to care. They were all treated successfully, one with pegylated interferon/ribavirin-based therapy and all the others with the more recent direct-acting antiviral therapies for hepatitis C. Despite proper counselling during the recruiting period, 50% of HCV-viraemic individuals were lost to follow-up and appropriate linkage to care. This has serious implications, as these are high-risk individuals who are likely to onwardly transmit the infection. Hepatitis C is invariably an asymptomatic infection, and if high-risk individuals are not screened periodically, we will fail to identify and treat HCV-infected individuals and prevent onward transmission. Elimination of viral hepatitis in the high-risk groups of MSM and PWID requires active harm reduction practices including hepatitis B vaccination, condom use and needle-syringe and opiate substitution programmes.
Study limitations
This was a small select group that lacked heterogeneity. A larger number more representative of SA, including provinces more affected by HIV infection, may yield different HCV seroprevalence rates.
Conclusions
This study alone raises concerns that HCV seroprevalence is indeed underestimated in SA in at-risk populations. Key populations, and particularly those who are HIV-infected, should access HCV screening. Furthermore, all patients with the risk profiles described above should be tested for HCV and be linked to care. Without addressing infections in key populations, attainment of the elimination ideal for hepatitis C will not be achieved.
Acknowledgements. The participants, who willingly gave of their time, are acknowledged, They have deepened our understanding of the challenges we face.
Author contributions. MWS conceived the study. NAG and TC executed the fieldwork for the study in participant recruitment and prepared the draft manuscript. All authors contributed to the development of the manuscript and final submission.
Funding. A Gastroenterology Foundation of South Africa (GFSA) grant funded the study.
Conflicts of interest. None.
References
1. Messina JP, Humphreys I, Flaxman A, et aL Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015;61(1):77-87. https://doi.org/10.1002/hep.27259 [ Links ]
2. Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013;57(6):2164-2170. https://doi.org/10.1002/hep.26218 [ Links ]
3. Van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis 2007;196(2):230-238. https://doi.org/10.1086/518796 [ Links ]
4. Daskalopoulou M, Rodger A, Thornton A, et al. Sexual behaviour, recreational drug use and hepatitis C co-infection in HIV-diagnosed men who have sex with men in the United Kingdom: Results from the ASTRA study. J Int AIDS Soc 2014;17(4 Suppl 3):19630. https://doi.org/10.7448/IAS.17.4.19630 [ Links ]
5. Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol Hepatol 2017;2(3):161-176. https://doi.org/10.1016/S2468-1253(16)30181-9 [ Links ]
6. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet 2011;378(9791):571-583. https://doi.org/10.1016/S0140-6736(11)61097-0 [ Links ]
7. Urbanus AT, van de Laar TJ, Geskus R, et al. Trends in hepatitis C virus infections among MSM attending a sexually transmitted infection clinic; 1995 - 2010. AIDS 2014;28(5):781-790. https://doi.org/10.1097/QAD.0000000000000126 [ Links ]
8. Zuma K, Shisana O, Rehle TM, et al. New insights into HIV epidemic in South Africa: Key findings from the National HIV Prevalence, Incidence and Behaviour Survey, 2012. Afr J AIDS Res 2016;15(1):67-75. https://doi.org/10.2989/16085906.2016.1153491 [ Links ]
9. Amin J, Kaye M, Skidmore S, Pillay D, Cooper DA, Dore GJ. HIV and hepatitis C coinfection within the CAESAR study. HIV Med 2004;5(3):174-179. https://doi.org/10.1111/j.1468-1293.2004.00207.x [ Links ]
10. Gjaerde LI, Shepherd L, Jablonowska E, et al. Trends in incidences and risk factors for hepatocellular carcinoma and other liver events in HIV and hepatitis C virus-coinfected individuals from 2001 to 2014: A multicohort study. Clin Infect Dis 2016;63(6):821-829. https://doi.org/10.1093/cid/ciw380 [ Links ]
11. Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Infect Dis 2015;15(7):819-824. https://doi.org/10.1016/S1473-3099(15)00006-7 [ Links ]
12. Tremeau-Bravard A, Ogbukagu IC, Ticao CJ, Abubakar JJ. Seroprevalence of hepatitis B and C infection among the HIV-positive population in Abuja, Nigeria. Afr Health Sci 2012;12(3):312-317. https://doi.org/10.4314/ahs.v12i3.10 [ Links ]
13. Newton OE, Oghene OA, Okonko IO. Anti-HCV antibody among newly diagnosed HIV patients in Ughelli, a suburban area of Delta State Nigeria. Afr Health Sci 2015;15(3):728-736. https://doi.org/10.4314/ahs.v15i3.5 [ Links ]
14. Zhuang X, Wang Y, Chow EP, Liang Y, Wilson DP, Zhang L. HIV and HCV prevalence among entrants to methadone maintenance treatment clinics in China: A systematic review and meta-analysis. BMC Infect Dis 2012;8(12):130. https://doi.org/10.1186/1471-2334-12-130 [ Links ]
15. Centers for Disease C, Prevention. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men - New York City, 2005 - 2010. MMWR Morb Mortal Wkly Rep 2011;60(28):945-950. [ Links ]
16. Fierer DS. Epidemic of sexually transmitted hepatitis C virus infection among HIV-infected men. Curr Infect Dis Rep 2010;12(2):118-125. https://doi.org/10.1007/s11908-010-0088-1 [ Links ]
17. Breskin A, Drobnik A, Pathela P, et al. Factors associated with hepatitis C infection among HIV-infected men who have sex with men with no reported injection drug use in New York City, 2000 - 2010. Sex Transm Dis 2015;42(7):382-386. https://doi.org/10.1097/OLQ.0000000000000293 [ Links ]
18. Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: An expanding epidemic. AIDS 2009;23(12):F1-F7. https://doi.org/10.1097/QAD.0b013e32832e5631 [ Links ]
19. Van de Laar TJ, Matthews GV, Prins M, Danta M. Acute hepatitis C in HIV-infected men who have sex with men: An emerging sexually transmitted infection. AIDS 2010;24(12):1799-1812. https://doi.org/10.1097/QAD.0b013e32833c11a5 [ Links ]
20. Chan DP, Lin AW, Wong KH, Wong NS, Lee SS. Diverse origins of hepatitis C virus in HIV co-infected men who have sex with men in Hong Kong. Virol J 2015;12:120. https://doi.org/10.1186/s12985-015-0355-8 [ Links ]
21. Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission; Hepatology 2010;52(4):1497-1505. https://doi.org/10.1002/hep.23808 [ Links ]
22. Semugoma NP, Rebe K, Sonderup MW, et al. Hepatitis C: A South African literature review and results from a burden of disease study among a cohort of drug-using men who have sex with men in Cape Town, South Africa. S Afr Med J 2017;107(12):1116-1120. https://doi.org/10.7196/SAMJ.2017.v107i12.12623 [ Links ]
23. Sonderup MW, Prabdial-Singh N, Manamela MJ, et al. Characteristics of hepatitis B and C prevalence in key populations in South Africa. Hepatology 2017;66(S1):561A-562A. https://doi.org/10.1002/hep.29501 [ Links ]
 Correspondence:
Correspondence:
M W Sonderup
mark.sonderup@uct.ac.za
Accepted 26 January 2018.














